A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Intra-Peritoneal Transplantation for Generating Acute Myeloid Leukemia in Mice
In This Article
Summary
Here, intra-peritoneal injection of leukemia cells is utilized to establish and propagate acute myeloid leukemia (AML) in mice. This new method is effective in the serial transplantation of AML cells and can serve as an alternative for those who may experience difficulties and inconsistencies with intravenous injection in mice.
Abstract
There is an unmet need for novel therapies to treat acute myeloid leukemia (AML) and the associated relapse that involves persistent leukemia stem cells (LSCs). An experimental AML rodent model to test therapies based on successfully transplanting these cells via retro-orbital injections in recipient mice is fraught with challenges. The aim of this study was to develop an easy, reliable, and consistent method to generate a robust murine model of AML using an intra-peritoneal route. In the present protocol, bone marrow cells were transduced with a retrovirus expressing human MLL-AF9 fusion oncoprotein. The efficiency of lineage negative (Lin-) and Lin-Sca-1+c-Kit+ (LSK) populations as donor LSCs in the development of primary AML was tested, and intra-peritoneal injection was adopted as a new method to generate AML. Comparison between intra-peritoneal and retro-orbital injections was done in serial transplantations to compare and contrast the two methods. Both Lin- and LSK cells transduced with human MLL-AF9 virus engrafted well in the bone marrow and spleen of recipients, leading to a full-blown AML. The intra-peritoneal injection of donor cells established AML in recipients upon serial transplantation, and the infiltration of AML cells was detected in the blood, bone marrow, spleen, and liver of recipients by flow cytometry, qPCR, and histological analyses. Thus, intra-peritoneal injection is an efficient method of AML induction using serial transplantation of donor leukemic cells.
Introduction
Acute myeloid leukemia (AML) is a type of hematologic malignancy of diverse etiology with poor prognosis1. The generation of AML animal models lays the foundation for the understanding of its complex variations and pathobiology in an effort to discover novel therapies2. Leukemogenesis in mice involves the transplantation of donor cells expressing fusion oncoproteins, including fusions involving the mixed lineage leukemia (MLL) gene to potently induce AML, to mimic the disease in humans3. Various cellular origins of donor cells have been reported in the transplantation of MLL gene-associated AML4, with very little being known about the cells responsible for the disease origin.
Multiple routes have been developed for transplantation in mice; rather than an intra-femoral injection, which directly introduces mutant donor cells into bone marrow5, an intravenous injection that utilizes the venous sinus plexus, tail vein, and jugular vein has been widely used to generate murine AML models6,7,8,9. In the case of retro-orbital (r.o.) injection, various inherent disadvantages, such as volume limitation, high technical demand, few chances for repeated attempts or error, and potential ocular injuries, have been major stumbling blocks with limited or no viable alternatives7. Tail vein injection can have similar problems besides local injuries; to facilitate the procedure, mice often need to be warmed up to dilate their tail veins10. It is also hard to locate the tail vein without an additional light source, particularly in the C57BL/6 strain of mice. For jugular vein injection, research personnel require sufficient training to locate the vein and limit possible complications. In addition, both venous sinus and jugular vein injections need to be performed under anesthesia, which adds another level of complexity. Thus, it is tempting to explore new routes for transplantation to facilitate the establishment of AML murine models.
Intra-peritoneal (i.p.) injection is commonly used to administer drugs, dyes, and anesthetics11,12,13,14,15; it has also been used to introduce hematopoietic cells for ectopic hematopoiesis16 and to transplant bone marrow-derived mesenchymal stem cells in various mouse models17,18,19,20,21. However, it has been infrequently used to establish hematopoietic malignancies in mice, particularly to study AML disease progression.
The present study describes the feasibility of i.p. injection in the generation of AML mouse models, in addition to comparing the transplantation efficiency of lineage negative (Lin-) and Lin-Sca-1+c-Kit+ (LSK) populations as donor cells. These findings provide a simple and efficient way to generate experimental models of AML and related myeloid leukemias. Such a method has the potential to further our understanding of the disease mechanisms as well as provide a relatively easy model to test experimental therapies.
Protocol
All experiments were preapproved by the Institutional Animal Care and Use Committee at the Pennsylvania State University.
1. Preparation of buffers and reagents
- Prepare ampicillin supplemented (AP) LB agar plates (sterile 10 cm plates). To do this, dissolve 10 g of LB broth with agar in 400 mL of distilled water, stir, and bring the volume up to 500 mL. Sterilize the solution by autoclaving, then allow the solution to cool down, add 0.5 mL of ampicillin (stock: 150 mg/mL) into solution, and shake it to mix. Immediately add 18 mL of solution to a sterile 10 cm plate near an alcohol lamp, allow to solidify at room temperature, and store the plates upside down at 4 °C until further usage.
- Prepare LB media by dissolving 10 g of LB without agar in 500 mL of distilled water. Sterilize the solution by autoclaving, allow the solution to cool down,add 0.5 mL of ampicillin (stock: 150 mg/mL) into solution, and shake it to mix.
- Prepare flow buffer by adding 5 mL of penicillin/streptomycin and 10 mL of heat-inactivated fetal bovine serum (hiFBS) into 485 mL of 1x Dulbecco's phosphate buffered saline (DPBS).
NOTE: To inactivate FBS by heating, place thawed FBS bottles in a 56 °C water bath. Ensure that the bottles don't tip over or otherwise become submerged in the water bath. The temperature is critical for complete degradation; to ensure this, wait until the temperature stabilizes at 56 ˚C after putting the bottles to the water bath. Gently swirl the bottles every 10 min three times. Do not allow the serum to incubate for more than 30 min. - Prepare maintenance media by adding 50 mL of hiFBS, 5 mL of L-glutamine, and 5 mL of penicillin/streptomycin into 440 mL of Dulbecco's modified eagle medium (DMEM). Prepare transfection media by adding 50 mL of hiFBS and 5 mL of L-glutamine into 445 mL of DMEM media.
- Prepare red blood cell (RBC) lysis buffer by adding 4.145 g of NH4Cl, 0.504 g of NaHCO3, and 16.81 mg of ethylenediaminetetraacetic acid (EDTA) into 500 mL of distilled water. Prepare incomplete Iscove's modified Dulbecco's medium (IMDM) media by adding 75 mL of hiFBS, 5 g of bovine serum albumin (BSA), 0.5 mL of 10 mg/mL insulin, 2.5 mL of 4 mg/mL holo-transferrin, 3.5 µL of β-mercaptoethanol, 5 mL of L-glutamine, and 0.5 mL of ciprofloxacin into 416.5 mL of IMDM media.
- Prepare 10 mL of 2x IMDM media, in which the concentration of cytokines is twice the amount in 1x IMDM media, by adding 10 µL of 50 ng/µL mr-SCF, 20 µL of 25 ng/µL mr-Flt3L, 20 µL of 10 ng/µL mr-IL-6, 20 µL of 10 ng/µL mr-IL-3, 10 µL of 10 mg/mL insulin, and 50 µL of 4 mg/mL holo-transferrin into 9.87 mL of incomplete IMDM media.
NOTE: Ensure that flow buffer, RBC lysis buffer, maintenance media, transfection media, and incomplete IMDM media are filter sterilized before use.
2. Plasmid transformation
- Thaw 20 µL of α-Select competent cells on ice. Add 1 µL (~2 ng) of MSCV-MLL-AF9-EF1α-luc2-P2A-EGFP-LC3 plasmid22 to thawed competent cells and gently mix by tapping the tube. Incubate the reaction on ice for 30 min.
- Heat shock the mixture by incubation for 40 s in a 42 °C heating block. Immediately transfer the tube on ice for 2 min.
- Add 1 mL of LB media (without ampicillin) to the tube and shake at 37 °C and 200 rpm for 1 h.
- Centrifuge the tube at room temperature at 500 x g for 4 min and discard 0.9 mL of supernatant. Resuspend the precipitate in the remaining 0.1 mL of LB media.
- Spread the transformed competent cells on pre-warmed (37 °C) AP LB agar plates. Incubate the plate upside down at 37 °C for 12-16 h.
- Pick a single colony and expend the transformed cells in 10 mL of AP LB media overnight at 37 ˚C and 200 rpm.
- Add 5 mL of expended transformed competent cells to 500 mL of AP LB media in a flask and incubate the flask overnight at 37 °C and 200 rpm.
- Extract the plasmid using a plasmid extraction kit according to manufacturer's instructions and resuspend in 0.5 mL of autoclaved ultrapure water. Quantify the plasmid using a spectrophotometer.
3. Transfection of Phoenix Ecotropic (pECO) cells
- Culture 2 x 106 pECO cells/plate in maintenance media in 10 cm plates in a humidified 5% CO2 incubator at 37 °C. Ensure that the pECO cells are maintained in exponential growth phase and actively dividing before passage.
- When the cells become 80% confluent, wash the plates with 5 mL of DPBS twice, add 1 mL of trypsin to the plate, and incubate in a humidified 5% CO2 incubator at 37 ˚C for 2 min. Harvest the cells with 5 mL of maintenance media in a sterile 15 mL tube and centrifuge at 4 °C and 400 x g for 3 min. Resuspend the cell pellet in 5 mL of maintenance media.
- Mix 10 µL of cell suspension and 10 µL of trypan blue and load 10 µL onto a hemocytometer to count the cells.
Total cells/mL = (Total cells counted x Dilution factor x 104 cells/mL)/ Number of squares counted)
Seed 2 x 106 cells/dish into 6 cm dishes using 5 mL of maintenance media for transfection and culture the cells in a humidified 5% CO2 incubator at 37 °C. - Replace the maintenance media with 5 mL of transfection media when the cells become 50%-60% confluent after 18 h of culture.
- Keep the transfection reagent at room temperature for at least 30 min before transfection.
- Add 5.5 µg of MSCV-MLL-AF9-EF1α-luc2-P2A-EGFP-LC3 plasmid22 to 0.5 mL of plain DMEM media in a sterile 1.5 mL tube. Mix it gently by tapping the tube and allow it to sit for 10 min.
- Add 14.6 µL (3x the plasmid amount; v/w) of transfection reagent to the tube and gently tap the tube every 10 min three times.
- Uniformly add the mixture dropwise to all areas of the dishes with the pECO cells in transfection media. Gently move the dishes forward and backward 10 times and sideways 10 times. Incubate the dishes in a humidified 5% CO2 incubator at 37 °C for 48 h.
- Measure the transfection efficiency by florescence microscopy and flow cytometry for green fluorescent protein (GFP) as described in22. The cells are first gated on FSC-A/FSC-H and FSC-A and SSC-A to acquire singlets. The GFP+ population is gated on FL1 plot by comparing it to non-transfected cells.
- Collect and filter the supernatants through a 0.45 µm syringe filter into a sterile 50 mL tube. Use the supernatants immediately for transduction or snap freeze them in liquid nitrogen and store them at -80 °C until further use.
NOTE: pECO cells have to be properly mixed and seeded in dishes uniformly. Allow the cells to spread by moving the dishes forward and backward 10 times and sideways 10 times while seeding. The cell number to be seeded may vary depending on the variations in counting. To find the optimal seeding cell number that can achieve 50%-60% confluence after 18 h of culture, seeding cells with serial dilutions is helpful.
4. Lentiviral transduction
- Euthanize 8-10-week-old CD45.1 female C57BL6/J mice (two to three donor mice per recipient mouse) in a CO2 chamber.
- Sterilize the whole body of the mice with 70% ethanol. Place the mice onto a sterile surgical pad on a Styrofoam board and pin the legs through the mouse paw pads.
- Cut the skin above the abdominal cavity at the midline and widen the subcutaneous space toward the hind legs with sharp-end sterile scissors.
- Extend the incision from the abdominal midline down to the ankles. Widen the subcutaneous space below the hind legs with the blades of sharp-end sterile scissors.
- Cut the Achilles's tendon with sharp-end sterile scissors. Hold the tendon using forceps with teeth and cut the other end attached to the femur to remove the gastrocnemius muscle.
- Cut the quadriceps tendon attached to the knee with sharp-end sterile scissors. Hold the tendon using forceps with teeth and cut the muscle heads attached to the femur to remove the gastrocnemius muscle.
- Cut the other muscles surrounding the femur at the end attached to the tibia with sharp-end sterile scissors.
- Cut the ankle with sharp-end sterile scissors, ensuring that the tibia remains intact. Hold the distal end of the femur using forceps with teeth and cut the hip joint with sharp-end sterile scissors, ensuring that the femoral head remains intact.
- Transfer the tibias and femurs to flow buffer in a sterile 15 mL tube.
- Separate the tibia and femur by breaking the knee by hand. Remove the patella, cartilage, and femoral condyles to expose the tibial plateau and distal femur by hand. Remove the muscles using a sterile gauze and then soak the bones in flow buffer.
- Cut the femoral neck and flush the bone marrow cells with flow buffer from both ends of the femur using a 10 mL syringe with a 23 G needle.
- Cut the tibial malleolus and flush the bone marrow cells with flow buffer from both ends of the tibia using a 10 mL syringe with a 23 G needle.
- Disperse the cells by pipetting up and down using a 10 mL syringe with an 18 G needle. Centrifuge the single cell suspension at 4 °C and 400 x g for 3 min.
- Discard the supernatant and resuspend the cells in 5 mL of RBC lysis buffer to lyse the RBCs for 3 min.
- Add 5 mL of flow buffer to stop the lysis and centrifuge the cell suspension at 4 °C and 400 x g for 3 min.
- Place a 70 µm cell strainer onto a sterile 50 mL tube. Suspend the pellet with 5 mL of flow buffer, mix, and pass through the cell strainer to collect the cells.
- Adjust the cell concentration with flow buffer to 1 x 108/mL in round bottom polypropylene tubes.
- Select Lin- cells using a mouse hematopoietic cell isolation kit according to the manufacturer's instruction.
- Keep aside three tubes of 1 x 104 cells in 100 µL of buffer for one unstained control and two single antibody-stained controls for APC-conjugated anti-mouse CD117 (c-Kit) and PE-Cy7-conjugated anti-mouse Ly-6A/E (Sca-1). Use 1 µL of antibody (from 0.2 mg/mL stock) for each of the single antibody-stained controls.
- Stain the rest of the cells in a tube with both antibodies (4 µL of each from 0.2 mg/mL stocks) in 400 µL. Incubate the tubes on ice in the dark for 0.5-1 h.
- After staining, wash the cells by adding 1 mL of flow buffer and centrifuge at 4 °C and 400 x g for 3 min.
- Resuspend the cells for the unstained control and the single antibody-stained controls in 100 µL of flow buffer. Resuspend double antibody-stained cells in 1 mL of flow buffer for sorting.
- Sort hematopoietic stem cells (HSCs) as an LSK population using a cell sorter as described in23,24.
- While staining, coat a sterile 6 cm dish with retronectin as follows: Prepare 100 µg/mL stock of retronectin in PBS and add 0.9 mL of PBS and 0.1 mL of retronectin to a 6 cm dish. Coat the dish in a sterile hood at room temperature for 2 h. Then, remove the retronectin and block the dish with 0.5 mL of filtered 2% BSA (in PBS) for 30 min. Wash the dish with 5 mL of PBS twice and the dish is ready for transduction.
- Centrifuge the sorted HSCs or unsorted Lin- cells at 4 °C and 400 x g for 3 min and resuspend in 3 mL of 2x (of cytokines) IMDM media and 3 mL of viral supernatant (generated from step 3.10) in a retronectin-coated dish.Incubate the dish in a humidified 5% CO2 incubator at 37 °C for 6 or 24 h.
NOTE: In the present study, Lin- cells were either sorted or unsorted depending on the experimental design.
5. Serial transplantation (Figure 1)
NOTE: Primary recipient mice were 8-10-week-old male C57BL6/J mice (CD45.2). They were provided water ad libitum containing antibiotics to prevent opportunistic digestive infections, from 3 days prior to transplantation to 7 days post transplantation. Primary recipient mice were sub-lethally irradiated (4.75 Gy) 3 h before transplantation25. Isoflurane was not applied to mice with intra-peritoneal injection.
- After transduction for 6 or 24 h, harvest the cells by centrifugation at room temperature and 400 x g for 3 min. Use trypsin to collect the cells attached to the dish bottom if needed. Discard the supernatant and resuspend the cells in prewarmed PBS. Determine the volume of PBS depending on the number of recipients (i.e., 0.1 mL/mouse and 0.5 mL/mouse for recipients with retro-orbital and intra-peritoneal injections, respectively).
- Place the sub-lethally irradiated recipient mice in an isoflurane chamber (flow rate of oxygen is set as 1.0 L/min and vaporizer of isoflurane is set as 5%). Apply wet ointment to the eyes to prevent dryness while under anesthesia. The mice are ready for further procedures when the heartbeat drops to 60 beats per min.
- Inject cells into primary recipient mice retro-orbitally (0.1 mL/mouse)7 or intra-peritoneally (0.5 mL/mouse)26 with a 27 G1/2 needle. Observe the mice continuously until they gain sufficient consciousness to maintain sternal recumbency. Monitor the mice daily for their well-being post transplantation.
- After 1 month, collect blood weekly by retro-orbital bleeding to monitor leukocytosis by evaluating complete blood count (CBC) on a hemavet as described below.
- Place the mouse laterally after anesthesia with isoflurane (flow rate of oxygen is set as 1.0 L/min and vaporizer of isoflurance is set as 5%). The mice are ready for further procedures when the heartbeat drops to 60 beats per min.
- Proptose the eye with the thumb and index finger. Penetrate the venous sinus plexus with asterile hemacrit capillary tube through the inner canthus.
- Collect 20-25 µL of blood into an EDTA blood collection tube and close the eyelids to stop the bleeding. Apply one drop of gentamicin sulfate ophthalmic solution to the eye.
- At the endpoint, when the white blood cells (WBCs) reach 4 x 104 cells/µL, euthanize the mouse in a CO2 chamber and isolate the bone marrow cells by flushing the femurs and tibias with flow buffer, followed by RBC lysis as mentioned in step 4.
- At the endpoint, harvest the splenocytes as mentioned below.
- Euthanize the mouse in a CO2 chamber. Place the mice onto a sterile surgical pad on a Styrofoam board and pin the legs through the mouse paw pads. Sterilize the whole body of the mice with 70% ethanol.
- Cut the skin and muscle at the midline to expose the abdominal cavity with sharp-end sterile scissors. Isolate the spleen with sharp-end sterile scissors and put it in flow buffer in a sterile 15 mL tube.
- Mesh the spleen through a 70 µm sterile strainer in a 6 cm dish with 3 mL of flow buffer. Transfer the cells from the dish to a sterile 15 mL tube and centrifuge the single cell suspension at 4 °C and 400 x g for 3 min.
- Discard the supernatant and resuspend the cells in 5 mL of RBC lysis buffer to lyse the RBCs for 3 min. Add 5 mL of flow buffer to stop the lysis and centrifuge the cell suspension at 4 °C and 400 x g for 3 min.
- Place a 70 µm cell strainer onto a sterile 50 mL tube. Suspend the pellet with 5 mL of flow buffer, mix, and pass through the cell strainer to collect cells.
- Identify primary (1˚) AML cells by staining the splenocytes and bone marrow cells with FITC-conjugated anti-mouse CD45.1 antibody and detecting on a flow cytometer. The cells are first gated on FSC-A/FSC-H and FSC-A and SSC-A to acquire singlets. The CD45.1+ population is gated on FL1 plot by comparing it to unstained cells.
- For secondary (2˚) transplantation, resuspend CD45.1 AML splenic cells from 1˚ r.o. recipients in PBS (0.1 mL/mouse) and inject them retro-orbitally into CD45.2 male C57BL6/J mice. Parallelly, resuspend AML splenic cells from 1˚ i.p. recipients in PBS (0.5 mL/mouse) and inject them intra-peritoneally into 8-12-week-old red fluorescence protein (RFP)-expressing Ai14TdTomato male mice27.
- For tertiary (3˚) transplantation, resuspend AML cells isolated from the bone marrow or peritoneal cavity of 2˚ i.p. recipients and inject them intra-peritoneally into Ai14TdTomato (RFP+) or CD45.2 mice, respectively. Resuspend AML cells isolated from the peritoneal cavity of 2˚ r.o. recipients and transplant them by r.o. injection into Ai14TdTomato (RFP+) mice.
NOTE: For 2˚ transplantation, we identified the disease progression in 2˚ recipients by monitoring the CBC in peripheral blood. To further confirm the establishment of AML, we collected whole peripheral blood by heart puncture as well as bone marrow, spleen, and liver. Furthermore, we performed i.p. lavage to collect i.p. cells. Single cell suspensions were acquired from bone marrow, spleen, and i.p. lavage as described above. Cells from these sites were analyzed on a flow cytometer following RBC lysis. AML cells were recognized as RFP negative (RFP-) cells. For 3˚ transplantation, we sampled blood, bone marrow, spleen, liver, and i.p. cells at the endpoint; RFP- or CD45.1+ cells were identified as AML cells and examined by flow cytometry. No irradiation or antibiotic water was given to 2˚ and 3˚ recipient mice.
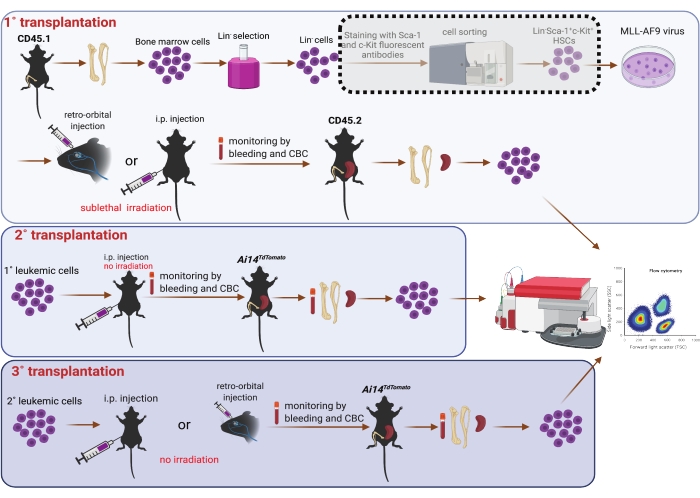
Figure 1: Schematic of MLL-AF9 viral transduction in bone marrow HSCs and serial transplantation (1˚, 2˚, and 3˚). Sorting of Sca-1 and c-Kit double positive population by using a cell sorter shown in the dotted shade box is considered optional, should resources allow. The figure was created using BioRender (https://biorender.com/). Please click here to view a larger version of this figure.
6. Intra-peritoneal lavage
- Inject 5 mL of incomplete IMDM media into the peritoneal cavity twice to collect the cells in a 15 mL sterile tube. Centrifuge the cell suspension at room temperature and 400 x g for 3 min. Transplant AML cells (4 x 105 cells/mouse) from the peritoneal cavity of 2˚ recipients via i.p. injection into 3˚ CD45.2 recipient mice (n = 3).
7. Histological analysis 28
- Isolate the spleens, livers, and femurs from mice upon euthanasia. Fix them in 5 mL of 10% (v/v) buffered formalin. Sample the spleens and livers from healthy counterparts for comparisons.
- Embed fixed tissues in paraffin and cut them into sections. Stain the sections with hematoxylin and eosin (H&E) dyes.
- Obtain the images under a microscope at 20x magnification installed with a compatible software for histological analysis.
8. Performing semi-quantitative PCR (qPCR)
- Prepare RNAs in RNA reagent according to the manufacturer's instructions.
- Use 0.5-1.0 µg RNAs to synthesize cDNA using a cDNA reverse transcription kit according to the manufacturer's instructions.
- Use cDNA to perform qPCR using a qPCR kit and run the samples in a qPCR system. Use the following pre-validated TaqMan probes: KMT2A (MLL; Ref Seq: NM_001197104(2), IDT)29 and 18S ribosomal RNA (Hs99999901_s1).
- Load the KMT2A and 18S amplicons onto a 2% agarose gel to visualize the expression. Acquire images in an imager installed with the compatible software program.
9. Data processing
- Analyze results using statistical analysis software and present the results as the mean ± SEM. Generate figures using a commercial illustrator tool.
Results
Comparison of the transplantation efficiency of murine AML cells using r.o. and i.p. routes of transplantation
Previously, the establishment of 1˚ AML was reported in recipient mice retro-orbitally transplanted with MLL-AF9-transduced LSK cells, and the transplantability of 1˚ AML cells was demonstrated by serial transplantation30. The present study is the first to evaluate the possibility of using bone marrow Lin- cells to perform transplantation. The p...
Discussion
These above-described studies provide supportive evidence that the transplantation of Lin- cells is comparable to LSK cells in the generation of 1˚ murine AML. In addition, the current data also shows that i.p. injection is an efficient and convenient method to establish murine AML compared to intravenous (or r.o.) injection.
In addition to LSK cells, other populations such as granulocyte-monocyte progenitor (GMP), common lymphoid progenitor (CLP), and common myeloid progenitor...
Disclosures
The authors declare no conflict of interest.
Acknowledgements
The authors thank Huck Institute's Flow Cytometry Core Facility and the Histopathology Core Facility of the Animal Diagnostic Laboratory, Department of Veterinary and Biomedical Sciences, The Pennsylvania State University, for providing timely technical support. This work was supported by grants from the American Institute for Cancer Research (KSP), Penn State College of Agricultural Sciences, Penn State Cancer Institute, USDA-NIFA project 4771, Accession number 00000005 to K.S.P. and R.F.P.
Materials
| Name | Company | Catalog Number | Comments |
| a-Select competent cells | Bioline | BIO-85027 | |
| Ammonium chloride (NH4Cl) | Sigma Aldrich | Cat# A-9434 | |
| Ampicillin | Sigma Aldrich | Cat# A0797 | |
| Bovine Serum Albumin (BSA), Fraction V—Low-Endotoxin Grade | Gemini bio-products | Cat# 700-102P | |
| Ciprofloxacin HCl | GoldBio.com | Cat# C-861-100 | |
| DMEM, high glucose, no glutamine | Gibco | Cat# 11960-044 | |
| Dulbecco’s Phosphate-Buffered Saline (PBS) | Corning | Cat# 21-031-CV | |
| EDTA, Disodium Salt (EDTA-2Na), Dihydrate, Molecular Biology Grade | Calbiochem | Cat# 324503 | |
| Fetal Bovine Serum - Premium Select | Atlanta Biologicals | Cat# S11550 | |
| Holo-transferrin, bovine | Sigma Aldrich | Cat# T1283 | |
| Insulin solution human | Sigma | Cat# I-9278 | |
| Iscove's Modified Dulbecco's Medium (IMDM) | Gibco | Cat# 12440-053 | |
| L-glutamine 200 mM (100×) solution | HyClone, Gelifesciences | Cat# SH30034.01 | |
| LB broth, Lennox | NEOGEN | Cat #: 7290A | |
| LB Broth with agar (Miller) | Sigma Aldrich | Cat# L-3147 | |
| Mouse anti-mouse CD45.1 (FITC) | Miltenyi Biotec | Cat# 130-124-211 | |
| Mouse Interleukin-3 (IL-3) | Gemini bio-products | Cat# 300-324P | |
| Mouse Interleukin-6 (IL-6) | Gemini bio-products | Cat# 300-327P | |
| Mouse Stem Cell Factor (SCF) | Gemini bio-products | Cat# 300-348P | |
| Penicillin-Streptomycin Solution, 100x | Corning | Cat# 30-002-CI | |
| Phenix-Eco (pECO) cells | ATCC | CRL-3214 | |
| Potassium Bicarbonate (KHCO3), Granular | JT. Baker | Cat# 2940-01 | |
| Rat anti-mouse CD117 (c-kit) (APC) | BioLegend | Cat # 105812 | |
| Rat anti-mouse Ly-6A/E (Sca-1) (PE-Cy7) | BD Pharmingen | Cat# 558162 | |
| Recombinant Murine Flt3-Ligand | Pepro Tech, INC. | Cat# 250-31L | |
| RetroNectin Recombinant Human Fibronectin Fragment | TaKaRa | Cat# T100A | |
| TransIT-293 Reagent | MirusBio | Cat# MIR 2705 | |
| TRI Reagent | Sigma Aldrich | Cat# T9424 | |
| Trypan Blue Solution, 0.4% | Gibco | Cat # 15250061 | |
| Trypsin-EDTA (0.25%), phenol red | Gibco | Cat# 25200-056 | |
| β-Mercaptoethanol (BME) | Sigma Aldrich | Cat# M3148 | |
| Commercial Assays | |||
| EasySep Mouse Hematopoietic Progenitor Cell Isolation Kit | StemCell technologies | Cat# 19856A | |
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher | Cat# 4368813 | |
| PerfeCTa qPCR SuperMix | Quanta Bio | Cat# 95051-500 | |
| Plasmid Maxi Kit (25) | Qiagen | Cat#:12163 | |
| Animals | |||
| Ai14TdTomato mice | Jackson Laboratory | Strain # 007914 | |
| CD45.1 C57BL6/J mice | Jackson Laboratory | Strain # 002014 | |
| CD45.2 C57BL6/J mice | Jackson Laboratory | Strain # 000664 | |
| Instruments and Softwares | |||
| Adobe illustrator | Version 25.2.3 | ||
| BD accuri C6 flow cytometer | BD Biosciences | ||
| FlowJo 10.8.0 | BD | ||
| GeneSys software program | Version 1.5.7.0 | ||
| GraphPad Prism version 6 | GraphPad | ||
| Hemavet 950FS | Drew Scientific | ||
| 7300 Real time PCR system | Applied Biosystems | ||
| Syngene G:BOX Chemi XR5 Chemiluminescence Fluorescence Imaging | G:Box Chemi |
References
- Dohner, H., Weisdorf, D. J., Bloomfield, C. D. Acute myeloid leukemia. The New England Journal of Medicine. 373 (12), 1136-1152 (2015).
- Fortier, J. M., Graubert, T. A. Murine models of human acute myeloid leukemia. Cancer Treatment and Research. 145, 183-196 (2010).
- Ernst, P., et al. Definitive hematopoiesis requires the mixed-lineage leukemia gene. Developmental Cell. 6 (3), 437-443 (2004).
- Fisher, J. N., Kalleda, N., Stavropoulou, V., Schwaller, J. The Impact of the cellular origin in acute myeloid leukemia: learning from mouse models. Hemasphere. 3 (1), 152 (2019).
- Zhan, Y., Zhao, Y. Hematopoietic stem cell transplant in mice by intra-femoral injection. Methods in Molecular Biology. 430, 161-169 (2008).
- Price, J. E., Barth, R. F., Johnson, C. W., Staubus, A. E. Injection of cells and monoclonal antibodies into mice: comparison of tail vein and retroorbital routes. Proceedings of the Society for Experimental Biology. 177 (2), 347-353 (1984).
- Yardeni, T., Eckhaus, M., Morris, H. D., Huizing, M., Hoogstraten-Miller, S. Retro-orbital injections in mice. Lab Animal. 40 (5), 155-160 (2011).
- Suckow, M. A., Danneman, P., Brayton, C. . The Laboratory Mouse. , (2001).
- Barr, J. E., Holmes, D. B., Ryan, L. J., Sharpless, S. K. Techniques for the chronic cannulation of the jugular vein in mice. Pharmacology, Biochemistry, and Behavior. 11 (1), 115-118 (1979).
- Kang, Y. Analysis of cancer stem cell metastasis in xenograft animal models. Methods in Molecular Biology. 568, 7-19 (2009).
- Nungestee, W., Wolf, A., Jourdonais, L. Effect of gastric mucin on virulence of bacteria in intraperitoneal injections in the mouse. Proceedings of the Society for Experimental Biology and Medicine. 30 (2), 120-121 (1932).
- Gargiulo, S., et al. Mice anesthesia, analgesia, and part I: anesthetic considerations in preclinical research. ILAR journal. 53 (1), 55-69 (2012).
- Leong, S. -. K., Ling, E. -. A. Labelling neurons with fluorescent dyes administered via intravenous, subcutaneous or intraperitoneal route. Journal of Neuroscience Methods. 32 (1), 15-23 (1990).
- Ma, P., et al. Intraperitoneal injection of magnetic Fe3O4-nanoparticle induces hepatic and renal tissue injury via oxidative stress in mice. International Journal of Nanomedicine. 7, 4809-4918 (2012).
- Schwarze, S. R., Ho, A., Vocero-Akbani, A., Dowdy, S. F. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 285 (5433), 1569-1572 (1999).
- Muench, M. O., Chen, J. C., Beyer, A. I., Fomin, M. E. Cellular therapies supplement: the peritoneum as an ectopic site of hematopoiesis following in utero transplantation. Transfusion. 51, 106-117 (2011).
- Zhao, W., et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World Journal of Gastroenterology. 18 (10), 1048 (2012).
- Yousefi, F., Ebtekar, M., Soleimani, M., Soudi, S., Hashemi, S. M. Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE). International Immunopharmacol. 17 (3), 608-616 (2013).
- Cheng, K., et al. Transplantation of bone marrow-derived MSCs improves cisplatinum-induced renal injury through paracrine mechanisms. Experimental and Molecular Pathology. 94 (3), 466-473 (2013).
- Castelo-Branco, M., et al. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 7 (3), 33360 (2012).
- Bazhanov, N., et al. Intraperitoneally infused human mesenchymal stem cells form aggregates with mouse immune cells and attach to peritoneal organs. Stem Cell Research & Therapy. 7, 27 (2016).
- Liu, Q., Chen, L., Atkinson, J. M., Claxton, D. F., Wang, H. G. Atg5-dependent autophagy contributes to the development of acute myeloid leukemia in an MLL-AF9-driven mouse model. Cell Death & Disease. 7 (9), 2361 (2016).
- Wognum, A. W., Eaves, A. C., Thomas, T. E. Identification and isolation of hematopoietic stem cells. Archives of Medical Research. 34 (6), 461-475 (2003).
- Randall, T. D., Weissman, I. L. Characterization of a population of cells in the bone marrow that phenotypically mimics hematopoietic stem cells: resting stem cells or mystery population. Stem Cells. 16 (1), 38-48 (1998).
- Gott, K. M., et al. A comparison of Cs-137 gamma rays and 320-kV X-rays in a mouse bone marrow transplantation model. Dose Response. 18 (2), 1559325820916572 (2020).
- Miner, N. A., Koehler, J., Greenaway, L. Intraperitoneal injection of mice. Applied Microbiology. 17 (2), 250-251 (1969).
- Madisen, L., et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neuroscience. 13 (1), 133-140 (2010).
- Cardiff, R. D., Miller, C. H., Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harbor Protocols. 2014 (6), 655-658 (2014).
- Ronan, J. L., Wu, W., Crabtree, G. R. From neural development to cognition: unexpected roles for chromatin. Nature Review Genetics. 14 (5), 347-359 (2013).
- Qian, F., et al. Interleukin-4 treatment reduces leukemia burden in acute myeloid leukemia. FASEB Journal. 36 (5), 22328 (2022).
- Krivtsov, A. V., et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 442 (7104), 818-822 (2006).
- Chen, W., et al. Malignant transformation initiated by Mll-AF9: gene dosage and critical target cells. Cancer Cell. 13 (5), 432-440 (2008).
- Somervaille, T. C. P., Cleary, M. L. Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell. 10 (4), 257-268 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved