A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Real-Time Measurement of the Mitochondrial Bioenergetic Profile of Neutrophils
In This Article
Summary
We describe stepwise protocols measuring the mitochondrial respiration of mouse and human neutrophils and HL60 cells using the metabolic extracellular flux analyzer.
Abstract
Neutrophils are the first line of defense and the most abundant leukocytes in humans. These effector cells perform functions such as phagocytosis and oxidative burst, and create neutrophil extracellular traps (NETs) for microbial clearance. New insights into the metabolic activities of neutrophils challenge the early concept that they primarily rely on glycolysis. Precise measurement of metabolic activities can unfold different metabolic requirements of neutrophils, including the tricarboxylic acid (TCA) cycle (also known as the Krebs cycle), oxidative phosphorylation (OXPHOS), pentose phosphate pathway (PPP), and fatty acid oxidation (FAO) under physiological conditions and in disease states. This paper describes a step-by-step protocol and prerequirements to measure oxygen consumption rate (OCR) as an indicator of mitochondrial respiration on mouse bone marrow-derived neutrophils, human blood-derived neutrophils, and the neutrophil-like HL60 cell line, using metabolic flux analysis on a metabolic extracellular flux analyzer. This method can be used for quantifying the mitochondrial functions of neutrophils under normal and disease conditions.
Introduction
Mitochondria play a major role in cell bioenergetics, which generates adenosine triphosphate (ATP) by oxidative phosphorylation (OXPHOS). In addition to this, the role of mitochondria extends into the generation and detoxification of reactive oxygen species, cytoplasmic and mitochondrial matrix calcium regulation, cellular synthesis, catabolism, and the transport of metabolites within the cell1. Mitochondrial respiration is essential in all cells, as their dysfunction can result in metabolic problems2, including cardiovascular diseases3 and a wide variety of neurodegenerative diseases, such as age-related macular degeneration4, Parkinson's and Alzheimer's diseases5, and Charcot-Marie-Tooth disease 2 A (CMT2A)6.
Electron microscopic studies on neutrophils revealed there are relatively few mitochondria7, and they rely heavily on glycolysis for their energy production as mitochondrial respiration rates are very low8. However, mitochondria are crucial for neutrophil functions, such as chemotaxis9 and apoptosis10,11,12. A previous study revealed a complex mitochondrial network in human neutrophils with high membrane potential. The mitochondrial membrane potential loss is an early indicator of neutrophil apoptosis10. Treatment with mitochondrial uncoupler carbonyl cyanide m-chlorophenyl hydrazone (CCCP) showed significant inhibition in chemotaxis, along with a change in mitochondrial morphology9,10.
Although the primary energy source for neutrophils is glycolysis, mitochondria provide the ATP that initiates neutrophil activation by fueling the first phase of purinergic signaling, which boosts Ca2+ signaling, amplifies mitochondrial ATP production, and initiates neutrophil functional responses13. Dysfunction of the mitochondrial respiratory chain results in excessive production of toxic reactive oxygen species (ROS) and leads to pathogenic damages14,15,16. NETosis, which is the process of forming neutrophil extracellular traps (NETs), is a critical property of neutrophils that helps them fight against pathogens17 and contributes to many pathological conditions, including cancer, thrombosis, and autoimmune disorders18. Mitochondrial-derived ROS contribute to NETosis19, mitochondrial DNA can be a component of NETs18, and altered mitochondrial homeostasis impairs NETosis20,21,22,23,24. Furthermore, during normal differentiation or maturation, neutrophil metabolic reprogramming gets reversed by limiting glycolytic activity, and they engage in mitochondrial respiration and mobilize intracellular lipids25,26.
The metabolic extracellular flux analyzer can continuously monitor and quantify live cell mitochondrial respiration and glycolysis. The analyzer utilizes a 96-well plate format sensor cartridge and two fluorophores to quantify oxygen (O2) concentration and pH changes. The sensor cartridge is above the cell monolayer during the assay and forms a ~200 nm high microchamber. The optical fiber bundles in the analyzer are used to excite the fluorophores and detect the fluorescent intensity changes. Real-time changes in O2 concentration and pH are automatically calculated and shown as oxygen consumption rate (OCR) and extracellular acidification rate (ECAR). There are four ports on the sensor cartridge that allow loading up to four compounds into each well during the assay measurements. This protocol focuses on quantifying the mitochondrial respiration of mouse and human neutrophils, as well as the neutrophil-like HL60 cells, using the metabolic extracellular flux analyzer.
Protocol
Heparinized whole-blood samples were obtained from healthy human donors after obtaining informed consent, as approved by the Institutional Review Board of UConn Health in accordance with the Declaration of Helsinki. All animal experiments followed the UConn Health Institutional Animal Care and Use Committee (IACUC) guidelines, and approval for the use of rodents was obtained from the UConn Health IACUC according to criteria outlined in the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. Male C57BL/6 mice at 6 weeks of age were used in this study.
1. Preparation of the 96-well plate for the metabolic extracellular flux assay
- A sensor cartridge is packaged on top of the specially designed 96-well plate for the metabolic extracellular flux assay. Hydrate the cartridge by carefully lifting it, and place 200 µL/well of calibration medium into each well of the underlying plate. Place the cartridge over the plate with calibrant in a non-CO2, humidified, 37 °C incubator overnight to hydrate.
- Based on the cell type, use a specific coating for the culture plate to ensure cell adhesion. For human neutrophils-undifferentiated and differentiated HL60 cells-coat the 96-well plate with sterile phosphate-buffered saline (PBS) containing 50 µL of 5 µg/mL purified mouse anti-human CD18 antibody (Clone TS1/18) at 4 °C overnight. For mouse neutrophils, coat the 96-well plate with 25 µL of 22.4 µg/mL Cell Tak at pH 8.0 in 0.1 M NaHCO3 at room temperature (RT) for 20 min.
- Wash the plates with 200 µL of sterile PBS twice.
- Add complete medium to the corner wells (A1, A12, H1, and H12) of the cell culture plate (without cells) for background correction in the cell culture plates while seeding.
NOTE: Evaporation during coating and calibration may affect the volume of the calibration media and normalization. Use a tray or chamber with sterile water-wetted tissue and place the plate with the calibrant above it to prevent evaporation.
2. Preparation and seeding of cells
- Preparation of assay medium
- Prepare assay medium by adding 1 mM pyruvate, 10 mM glucose, and 2 mM glutamine to Dulbecco's modified Eagle medium (DMEM).
- Isolation of mouse neutrophils from bone marrow
- Isolate mouse neutrophils from bone marrow using the Mouse Neutrophil Enrichment Kit, according to the manufacturer's instructions.
- Anesthetize the mice by intraperitoneal (i.p.) injection of ketamine (125 mg/kg) and xylazine (12.5 mg/kg) and then euthanize the mice by cervical dislocation.
- Harvest the femurs and tibias, as described previously27. Briefly, cut the skin to expose the leg with the muscles. Cut the hip joint to remove the leg from the body. Then, remove the muscles to collect the femur and tibia.
- Cut the smaller ends of the femurs and tibias. Holding the cut ends down, place them in a 0.5 mL centrifuge tube with two 25 G syringe needle-punched holes in the bottom, and place the 0.5 mL tube into a 1.5 mL centrifuge tube (Figure 1A). Add 50 µL of PBS into the 0.5 mL tube to prevent the drying of bone marrow cells.
- Centrifuge at 5,900 × g at RT for 15 s to collect the bone marrow at the bottom of the 1.5 mL tube. Resuspend the bone marrow cells with 1 mL of DMEM. Add 50 µL of rat serum from the kit, gently mix by pipetting, and transfer the cell suspension into a 5 mL polystyrene culture test tube.
- Add 50 µL of enrichment cocktail from the Mouse Neutrophil Enrichment Kit and incubate for 15 min at RT. Centrifuge at 300 × g at RT for 5 min.
- Resuspend the cells with 1 mL of DMEM, add 50 µL of biotin selection cocktail from the kit, gently mix by pipetting, and incubate for 15 min at RT.
- Add 150 µL of vortexed magnetic particles from the kit, gently mix by pipetting, and incubate for 10 min at RT.
- Add ~1.3 mL of DMEM, gently mix by pipetting, place the tube in the magnet for 3 min, and transfer the supernatant containing purified mouse neutrophils to a new 5 mL polystyrene culture test tube by inverting the original tube along with the magnet.
- Centrifuge at 300 × g at RT for 5 min. After removing the supernatant by vacuum aspiration, resuspend the cells with 1 mL of assay medium.
- Count the cells manually using a hemocytometer.
- Adjust the cell density to 1.1 × 106 cells/mL by adding assay medium, seed 180 µL of the mouse neutrophil suspension (2 × 105 cells) per well into the prepared 96-well plate (steps 1.2-1.4), and centrifuge the plate at 300 × g at RT for 3 min without brake to ensure proper adherence of the cells on the bottom of the plate.
- Incubate the plate in a non-CO2, humidified, 37 °C incubator for 1 hr to preequilibrate the cells with the assay medium.
NOTE: The purity of the neutrophils is critical for the assay since it is a potential bias. The purity of the mouse neutrophil isolation is 69.9%-88.7% by this protocol. There are other methods for isolating neutrophils from mouse bone marrow, such as density gradient centrifugation28. There are also alternative neutrophil isolation kits from other vendors, based on the negative magnetic selection using the monoclonal antibodies against antigens that are not expressed on neutrophils.
- Isolation of human neutrophils from peripheral blood
- Add 8 mL of polysaccharide solution into a 15 mL centrifuge tube, then layer over 4 mL of peripheral blood on top of the polysaccharide solution without mixing.
- Centrifuge at 550 × g at 20 °C for 30 min. Make the rotor decelerate at 1.
NOTE: The neutrophil separation time may vary between donors. It is a minimum of 30 min, and an additional 10-20 min may be needed if neutrophil separation is unsuccessful. - Observe the separation of plasma/platelets and mononuclear cells after the centrifugation, as shown in Figure 1B. Carefully remove the upper yellow liquid on top (plasma and platelets) and the upper cloudy band (mononuclear cells) without disturbing the lower cloudy band (neutrophils) using a 1 mL pipette.
- Collect the lower cloudy band and ~3-4 mL of the clear liquid below into a new 15 mL centrifuge tube containing 10 mL of PBS.
- Centrifuge at 400 × g at 20 °C for 10 min and remove the supernatant by vacuum aspiration.
- Resuspend the cells with 5 mL of PBS and centrifuge at 300 × g at 20 °C for 5 min.
- After removing the supernatant, resuspend the cells with 1 mL of assay medium.
- Count the cells manually using a hemocytometer.
NOTE: Since erythrocytes in the blood do not have any mitochondria, erythrocyte contamination does not affect the mitochondrial stress test assay and prevents neutrophil activation/priming29,30. Erythrocytes need to be lysed during cell counting to get an accurate neutrophil concentration. Add 10 µL of the cell suspension to 891 µL of deionized water for 10-30 s to lyse the erythrocytes, then add 99 µL of 10x PBS to balance the osmotic pressure, avoiding lysis of the neutrophils. - Adjust the cell density to 2.2 × 106 cells/mL by adding assay medium, seed 180 µL of human neutrophils (~4 × 105) per well into the prepared 96-well plate, and centrifuge the plate at 300 × g at RT for 3 min without brake to ensure proper adherence of the cells on the bottom of the plate.
- Incubate the plate in a non -CO2, humidified, 37 °C incubator for 1 hr to preequilibrate the cells with the assay medium.
NOTE: The purity of neutrophils is critical for the assay since it is a potential bias. The purity of human neutrophil isolation is 86.6%-96.8%. There are other density gradient centrifugation methods for isolating neutrophils from human blood, including Percoll isolation as well as a combination of Ficoll isolation and dextran sedimentation31.
- HL60 cell culture and neutrophil-directed differentiation
- Maintain HL60 cells in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine serum (FBS), 100 µg/mL penicillin, 100 µg/mL streptomycin, and 250 ng/mL amphotericin B at 37 °C and 5% CO2.
- For neutrophil-directed differentiation, maintain the HL60 cells (suspended cells) in a T25 flask at a density of 1 × 105 cells/mL in RPMI-1640 medium containing 10% FBS, 100 µg/mL penicillin, 100 µg/mL streptomycin, 250 ng/mL amphotericin B, and 1.3% dimethyl sulfoxide (DMSO) at 37 °C and 5% CO2 for 6 days.
- On the day of the assay, count the cells manually using the hemocytometer, centrifuge differentiated or undifferentiated HL60 cells at 300 × g at RT for 5 min, wash with DMEM once, and resuspend the cells with the assay medium to get a cell density of 1.39 × 106 cells/mL.
- Seed 180 µL of differentiated or undifferentiated HL60 cells (~2.5 × 105) per well into the prepared 96-well plate and centrifuge the plate at 300 × g at RT for 3 min without brake to ensure proper adherence of the cells on the bottom of the plate.
- Incubate the plate in a non-CO2, humidified, 37 °C incubator for 1 hr to preequilibrate the cells with the assay medium.
NOTE: Confirm the complete adhesion of cells using a microscope before the next step.
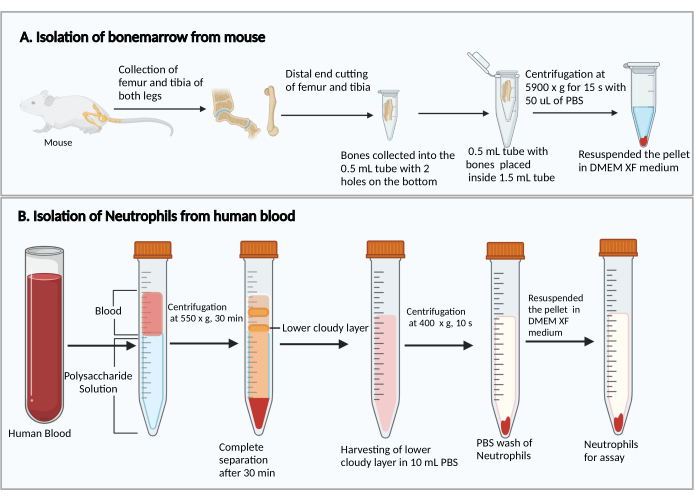
Figure 1: Schematic diagram of the isolation of bone marrow cells and neutrophils. (A) Harvesting bone marrow cells from a mouse and (B) isolating neutrophils from human blood. Please click here to view a larger version of this figure.
| Cell type | Cells per well | Compounds/Reagents | Working solution concentration | Injection volume to ports | Final concentration in wells |
| Mouse neutrophils | 2 × 105 | Oligomycin | 25 µM | 20 µL | 2.5 µM |
| FCCP | 7.5 µM | 17.6 µL | 0.61 µM | ||
| Rotenone Antimycin A mixture | 10 µM | 24 µL | 1 µM | ||
| Human neutrophils | 4 × 105 | Oligomycin | 10 µM | 20 µL | 1 µM |
| FCCP | 12.5 µM | 22 µL | 1.25 µM | ||
| Rotenone Antimycin A mixture | 10 µM | 24 µL | 1 µM | ||
| Undifferentiated or differentiated HL60 cells | 2.5 × 105 | Oligomycin | 25 µM | 20 µL | 2.5 µM |
| FCCP | 15 µM | 22 µL | 1.5 µM | ||
| Rotenone Antimycin A mixture | 10 µM | 24 µL | 1 µM |
Table 1: Cell numbers and reagent concentrations for the mitochondrial stress test.
3. Preparing compounds in the mitochondrial stress test kit
- Open the mitochondrial stress test kit and prepare the reagents.
NOTE: The different working solution concentrations, injection volume into the wells, and final concentrations in the wells of oligomycin, carbonylcyanide p-trifluoromethoxy phenylhydrazone (FCCP), and rotenone/antimycin A mixture used for mouse neutrophils, human neutrophils, and HL60 cells are shown in Table 1.- Prepare oligomycin stock solution by reconstituting oligomycin with 630 µL of assay medium to obtain a 100 µM stock solution.
NOTE: It is recommended to use the stock solution on the same day.- Prepare oligomycin working solution for mouse neutrophil and HL60 cell assays by mixing 630 µL of stock solution with 1,890 µL of assay medium to obtain a 25 µM working solution.
- Prepare oligomycin working solution for the human neutrophil assay by mixing 300 µL of stock solution with 2,700 µL of assay medium to obtain a 10 µM working solution.
NOTE: The binding of oligomycin to the Fo baseplate subunit of the Fo/F1 ATPase (ATP synthase) prevents the re-entry of protons into mitochondria and inhibits ATP synthesis32. This significantly reduces electron flow through the electron transport chain and the OCR. However, electron flow does not stop completely due to proton leak across the inner mitochondrial membrane33.
- Prepare FCCP stock solution by reconstituting FCCP with 720 µL of assay medium to obtain a 100 µM stock solution.
- Prepare working solution for the mouse neutrophil assay by mixing 300 µL of stock solution with 3,700 µL of assay medium to obtain a 7.5 µM working solution.
- Prepare working solution for the human neutrophil assay by mixing 375 µL of stock solution with 2,625 µL of assay medium to obtain a 12.5 µM working solution.
- Prepare working solution for the HL60 cell assay by mixing 720 µL of stock solution with 4,080 µL of assay medium to obtain a 15 µM working solution.
NOTE: The addition of FCCP reveals the maximum capacity of the mitochondria to use OXPHOS. It is a lipid-soluble, weak acid permeable to mitochondria results in the dissipitation of transmembrane potential. Discharging the proton gradient across the mitochondrial inner membrane and diverting the proton flux from Fo/F1 ATP synthase results in mitochondrial uncoupling. This uncoupling effect abruptly increases mitochondrial oxygen consumption to preserve the proton gradient34.
- Prepare rotenone/antimycin A stock solution by reconstituting the rotenone/antimycin A mixture with 540 µL of assay medium to obtain a 50 µM stock solution.
- Prepare rotenone/antimycin A working solution for all assays by mixing 540 µL of stock solution with 2,160 µL of assay medium to obtain a 10 µM working solution.
NOTE: Rotenone blocks complex I by inhibiting electron transfer from the iron-sulfur centers in complex I to ubiquinone, while antimycin A blocks complex III of the electron transport chain, leading to a blockade of OXPHOS with a limited synthesis of ATP35. Thereby, this reveals non-mitochondrial respiration36,37.
- Prepare rotenone/antimycin A working solution for all assays by mixing 540 µL of stock solution with 2,160 µL of assay medium to obtain a 10 µM working solution.
- Prepare oligomycin stock solution by reconstituting oligomycin with 630 µL of assay medium to obtain a 100 µM stock solution.
- Load the cartridge with reagents; load prepared oligomycin, FCCP, and rotenone/antimycin A mixture into the A, B, and C ports, respectively, in the cartridge for injections (Figure 2). Use the template insert available along with the cartridge to facilitate the loading of reagents to the ports. Fill the unused ports with 20 µL of assay medium.
- For the mouse neutrophil assay, load 20 µL of oligomycin (25 µM; each well of the 96-well plate has 180 µL of assay medium at the beginning, thus the final concentration is 2.5 µM) into the A ports. Load 17.6 µL of FCCP (7.5 µM; final concentration: ~0.61 µM) into the B ports. Load 24 µL of rotenone/antimycin A mixture (10 µM; final concentration: ~1 µM) into the C ports.
- For the human neutrophil assay, load 20 µL of oligomycin (10 µM; each well of the 96-well plate has 180 µL of assay medium at the beginning, thus the final concentration is 1 µM) into the A ports. Load 22 µL of FCCP (12.5 µM; final concentration: ~1.25 µM) into the B ports. Load 24 µL of rotenone/antimycin A mixture (10 µM; final concentration: ~1 µM) into the C ports.
- For the HL60 and dHL60 cell assay, load 20 µL of oligomycin (25 µM; each well of the 96-well plate has 180 µL of assay medium at the beginning, thus the final concentration is 2.5 µM) into the A ports. Load 22 µL of FCCP (15 µM; final concentration: ~1.5 µM) into the B ports. Load 24 µL of rotenone/antimycin A mixture (10 µM; final concentration: ~1 µM) into the C ports.
NOTE: Pipette the drug solution in the port without touching the bottom of the port. Do not tap the plate after loading to avoid leakage, as the liquids are held by the capillary forces. The background wells of the culture plate and the ports on the sensor cartridge are loaded with assay medium or with the same port of the loading of reagents as in the sample wells to normalize the effect of reagents on the background values. The reagents must be added to the respective ports without lifting the cartridge from the calibrant-containing utility plate to avoid any air trap. Fill the last port of all wells with the medium as shown in Figure 2.
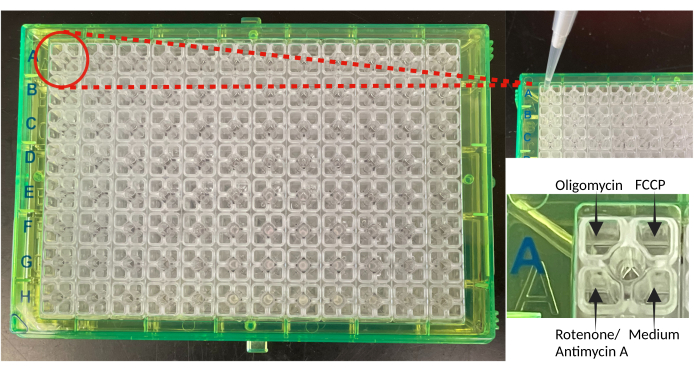
Figure 2: The mitochondrial stress assay cartridge and their ports of injection. The image shows the cartridge of the mitochondrial stress assay and an enlarged image showing the loading of individual drugs/medium to the ports. Abbreviation: FCCP = carbonylcyanide p-trifluoromethoxy phenylhydrazone. Please click here to view a larger version of this figure.
4. Running the mitochondrial stress assay
- Power on the metabolic analyzer and computer, open the Wave software, and click on Heater On to set up the machine at 37 °C at least 5 hr in advance. After reaching the temperature, the lower-left corner of the wave software shows ready.
- Open a template for the mitochondrial stress assay kit in the software. Click on Group Definition in the top menu bar.
- Separately pre-set up each definition on the left side, such as injection strategies, pretreatments, assay media, and (biomaterial used) cell types.
- Click on injection strategies on the left side, then click on Add and name the injection condition as Human neutrophils/Mouse neutrophils/HL60. Select port A-D by clicking on each and click on Add compound and enter Oligomycin/FCCP/Rotenone Antimycin A with the respective concentrations (Table 1).
- Click on pretreatment on the left side, then click on Add and name them as CD18 and Cell Tak separately. Click on the biomaterial used on left side, then click on Add and name them as Human neutrophils, Mouse neutrophils, and HL60/dHL60 with seeding density.
- Define the groups by clicking on Add Group (e.g., for the human neutrophil assay) and by clicking on the definition underlying it (e.g., Injection strategies as per Table 1-Human neutrophils, Pretreatment as CD18, and cell type as Human neutrophils).
- Click on Plate Map in the top menu to observe all the groups, with definitions on the left side and the plate map on the right. Drag and drop to add each well to the group while maintaining the four corner wells as Background (default).
- Set up the protocol by clicking on Protocol in the top menu and define three cycles of baseline, oligomycin, FCCP, rotenone, and antimycin A mixture by setting the time to Mix as 3 min, Rest as 0 min, and Measurement as 7 min.
- Go to the Run Assay page, provide the project summary information for reference, and click on Start Run.
- Give the location to save the files, so that all the results are saved after completion of the assay.
- After automatic loading of the assay, wait for the tray to open to place the sensor cartridge and plate with 200 µL of calibrant (step 1.1). Set the cartridge barcode direction facing to the right. Run the calibration by clicking on I'm ready, which takes approximately 20 min.
- After the calibration, click on Open Tray. Replace the plate with the cell-seeded plate and click on Load Cell Plate to continue the assay.
- After completing the assay, the data is automatically saved. Click on View results and export it as a spreadsheet or other analysis software file.
- Graph and analyze the data (Figure 3 and Figure 4).
- Calculate respiration parameters, including basal mitochondrial respiration, proton leak-linked respiration, ATP-linked respiration, maximal respiration, spare respiratory capacity, and non-mitochondrial respiration (Figure 4A)38,39,40,41.
- Calculate the basal mitochondrial respiration by subtracting the OCR value measured after adding rotenone/antimycin A mixture from the OCR before injecting oligomycin (Figure 4A, a).
- Calculate the proton leak-linked respiration by subtracting the OCR value after rotenone/antimycin A mixture injection from the OCR value measured after oligomycin injection (Figure 4A,b).
- Estimate the ATP-linked respiration by calculating the difference between the basal mitochondrial respiration and proton leak-linked respiration. Subtract the first OCR value measured after oligomycin injection from the first OCR value before oligomycin injection (Figure 4A,c).
- Maximal respiration is the maximal respiration rate that a cell can achieve after adding FCCP. Calculate this by subtracting the OCR value after rotenone/antimycin A mixture injection from the OCR value measured after FCCP injection (Figure 4A,d).
- Spare respiratory capacity refers to tha capacity of a cell to meet the higher energy demand through OXPHOS. Calculate this by finding the difference between the maximal respiration and basal mitochondrial respiration (Figure 4A, e).
- Non-mitochondrial respiration is the amount of oxygen consumed by non-mitochondrial sources. Measure this after the addition of rotenone/antimycin A mixture (Figure 4A,f).
- Perform statistical analysis using Student's t-test to compare different respiration parameters of undifferentiated and differentiated HL60 cells. Consider p < 0.05 to be statistically significant.
NOTE: Replicates with OCR or ECAR values below zero are considered an error in sample preparation, compound injection, or measurement. They are excluded from future analysis.
Results
Representative OCR dynamics are shown indicating the mitochondrial respiration changes in response to oligomycin, FCCP, and rotenone/antimycin A mixture of mouse neutrophils (Figure 3A), human neutrophils (Figure 3B), and undifferentiated and differentiated HL60 cells (Figure 3C). In all cells, oligomycin treatment decreases the OCR value by inhibiting the proton channel of ATP synthase; FCCP treatment restores the OCR value by incr...
Discussion
The standard procedure that measures the mitochondrial respiration of neutrophils using the metabolic extracellular flux analyzer is limited by many factors, including cell number, cell growth, and viability. Each compound concentration varies among the type and source of cells in this assay. Oligomycin and rotenone/antimycin A are mostly used in a similar concentration among most cell types. However, as the FCCP-induced maximum respiratory rate varies among different cells, careful titration of FCCP is required to ...
Disclosures
The authors declare no competing financial interest.
Acknowledgements
We acknowledge Dr. Anthony T. Vella and Dr. Federica Aglianoin from the Department of Immunology at UConn Health for their training in using the metabolic extracellular flux analyzer, and Dr. Lynn Puddington in the Department of Immunology at UConn Health for her support of the instruments. We acknowledge Dr. Geneva Hargis from UConn School of Medicine for her help with scientific writing and editing of this manuscript. This research was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (R01HL145454), National Institute of General Medical Sciences (R35GM147713 and P20GM139763), a startup fund from UConn Health, and a Career re-entry fellowship from the American Association of Immunologists.
Materials
| Name | Company | Catalog Number | Comments |
| 37 °C non-CO2 incubator | Precision | Economy Model 2EG | Instrument |
| Biorender | Software Application | ||
| Centrifuge | Eppendorf | Model 5810R | Instrument |
| Corning Cell-Tak Cell and Tissue Adhesive | Corning | 102416-100 | Reagent |
| EasySep Magnet | STEMCELL | 18000 | Magnet |
| EasySepMouse Neutrophil Enrichment kit | STEMCELL | 19762A | Reagents |
| Graphpad Prism 9 | Software Application | ||
| Human Serum Albumin Solution (25%) | GeminiBio | 800-120 | Reagents |
| Ketamine (VetaKet) | DAILYMED | NDC 59399-114-10 | Anesthetic |
| PBS | Cytiva | SH30256.01 | Reagents |
| Plate buckets | Eppendorf | UL155 | Accessory |
| PolymorphPrep | PROGEN | 1895 (previous 1114683) | polysaccharide solution |
| Purified mouse anti-human CD18 antibody | Biolegend | 302102 | Clone TS1/18 |
| RPMI 1640 Medium | Gibco | 11-875-093 | Reagents |
| Seahorse metabolic extracellular flux analyzer | Agilent | XFe96 | Instrument |
| Seahorse XF Cell Mito Stress Test Kit | Agilent | 103015-100 | mitochondrial stress test Kit |
| Swing-bucket rotor | Eppendorf | A-4-62 | Rotor |
| Vactrap 2 Vacum Trap | Fox Lifesciences | 3052101-FLS | Instrument |
| Wave | Software Application | ||
| XF 1.0 M Glucose Solution | Agilent | 103577-100 | Reagent |
| XF 100 mM Pyruvate Solution | Agilent | 103578-100 | Reagent |
| XF 200 mM Glutamine Solution | Agilent | 103579-100 | Reagent |
| XF DMEM medium | Agilent | 103575-100 | Reagent |
| XFe96 FluxPak | Agilent | 102601-100 | Material |
| Xylazine (AnaSed Injection) | DAILYMED | NDC 59399-110-20 | Anesthetic |
References
- Demine, S., Renard, P., Arnould, T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 8 (8), 795 (2019).
- Noguchi, M., Kasahara, A. Mitochondrial dynamics coordinate cell differentiation. Biochemical and Biophysical Research Communications. 500 (1), 59-64 (2018).
- Zhu, L., et al. Correlation between mitochondrial dysfunction, cardiovascular diseases, and traditional Chinese medicine. Evidence-Based Complementary and Alternative Medicine. 2020, e2902136 (2020).
- Kaarniranta, K., et al. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Progress in Retinal and Eye Research. 79, 100858 (2020).
- Onyango, I. G., Khan, S. M., Bennett, J. P. Mitochondria in the pathophysiology of Alzheimer's and Parkinson's diseases. Frontiers in Bioscience. 22 (5), 854-872 (2017).
- Loiseau, D., et al. Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Annals of Neurology. 61 (4), 315-323 (2007).
- Zucker-Franklin, D. Electron microscopic studies of human granulocytes: structural variations related to function. Seminars in Hematology. 5 (2), 109-133 (1968).
- Karnovsky, M. L. The metabolism of leukocytes. Seminars in Hematology. 5 (2), 156-165 (1968).
- Bao, Y., et al. mTOR and differential activation of mitochondria orchestrate neutrophil chemotaxis. The Journal of Cell Biology. 210 (7), 1153-1164 (2015).
- Fossati, G., et al. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. Journal of Immunology. 170 (4), 1964-1972 (2003).
- Pryde, J. G., Walker, A., Rossi, A. G., Hannah, S., Haslett, C. Temperature-dependent arrest of neutrophil apoptosis. Failure of Bax insertion into mitochondria at 15 degrees C prevents the release of cytochrome c. The Journal of Biological Chemistry. 275 (43), 33574-33584 (2000).
- Maianski, N. A., Mul, F. P. J., van Buul, J. D., Roos, D., Kuijpers, T. W. Granulocyte colony-stimulating factor inhibits the mitochondria-dependent activation of caspase-3 in neutrophils. Blood. 99 (2), 672-679 (2002).
- Bao, Y., et al. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. The Journal of Biological Chemistry. 289 (39), 26794-26803 (2014).
- Chouchani, E. T., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 515 (7527), 431-435 (2014).
- Hayashi, G., Cortopassi, G. Oxidative stress in inherited mitochondrial diseases. Free Radical Biology and Medicine. 88, 10-17 (2015).
- Mailloux, R. J. An update on mitochondrial reactive oxygen species production. Antioxidants. 9 (6), 472 (2020).
- Abuaita, B. H., et al. The IRE1α stress signaling axis is a key regulator of neutrophil antimicrobial effector function. Journal of Immunology. 207 (1), 210-220 (2021).
- Lood, C., et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nature Medicine. 22 (2), 146-153 (2016).
- Douda, D. N., Khan, M. A., Grasemann, H., Palaniyar, N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proceedings of the National Academy of Sciencesa. 112 (9), 2817-2822 (2015).
- Monteith, A. J., et al. Altered mitochondrial homeostasis during systemic lupus erythematosus impairs neutrophil extracellular trap formation rendering neutrophils ineffective at combating Staphylococcus aureus. Journal of Immunology. 208 (2), 454-463 (2022).
- Monteith, A. J., Miller, J. M., Beavers, W. N., Juttukonda, L. J., Skaar, E. P. Increased dietary manganese impairs neutrophil extracellular trap formation rendering neutrophils ineffective at combating Staphylococcus aureus. Infection and Immunity. 90 (3), 0068521 (2022).
- Monteith, A. J., et al. Mitochondrial calcium uniporter affects neutrophil bactericidal activity during Staphylococcus aureus infection. Infection and Immunity. 90 (2), 0055121 (2022).
- Cao, Z., et al. Roles of mitochondria in neutrophils. Frontiers in Immunology. 13, 934444 (2022).
- Papayannopoulos, V., Metzler, K. D., Hakkim, A., Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. The Journal of Cell Biology. 191 (3), 677-691 (2010).
- Fan, Z., Ley, K. Developing neutrophils must eat…themselves. Immunity. 47 (3), 393-395 (2017).
- Riffelmacher, T., et al. Autophagy-dependent generation of free fatty acids is critical for normal neutrophil differentiation. Immunity. 47 (3), 466-480 (2017).
- Amend, S. R., Valkenburg, K. C., Pienta, K. J. Murine hind limb long bone dissection and bone marrow isolation. Journal of Visualized Experiments. (110), e53936 (2016).
- Swamydas, M., Isolation Lionakis, M. S. purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. Journal of Visualized Experiments. (77), e50586 (2013).
- Gerner, M. C., et al. Packed red blood cells inhibit T-cell activation via ROS-dependent signaling pathways. The Journal of Biological Chemistry. 296, 100487 (2021).
- Zhang, Z. -. W., et al. Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB Life. 63 (7), 560-565 (2011).
- Kuhns, D. B., Priel, D. A. L., Chu, J., Zarember, K. A. Isolation and functional analysis of human neutrophils. Current Protocols in Immunology. 111, 1-16 (2015).
- Hearne, A., Chen, H., Monarchino, A., Wiseman, J. S. Oligomycin-induced proton uncoupling. Toxicology In Vitro. 67, 104907 (2020).
- Plitzko, B., Loesgen, S. Measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in culture cells for assessment of the energy metabolism. Bio-Protocol. 8 (10), e2850 (2018).
- Nath, S. The molecular mechanism of ATP synthesis by F1F0-ATP synthase: a scrutiny of the major possibilities. Advances in Biochemical Engineering/Biotechnology. 74, 65-98 (2002).
- Heinz, S., et al. Mechanistic investigations of the mitochondrial complex I inhibitor rotenone in the context of pharmacological and safety evaluation. Scientific Reports. 7 (1), 45465 (2017).
- Hytti, M., et al. Antimycin A-induced mitochondrial damage causes human RPE cell death despite activation of autophagy. Oxidative Medicine and Cellular Longevity. 2019, 1583656 (2019).
- Malecki, M., Kamrad, S., Ralser, M., Bähler, J. Mitochondrial respiration is required to provide amino acids during fermentative proliferation of fission yeast. EMBO Reports. 21 (11), e50845 (2020).
- Divakaruni, A. S., Paradyse, A., Ferrick, D. A., Murphy, A. N., Jastroch, M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods in Enzymology. 547, 309-354 (2014).
- Marchetti, P., Fovez, Q., Germain, N., Khamari, R., Kluza, J. Mitochondrial spare respiratory capacity: Mechanisms, regulation, and significance in non-transformed and cancer cells. The FASEB Journal. 34 (10), 13106-13124 (2020).
- Nicholas, D., et al. Advances in the quantification of mitochondrial function in primary human immune cells through extracellular flux analysis. PLoS One. 12 (2), e0170975 (2017).
- Tur, J., et al. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Reports. 32 (8), 108079 (2020).
- Benz, R., McLaughlin, S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophysical Journal. 41 (3), 381-398 (1983).
- Wettmarshausen, J., Perocchi, F. Assessing calcium-stimulated mitochondrial bioenergetics using the seahorse XF96 analyzer. Methods in Molecular Biology. 1925, 197-222 (2019).
- Forkink, M., et al. Mitochondrial hyperpolarization during chronic complex I inhibition is sustained by low activity of complex II, III, IV and V. Biochimica et Biophysica Acta. 1837 (8), 1247-1256 (2014).
- . Methods for Reducing Cell Growth Edge Effects in Agilent Seahorse XF Cell Culture Microplates Available from: https://www.agilent.com/cs/library/usermanuals/public/user-manual-methods-for-reducing-cell-growth-edge-effect-cell-analysis-5994-0240en-agilent.pdf (2019)
- Lundholt, B. K., Scudder, K. M., Pagliaro, L. A simple technique for reducing edge effect in cell-based assays. Journal of Biomolecular Screening. 8 (5), 566-570 (2003).
- Wu, D., Yotnda, P. Induction and testing of hypoxia in cell culture. Journal of Visualized Experiments. (54), e2899 (2011).
- Normalisation of Seahorse XFe96 metabolic assaysto cell number with Hoechst stain using well-scan mode on the CLARIOstar Plus. BMG Labtech Available from: https://www.bmglabtech.com/cn/normalisation-of-seahorse-xfe96-metabolic-assays-to-cell-number-with-hoechst-stain/ (2020)
- Yetkin-Arik, B., et al. The role of glycolysis and mitochondrial respiration in the formation and functioning of endothelial tip cells during angiogenesis. Scientific Reports. 9 (1), 12608 (2019).
- Jastroch, M., Divakaruni, A. S., Mookerjee, S., Treberg, J. R., Brand, M. D. Mitochondrial proton and electron leaks. Essays in Biochemistry. 47, 53-67 (2010).
- Jandl, R. C., et al. Termination of the respiratory burst in human neutrophils. The Journal of Clinical Investigation. 61 (5), 1176-1185 (1978).
- Azevedo, E. P., et al. A metabolic shift toward pentose phosphate pathway is necessary for amyloid fibril- and phorbol 12-myristate 13-acetate-induced neutrophil extracellular trap (NET) formation. The Journal of Biological Chemistry. 290 (36), 22174-22183 (2015).
- Six, E., et al. AK2 deficiency compromises the mitochondrial energy metabolism required for differentiation of human neutrophil and lymphoid lineages. Cell Death & Disease. 6 (8), e1856 (2015).
- Kumar, S., Dikshit, M. Metabolic insight of neutrophils in health and disease. Frontiers in Immunology. 10, 2099 (2019).
- Rodríguez-Espinosa, O., Rojas-Espinosa, O., Moreno-Altamirano, M. M. B., López-Villegas, E. O., Sánchez-García, F. J. Metabolic requirements for neutrophil extracellular traps formation. Immunology. 145 (2), 213-224 (2015).
- Invernizzi, F., et al. Microscale oxygraphy reveals OXPHOS impairment in MRC mutant cells. Mitochondrion. 12 (2), 328-335 (2012).
- Zenaro, E., et al. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nature Medicine. 21 (8), 880-886 (2015).
- Maianski, N. A., et al. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death and Differentiation. 11 (2), 143-153 (2004).
- Bergman, O., Ben-Shachar, D. Mitochondrial oxidative phosphorylation system (OXPHOS) deficits in schizophrenia. Canadian Journal of Psychiatry. 61 (8), 457-469 (2016).
- Zhou, W., Qu, J., Xie, S., Sun, Y., Yao, H. Mitochondrial dysfunction in chronic respiratory diseases: implications for the pathogenesis and potential therapeutics. Oxidative Medicine and Cellular Longevity. 2021, 5188306 (2021).
- Hirano, M., Emmanuele, V., Quinzii, C. M. Emerging therapies for mitochondrial diseases. Essays in Biochemistry. 62 (3), 467-481 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved