Method Article
Co-Culture and Transduction of Murine Thymocytes on Delta-Like 4-Expressing Stromal Cells to Study Oncogenes in T-Cell Leukemia
In This Article
Summary
This protocol describes the isolation of double-negative thymocytes from the mouse thymus followed by retroviral transduction and co-culture on the delta-like 4-expressing bone marrow stromal cell line co-culture system (OP9-DL4) for further functional analysis.
Abstract
Transduced mouse immature thymocytes can be differentiated into T cells in vitro using the delta-like 4-expressing bone marrow stromal cell line co-culture system (OP9-DL4). As retroviral transduction requires dividing cells for transgene integration, OP9-DL4 provides a suitable in vitro environment for cultivating hematopoietic progenitor cells. This is particularly advantageous when studying the effects of the expression of a specific gene during normal T cell development and leukemogenesis, as it allows researchers to circumvent the time-consuming process of generating transgenic mice. To achieve successful outcomes, a series of coordinated steps involving the simultaneous manipulation of different types of cells must be carefully performed. Although these are very well-established procedures, the lack of a common source in the literature often means a series of optimizations are required, which can be time-consuming. This protocol has been shown to be efficient in transducing primary thymocytes followed by differentiation on OP9-DL4 cells. Detailed here is a protocol that can serve as a quick and optimized guide for the co-culture of retrovirally transduced thymocytes on OP9-DL4 stromal cells.
Introduction
The OP9 bone marrow stromal cell line provides a useful in vitro system for the induction of lymphopoiesis from several sources of progenitors1. The first studies that used OP9 cells demonstrated that the lack of macrophage colony-stimulating factor (MCSF) expression contributed to the ability of the OP9 cell line to support hematopoiesis and B cell differentiation from bone marrow-derived hematopoietic stem cells (HSCs), as was later also shown for embryonic stem cells (ESCs)2,3,4,5. In previous studies, the generation of delta-like 1/4-expressing OP9 cells (OP9-DL1/OP9-DL4) enabled the induction of T cell lineage commitment6 and demonstrated the ability to successfully recapitulate thymic maturation7,8. Briefly, T cell development has been described by the sequential expression of CD4 and CD8 molecules. Immature thymocytes are "double-negative" (DN, CD4− CD8−) and can be further subdivided according to the surface expression of CD44 and CD25. DN thymocytes differentiate through the immature single-positive (ISP) stage, characterized by CD8 expression in mice and CD4 in humans, followed by the double-positive (DP) stage, characterized by the co-expression of CD4 and CD8, and, finally, the mature single-positive stage, characterized by the expression of CD4 or CD89. HSCs express the Notch1 receptor, which normally interacts with the delta-like 4 (DL4) expressed on thymic epithelial cells to induce T lineage differentiation10. Hence, interest in the OP9-DL1/4 model has progressively increased, leading to the extensive use of this approach in a wide variety of applications in the past two decades8,11,12,13. Although DL1 and DL4 are both able to support T cell differentiation in vitro, they show differential requirements in vivo, and a few studies have suggested that OP9_DL4 is more efficient than OP9_DL1 in recapitulating the mouse thymic environment7,14.
Among the potential applications of the OP9-DL1/4 system, there is particular interest in the combination of this system with the transduction of DN cells or HSCs with retroviral vectors. This combination is an effective way to manipulate gene expression during normal T cell development and leukemogenesis and has been shown to be an efficient method to induce or inhibit the function of a gene of interest15,16,17. This model has been particularly successfully used to study the collaboration between the oncogenes driving leukemia15 as it is flexible and enables the examination of the effects of multiple gene combinations in a reasonable time, in contrast to generating transgenic mice. Moreover, similar models have been used previously to assess the effects of introducing oncogenes into normal cells15,16,17. Additionally, retroviral transduction requires dividing cells for transgene integration18, and while lentiviral transduction would overcome this limitation by eliminating the need for dividing cells for transgene integration, we have been unable to achieve the successful transduction of DN thymocytes using lentiviral vectors. Thus, OP9-DL1/DL4 is a suitable tool for growing hematopoietic progenitor cells.
The standard protocol for the lymphopoiesis of transduced thymocytes on OP9-DL4 involves a series of coordinated steps that must be carefully performed to achieve a successful outcome. Although these are techniques that have been serving the community well for many years, often the protocols available in the literature are fragmented. As a result, each laboratory is forced to adapt and optimize different stages of the procedure, which can be time-consuming. Here, this protocol describes the isolation of DN thymocytes from the mouse thymus, followed by retroviral transduction and co-culture on OP9-DL4 stromal cells for further functional analysis. This established protocol has been shown to be efficient and reproducible in transducing primary thymocytes, followed by differentiation on OP9-DL4 cells or the induction of T cell acute lymphoblastic leukemia15.
Protocol
All animal experiments described were approved by the NIH Institutional Biosafety Committee (IBC) and Animal Care and Use Committee (ACUC). See the Table of Materials for details related to all the reagents and materials used in this protocol. Refer to published guidelines19 for more details on retrovirus-producer cell culture and maintenance procedures. See Figure 1 for an overview of the protocol.
1. Starting the culture of OP9-DL4 cells (Day 1)
- Thaw OP9-DL4 cells rapidly by holding the vial and gently shaking in a water bath at 37 °C. Immediately transfer the cells to a centrifuge tube containing 5 mL of the OP9 medium (MEM-alpha medium, 10% FBS, 50 U/mL penicillin/streptomycin, 55 µM 2-mercaptoethanol, 2 mM L-glutamine) to remove the cryoprotectant agents.
- Centrifuge at 300 × g for 5 min at room temperature. Discard the supernatant, and resuspend the cells in 1 mL of OP9 medium.
- Place 5 mL of OP9 medium into a T25 flask, and add the resuspended cells to this flask. Culture at 37 °C and 5% CO2.
- After 2–3 days, subculture the cells using a 1:3 split (passage the cells every Monday, Wednesday, and Friday).
NOTE: Split the OP9-DL4 cells before they reach confluency. The OP9-DL4 flask must be 80%–90% confluent on days 6–7 and on days 8–9+ for co-culture with thymocytes. Hence, it is important to plan how many OP9-DL4 cells will be required on those days while splitting the OP9-DL4 cells.- Discard the medium from the flask, and wash the monolayer with 1x PBS. Discard the PBS, add 1 mL of 0.25% trypsin, and incubate for 1–5 min at 37 °C, or until the cells have dislodged from the flask. Thump the flask to dislodge the cells.
NOTE: (Optional) The progress of cell dissociation can be checked by microscopy. - Add 5 mL of OP9 medium to inactivate the trypsin, and resuspend the cells by rinsing the cell-covered surface of the flask during resuspension.
- Centrifuge the cell suspension at 300 × g for 5 min at room temperature and resuspend in 3 mL of OP9 medium (for a 1:3 split).
- Place 1 mL of the cell suspension into a T25 flask that already contains 5 mL of fresh OP9 medium. Freeze/discard the remaining cells.
- To freeze the OP9-DL4 cells, resuspend the cells in 90% FBS/10% DMSO at a ratio of 1 mL of freezing medium to one vial of cells.
- Freeze in 1 mL cryovials at −80 °C or in liquid nitrogen, depending on the future requirements. Store in liquid nitrogen for prolonged storage.
- Discard the medium from the flask, and wash the monolayer with 1x PBS. Discard the PBS, add 1 mL of 0.25% trypsin, and incubate for 1–5 min at 37 °C, or until the cells have dislodged from the flask. Thump the flask to dislodge the cells.
2. Starting the culture of the retrovirus-producer cell line (Day 1)
- Thaw the retrovirus-producer cell line rapidly by holding the vial and gently shaking in a water bath at 37 °C. Immediately transfer the cells to a centrifuge tube containing 5 mL of retrovirus-producer cell medium (RPC: DMEM, 10% FBS) to remove the cryoprotectant agent.
- Centrifuge at 300 × g for 5 min at room temperature. Discard the supernatant, and resuspend the cells in 1 mL of RPC medium.
- Place 5 mL of RPC medium into a T25 flask, and add the resuspended cells to this flask. Culture at 37 °C and 5% CO2.
- Passage the cells every 2–3 days at a 1:5 to 1:8 split.
- Thump the flask to dislodge the cells, and resuspend the cells by pipetting aggressively.
NOTE: These cells can be removed from the flask surface by aggressive pipetting without trypsin. Alternatively, trypsinize (0.05% trypsin/0.53 mM EDTA) until the cells easily detach and can be readily pipetted into a single-cell suspension.
- Thump the flask to dislodge the cells, and resuspend the cells by pipetting aggressively.
- Centrifuge the cell suspension at 300 × g for 5 min, and resuspend in 5 mL of RPC medium (for a 1:5 split).
- Place 1 mL of the cell suspension into a T25 flask that already contains 5 mL of fresh RPC medium. Freeze/discard the remaining cells. Freeze the retrovirus-producer cells in the same way as described for OP9-DL4 cells (steps 1.4.4.1–1.4.4.2).
3. Beginning the culture of the retrovirus-producer cells in 6-well plates (Days 4–5)
- Seed the retrovirus-producer cells 18–24 h prior to transfection at 70%–90% confluency in each well of a 6-well tissue culture plate in 2 mL of RPC medium,and incubate at 37 °C and 5% CO2.
NOTE: Transfect two wells of the retrovirus-producer cells for every 2–5 × 105 to 1 × 106 thymocytes that will be transduced.
4. Transfecting the retrovirus-producer cells to generate the retrovirus containing the genes of interest (Days 5–6)
- Replace the medium with fresh RPC medium 1 h before transfection.
- Prepare lipofection mixtures (for each well of a 6-well plate—scale up as needed): Dilute 4 µg of DNA (2 µg of helper plasmid (pCL-Eco) and 2 µg of transfer plasmid (pMIG) in 250 µL of reduced-serum medium. Mix gently.
- Mix 10 µL of transfection reagent with 250 uL of the reduced-serum medium from step 4.2. Incubate for 5 min at room temperature.
- After 5 min of incubation, combine the diluted DNA with diluted transfection reagent (total volume = 500 µL). Mix gently, and incubate for 20–25 min at room temperature.
- Add the 500 µL of DNA/transfection reagent mixtures gently to the well containing the retrovirus-producer cells by dropping onto the cells with a circular movement. Mix gently by rocking the plate back and forth, and incubate the plate in a 37 °C incubator for 16–24 h.
NOTE: Subconfluent cells (80%–90%) are best suited for transfection and potentially generate the highest viral titer. As the retrovirus-producer cells dislodge very easily from the flask, avoid sudden movements when handling these cells.
5. Changing the retrovirus-producer cell medium (Days 6–7)
- Replace the old medium approximately 16 h after transfection with 2 mL of fresh RPC medium. Continue to incubate cells at 37 °C for 20–24 h.
- Evaluate the transfection efficiency under a fluorescence microscope (optional).
NOTE: In this protocol, GFP-positive cells are the cells of interest (see Figure 2). This step depends on the retrovirus vector selected to use in this protocol (whether a reporter gene is present in the backbone).
6. Thymocyte preparation and depletion of CD4+ and CD8+ cells (Days 6–7)
- Harvest the thymus from a 4–6 week old C57BL/6 mouse. For more details on thymus harvesting, refer to Xing and Hogquist20.
NOTE: Mice were euthanized by CO2 inhalation followed by cervical dislocation. - Prepare a thymocyte single-cell suspension by placing the thymus in 5 mL of PBS in a Petri dish. Using sterile glass slides, place the thymus between the frosted surfaces of the slides, and gently rub the slides together, rolling the thymus between the two slides. Rinse the glass slides to collect the cells, and discard the remnant thymic stromal tissue.
NOTE: The estimated yield from one thymus is 90 × 106–100 × 106 cells, and approximately 1% of the cells will remain after CD4 and CD8 depletion. Use thymus tissue from younger mice for a better yield of cells, since the cellularity of the mouse thymus in the first weeks after birth increases quickly, reaches a plateau at 4–6 weeks of age, and progressively involutes subsequently21. Count the cells using an automatic blood cell counter or any alternative method. - Filter the thymic suspension through a 30 µm or 40 µm filter by pipetting 5 mL of the thymic suspension through a cell strainer. Centrifuge the cells at 300 × g for 10 min.
- Remove the supernatant, and lyse the red blood cells with ACK lysis buffer by adding (depending on the sample size) 1 mL of buffer per tube for 1 min. Add 5 mL of cell depletion buffer (500 mL of PBS [pH 7.2], 0.5% BSA, 2 mM 0.5M EDTA, pH 8.0) to deactivate the ACK lysis buffer, centrifuge at 300 × g for 10 min, and resuspend in 1–5 mL of cell depletion buffer for counting.
NOTE: Set aside the following on ice before depletion (pre-depletion): 70 µL of cell suspension to count (vary this depending on the counting cell method chosen) and 200 µL of cell suspension to determine the efficiency of depletion by staining for CD4 and CD8 and analyzing by flow cytometry22,23. - Count the cells, and centrifuge at 300 × g for 10 min. Resuspend the cells at 1 × 107/80 µL of cell depletion buffer. Add 10 µL each of CD4 and CD8 microbeads per 1 × 107 cells. Mix well, and incubate for 15 min in the dark in the refrigerator (2–8 °C).
- Prepare a depletion column by rinsing it with 2 mL of depletion buffer and discarding the flowthrough.
- Wash the cells from step 6.6 by adding 1–2 mL of depletion buffer per 1 × 107 cells, and centrifuge at 300 × g for 10 min. Remove and discard the supernatant.
- Resuspend up to 1.25 × 108 cells in 500 µL of depletion buffer. Apply the cell suspension to the column, and collect the flowthrough (unlabeled cells). Wash the column 2x with 1 mL of buffer, and collect the flowthrough.
NOTE: Only add new buffer when the column reservoir is empty. Set aside the following on ice after depletion (post-depletion control): 70 µL of cell suspension to count (vary this depending on the counting cell method chosen) and 1,000 µL of cell suspension to determine the efficiency of depletion by staining for CD4 and CD8 and analyzing by flow cytometry22,23. - Stain the depletion efficiency controls by splitting the 200 µL of cells collected before depletion into four FACS tubes: unstained, CD4 single-stained, CD8 single-stained, and CD4/CD8 double-stained (use the unstained and single-stained samples to set up the flow cytometry parameters). Use the 1,000 µL of cells collected after depletion to stain for CD4 and CD8 and compare with the double-stained sample collected before depletion (see typical depletion results in Figure 3).
NOTE: Adjust the volumes for flow cytometry staining according to the antibody manufacturer’s recommendation (e.g., 1 µL of anti-CD4-FITC + 0.5 µL of anti-CD8-PE per 1 x 106 cells in 50 µL of buffer).
7. Culturing thymocytes on OP9-DL4 cells (Days 6–7)
- Place 2–5 × 105 to 1 × 106 post-depletion thymocytes into a T25 flask of 80%–90% confluent OP9-DL4 cells in OP9 medium containing cytokines (10 ng/mL recombinant IL-7 and recombinant hFLT-3). Culture at 37 °C and 5% CO2. Grow for approximately 24 h on OP9-DL4 cells.
NOTE: This duration is required to make the T cells transducible (see a typical result in Figure 4). Reserve one T25 flask of the co-culture of thymocytes and OP9-DL4 cells to be used as a control (untransduced). The untransduced thymocytes will be stained together with the transduced thymocytes (step 9.1) as a negative control to evaluate the transduction efficiency. Untransduced thymocytes can also be used to measure the effect of the transgene expression on cell differentiation. Discard the cytokine-containing medium after 1 month.
8. Harvesting the retrovirus from the supernatant and using it to transduce the thymocytes (Days 8–9)
- Collect the supernatant containing the retroviruses from the transfected cells by tilting the 6-well plate and positioning a 3–5 mL syringe at the bottom of the plate while pulling the plunger to aspirate the supernatant. Replace the medium with 2 mL of fresh RPC medium. Continue to incubate the cells at 37 °C for 20–24 h for the second transduction in step 8.9.
- Filter the retrovirus supernatant through a 0.45 µm syringe filter, and collect the filtrate in a 50 mL tube.
NOTE: Do not freeze the retroviral supernatant. Use a freshly made virus preparation for transduction. - Collect thymocytes from the OP9-DL4 culture by aggressive pipetting to remove the thymocytes and OP9-DL4 cells from the flask surface. Filter the cell suspension through a 40 µm cell strainer to remove most of the OP9-DL4 cells, and collect the filtrate in a 50 mL tube.
NOTE: OP9 cells are very adherent. Although aggressive pipetting may remove some of the OP9-DL4 cells from the flask, the disruption of the OP9-DL4 monolayer during this process is minimal, and the OP9-DL4 cells that come off will be filtered out with the 40 µm cell strainer, since the OP9-DL4 cells are much larger than the primary thymocytes. If the OP9-DL4 cells are still 80%–90% confluent, remove the thymocytes, and re-plate them in the same OP9-DL4 flask. Alternatively, a new OP9-DL4 flask must be prepared. - Centrifuge the thymocytes in the filtrate from step 8.3 at 300 × g for 5 min. Discard the supernatant.
- In the 50 mL tube, resuspend the thymocytes in 0.5–1 mL of OP9 medium + cytokines (see step 7.1). Add 1–2 mL of RPC medium containing the virus (twice as much RPC medium as thymocyte medium). Add hexamethrine bromide (10 µg/µL stock concentration) to yield 8 µg of hexamethrine bromide/mL of total cell suspension.
- Spinoculate by centrifuging the cells at 850 × g for 1 h at room temperature.
- Resuspend the cells in 6 mL of OP9 medium + cytokines per flask (see step 7.1), and add the suspension back onto the OP9-DL4 cell monolayer.
NOTE: Alternatively, have new OP9-DL4 flasks ready to receive the transduced thymocytes. If the thymocytes are to be placed back into a used OP9-DL4 flask, make sure to add fresh OP9 medium to the OP9-DL4 cells during the 1 h of thymocyte spinoculation to keep the cells healthy and ensure 80%–90% confluency. - Incubate at 37 °C overnight.
- Repeat steps 8.2–8.7 using a new well of the retrovirus-containing cell supernatant.
9. Maintaining transduced thymocytes on OP9-DL4 culture for 2–5 days or freezing as needed (Day 9+)
- Assess the thymocyte differentiation by flow cytometry by staining thymocytes for T cell development markers such as CD4, CD8, CD25, and CD4423. See typical thymocyte differentiation results in Figure 5.
Results
The depletion efficiency can be assessed flow cytometrically by labeling the magnetically unlabeled cell fraction for CD4 and CD8 after immunomagnetic cell separation (MACS) and analyzing this on a two-dimensional bivariate dot-plot (Figure 3). A good yield of double negative (CD4−, CD8−) cells is 95% or above, as represented in Figure 3. Two of the most common causes of lower yield are the miscalculation of the microbeads based on the number of cells and the number of labeled cells exceeding the column capacity. It is recommended to choose the correct number of MACS columns according to the number of labeled cells. When working with thymocytes, the number of labeled cells (DP and SP cells) is almost equal to the number of total cells (more than 96%). Cell counting can be done in an automatic cell counter, a Neubauer chamber, or with any alternative method. As a result, the volumes allocated for cell counting may need to be adjusted depending on the specific machine and the chosen counting method.
It is important to determine the number of cells present before and after depletion. These cell counts are needed to calculate the number of LD depletion columns needed and to evenly distribute the DN thymocytes into the appropriate number of OP9-DL4 flasks. The controls for flow cytometry (unlabeled cells, cells labeled for CD4, and cells labeled for CD8) can be performed with the reserved pre-depletion sample, as this contains more cells. However, as most of the cells are expected to be retained within the column, the post-depletion sample to be labeled will require a higher volume. Consequently, adjustments may need to be made according to the labeling protocol selected.
When using vectors that express screenable markers, such as a fluorescence gene, the transfection and transduction can be roughly and empirically assessed by fluorescence microscopy (Figure 2). The transduction efficiency can be analyzed by harvesting the thymocyte from the OP9-DL4 monolayer, as described in step 8.3, and looking at the expression of a fluorescence gene by flow cytometry. The efficiency of transduction using an empty retroviral vector with GFP as the reporter gene was 84.2% (Figure 4).
T cell differentiation on OP9-DL4 cells can be observed 4 days after transduction. Flow cytometry is usually performed to assess the cell differentiation induced by the co-culture on OP9-DL4 cells and/or the transgene expression, as represented in Figure 5, where the cells were labeled for CD4, CD8, CD44, and CD25. There are several possible combinations of cell surface molecule labeling that have been shown to be useful for investigating the molecular and cellular mechanisms of T cell development in mice24,25,26,27. Therefore, the panels of fluorescent antibodies may vary according to the question of interest being addressed. The transduction of DN thymocytes with the empty retroviral vector pMIG, shown in panel B, presented approximately the same proportions of single positives (CD4+ or CD8+), double positives (CD4+/CD8+), double negatives (CD4−/CD8−), and its substages double-negative 1–4 (DN1–DN4) as the untransduced thymocytes, shown in panel A, indicating that T cell development was not affected by the transduction process.
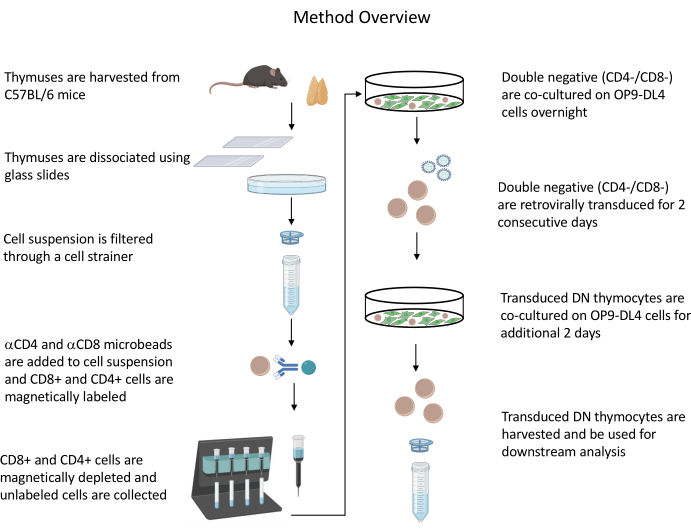
Figure 1: Diagram of the steps of thymocyte isolation, transduction, and co-culture. Abbreviation: DN = double-negative. Please click here to view a larger version of this figure.
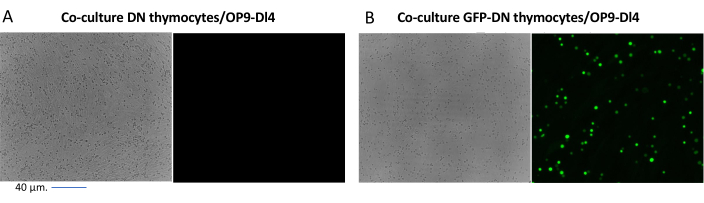
Figure 2: Co-culture fluorescence microscopy of GFP-transduced thymocytes or untransduced thymocytes and OP9-DL4 cells. (A) Untransduced thymocytes and (B) stable GFP-expressing murine thymocytes on OP9-DL4 at day 3 after the second transduction. An Olympus-IX71 fluorescence microscope with a 40x lens and a 480/30 filter suitable for GFP detection was used. Scale bar = 40 µm. Abbreviations: GFP = green fluorescent protein; DN = double-negative. Please click here to view a larger version of this figure.
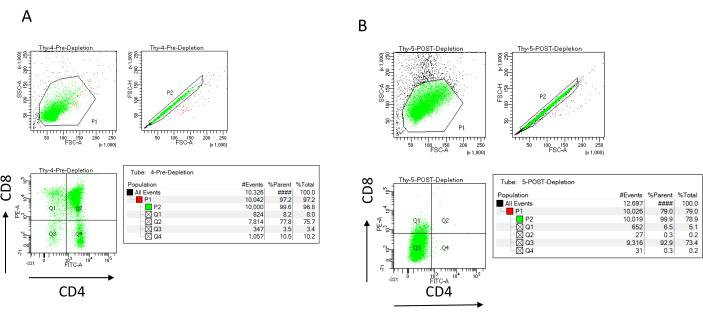
Figure 3: Flow cytometry of thymocyte depletion. Representative flow cytometry plots analyzing the expression of CD4 and CD8 on thymocytes obtained from 7-8 week old C57BL/6J female mice (A) pre-depletion and (B) post-depletion of CD4+ and CD8+ using microbeads and LD columns according to the manufacturer's instructions. The dot plots on the left show gates based on the size and complexity of the event (FCS-A and SSC-A, respectively). The middle panel shows FSC-H versus FSC-A to gate single cells and exclude doublets. In the plots on the right, cells were defined as CD4 and CD8 from the single-cell gate. Abbreviations: FSC-A = forward scatter-peak area; SSC-A = side scatter-peak area; FSC-H = forward scatter-peak height; FITC = fluorescein isothiocyanate; PE = phycoerythrin. Please click here to view a larger version of this figure.
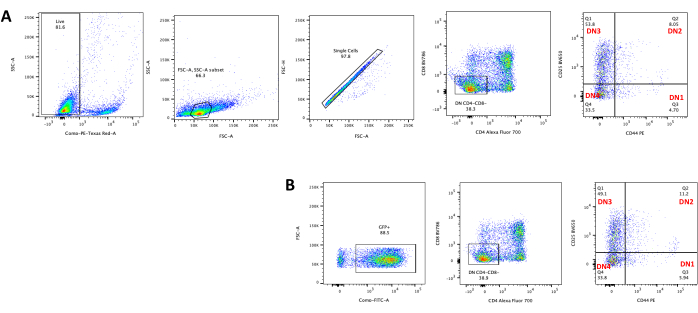
Figure 4: Flow cytometry of the thymocyte retroviral transduction efficiency. (A) Untransduced thymocytes co-cultured on OP9-DL4; (B) retrovirally transduced thymocytes on OP9-DL4 at day 3 post transduction. Abbreviations: FSC-A = forward scatter-peak area; SSC-A = side scatter-peak area; FSC-H = forward scatter-peak height; FITC = fluorescein isothiocyanate; PE = phycoerythrin; DN = double-negative. Please click here to view a larger version of this figure.
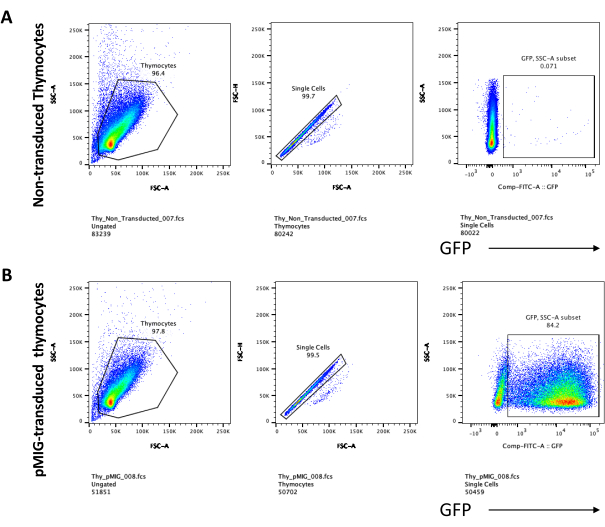
Figure 5: Flowcytometry of transduced thymocytes after 4 days of OP9-DL4 co-culture. (A) Untransduced and (B) transduced with pMIG retrovirus. The cells were first gated on live cells and then gated based on size and complexity (FCS-A and SSC-A, respectively), followed by plotting FSC-H versus FSC-A to gate on single cells and exclude doublets. For untransduced cells, we used the following gate strategy. From the single-cell gate, the cells were defined as single positives (CD4+ or CD8+), double positives (CD4+/CD8+), and double negatives (CD4−/CD8−). Then, from the double negatives (CD4−/CD8−) gate, the cells were defined as CD44+, CD25+, CD44+/CD25+, and CD44−/CD25−, as shown in panel A. For the pMIG transduced cells, shown in panel B, from the single-cell gate, the cells were first defined as GFP+/GFP−, and then from GFP+ cells, the cell population distribution into the major T cell development stages, as defined by CD4, CD8, CD44, and CD25 expression, was determined using the same gate strategy as for the untraduced cells. Abbreviations: FSC-A = forward scatter-peak area; SSC-A = side scatter-peak area; FSC-H = forward scatter-peak height; FITC = fluorescein isothiocyanate; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
Discussion
The protocol described here was developed specifically for thymus-derived DN (CD4−/CD8−) T cell studies with retroviral transfection followed by an OP9-DL4 differentiation model. However, it is likely that the target cells that will be subjected to this protocol of transduction followed by cell differentiation will have a wider interdisciplinary utility. Thus, in addition to immature thymocytes, hematopoietic stem cells, such as cells derived from either fetal liver or bone marrow, could potentially be used in this protocol.
The OP9-DL4 system has proven to be an effective model to study gene function in a variety of aspects, including cell differentiation17 and oncogenesis15. While the retroviral modification of hematopoietic progenitors is a well-established technique that enables stable genetic modification, combining the induction of cell differentiation on the OP9-DL4 system and retroviral transduction requires care and skill. The critical aspect for achieving success with this protocol is ensuring all the steps are well-coordinated, since the protocol involves using three different cell types that need to be kept healthy and at the ideal confluence required for each specific stage. With that in mind, it is important to perform all the quality control checkpoint analyses after the execution of each step before proceeding to the next step. This will ensure that all the steps are working. Therefore, checking the depletion, transfection, and transduction efficiencies is important for the successful execution of this protocol (see a typical result for transduction efficiency in Figure 4). Good primary cell transduction efficiency is linked to a high viral titer. Typically, larger inserts result in lower virus titers18. For training purposes, we use an empty vector to represent the results that can be obtained with this protocol. In our experience, the transduction and transfection efficiencies vary according to the insert size, especially considering the viral backbone-expressing reporter genes, such as GFP. One strategy that can be used when studying the interaction of more than one gene is to clone each gene into a different transfer vector, followed by individual virus production, and finally, co-transduction of the target cell. In that case, a selection step may be applied to eliminate the singly transduced cells and retain only the co-transduced cells.
It is worth noting that the majority of OP9-DL1/DL4 stromal feeder layer cell lines have been genetically engineered to express GFP as part of the DL1 or DL4 constructs7. In this protocol, we used a retroviral vector that also expresses GFP; however, it is brighter than the GFP protein expressed by OP9-DL4 cells and does not interfere with the transduction inspection when visualizing the co-culture under the fluorescence microscope.
OP9 cells differentiate into adipocytes after many passages, long periods in culture, or under conditions of over-confluency19. This is evidenced by the development of large vacuoles. Thus, OP9 cells presenting these characteristics should not be used in this protocol. Transfecting overly confluent retrovirus-producer cells will result in a low virus titer. Indeed, the subconfluent stage is when the cells are most transfectable. Furthermore, transfecting low-confluence retrovirus-producer cells will decrease the cell stress in the transfection process and give the highest virus titer.
While, in this protocol, we do not titrate the virus supernatant, virus supernatant titration must be considered in some cases, such as in the absence of a reporter gene in the retroviral vector, which would prevent the indirect determination of viral production, or in cases where the experimental design requires a more accurate number of the transgene copies to be integrated into the target cell genome. However, it is important to note that the titer of retroviral vector supernatant decreases significantly when stored at −80 °C or 4 °C until the titration results are obtained. Therefore, using freshly prepared virus supernatant for transduction will yield better transduction efficiency.
The thymus contains a large number of double-positive (DP) thymocytes (more than 85%) and about 10% single-positive15 cells (CD4 or CD8), which are the post-DN stage thymocytes. The DP cells cannot survive in vitro for retroviral manipulation, while the SP cells are untransducible. Therefore, this protocol can be applied to generate retroviral vector transducible DN thymocytes.
Disclosures
The authors have no conflicts of interest to disclose
Acknowledgements
This work was supported by the intramural program of the National Cancer Institute, project ZIABC009287. OP9-DL4 was obtained from Dr. Juan Carlos Zúñiga-Pflücker (Sunnybrook Health Sciences Centre, Toronto, ON, Canada). The authors thank the NCI-Frederick Laboratory Animal Sciences Program for their continued technical assistance and experimental advice and input, as well as Jeff Carrel, Megan Karwan, and Kimberly Klarmann for flow cytometry assistance. We are grateful to Howard Young for critical advice and input.
Materials
| Name | Company | Catalog Number | Comments |
| 2-mercaptoethanol | Sigma | M3148 | |
| ACK Lysis buffer | Lonza | 10-548E | |
| BSA | Cell Signaling Technology | 9998S | |
| CD4 Microbeads | Miltenyi | 130-049-201 | |
| CD8 Microbeads | Miltenyi | 130-049-401 | |
| Centrifuge 5910R | eppendorf | 5942IP802353 | |
| DMEM | Corning | 10-013-CV | |
| EDTA | Invitrogen | 15575-038 | |
| Fetal calf serum HyClone FBS | ThermoScientific | SH30910.03 | |
| LD columns | Miltenyi | 130-042-901 | |
| L-glutamine | Sigma | G7513 | Freeze glutamine in aliquots and use freshly-thawed glutamine |
| Lipofectamine 2000 | Invitrogen | P/N 52887 | |
| MEM-alpha Medium | Gibco | 12561-072 | |
| OPTI-MEM I Reducing Serum Medium | Invitrogen | 31985-062 | |
| PBS pH 7.2 | Corning | 21-040-CV | |
| pcL-Eco Plasmid | Addgene | 12371 | |
| penicillin/streptomycin | Gibco | 15140-122 | |
| pMIG Plasmid | Addgene | 6492 | |
| Polybrene | Chemicon | TR-1003-G | |
| Pre-Separation Filters | Miltenyi | 130-041-407 | |
| recombinant hFLT-3L | PeproTech | 300-19 | |
| recombinant IL-7 | Peprotech | 217-17 | |
| Retrovial packaging cell line Phoenix-Eco | Orbigen | RVC-10002 | |
| Syringe filter (0.45 µm) | Millipore | SLHV033RS |
References
- Zuniga-Pflucker, J. C. T-cell development made simple. Nature Reviews Immunology. 4 (1), 67 (2004).
- Kodama, H., Nose, M., Niida, S., Nishikawa, S., Nishikawa, S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Experimental Hematology. 22 (10), 979-984 (1994).
- Nakano, T., Kodama, H., Honjo, T. Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science. 265 (5175), 1098-1101 (1994).
- Nakano, T., Kodama, H., Honjo, T. In vitro development of primitive and definitive erythrocytes from different precursors. Science. 272 (5262), 722-724 (1996).
- Ueno, H., et al. A stromal cell-derived membrane protein that supports hematopoietic stem cells. Nature Immunology. 4 (5), 457-463 (2003).
- Jaleco, A. C., et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. Journal of Experimental Medicine. 194 (7), 991-1002 (2001).
- Mohtashami, M., et al. Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. Journal of Immunology. 185 (2), 867-876 (2010).
- Schmitt, T. M., Zuniga-Pflucker, J. C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 17 (6), 749-756 (2002).
- Mazzucchelli, R., Durum, S. K. Interleukin-7 receptor expression: Intelligent design. Nature Reviews Immunology. 7 (2), 144-154 (2007).
- Robey, E. A., Bluestone, J. A. Notch signaling in lymphocyte development and function. Current Opinion in Immunology. 16 (3), 360-366 (2004).
- Lavaert, M., et al. Integrated scRNA-Seq identifies human postnatal thymus seeding progenitors and regulatory dynamics of differentiating immature thymocytes. Immunity. 52 (6), 1088-1104 (2020).
- Roh, K. H., Roy, K. Engineering approaches for regeneration of T lymphopoiesis. Biomaterials Research. 20 (20), (2016).
- Zlotoff, D. A., et al. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 115 (10), 1897-1905 (2010).
- Holmes, R., Zuniga-Pflucker, J. C. The OP9-DL1 system: Generation of T-lymphocytes from embryonic or hematopoietic stem cells in vitro. Cold Spring Harbor Protocols. 2009 (2), (2009).
- Cramer, S. D., et al. Mutant IL-7Ralpha and mutant NRas are sufficient to induce murine T cell acute lymphoblastic leukemia. Leukemia. 32 (8), 1795-1882 (2018).
- Treanor, L. M., et al. Interleukin-7 receptor mutants initiate early T cell precursor leukemia in murine thymocyte progenitors with multipotent potential. Journal of Experimental Medicine. 211 (4), 701-713 (2014).
- Yokoyama, K., et al. In vivo leukemogenic potential of an interleukin 7 receptor alpha chain mutant in hematopoietic stem and progenitor cells. Blood. 122 (26), 4259-4263 (2013).
- Simmons, A., Alberola-Ila, J. Retroviral transduction of T cells and T cell precursors. Methods in Molecular Biology. 1323, 99-108 (2016).
- . Retroviral systems Available from: https://web.stanford.edu/group/nolan/_OldWebsite/retroviral_systems/retsys.html (2022)
- Xing, Y., Hogquist, K. A. Isolation, identification, and purification of murine thymic epithelial cells. Journal of Visualized Experiments. (90), e51780 (2014).
- Gray, D. H., et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood. 108 (12), 3777-3785 (2006).
- Godfrey, D. I., Kennedy, J., Suda, T., Zlotnik, A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. Journal of Immunology. 150 (10), 4244-4252 (1993).
- Wang, Y. B., Edinger, M., Mittar, D., McIntyre, C. Studying mouse thymocyte development using multiparametric flow cytometry: An efficient method to improve an 8-color panel on the BD FACSVerse™ system. BD Biosciences–Application Note. , (2012).
- Sakaguchi, N., et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature. 426 (6965), 454-460 (2003).
- He, X., Park, K., Kappes, D. J. The role of ThPOK in control of CD4/CD8 lineage commitment. Annual Review of Immunology. 28, 295-320 (2010).
- Wang, Y., et al. A conserved CXXC motif in CD3epsilon is critical for T cell development and TCR signaling. PLoS Biology. 7 (12), e1000253 (2009).
- Aliahmad, P., Kadavallore, A., de la Torre, B., Kappes, D., Kaye, J. TOX is required for development of the CD4 T cell lineage gene program. Journal of Immunology. 187 (11), 5931-5940 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved