A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Real-Time High Throughput Technique to Quantify Neutrophil Extracellular Traps Formation in Human Neutrophils
In This Article
Summary
We present an automated high-throughput method to quantify neutrophil extracellular traps (NETs) utilizing the live cell analysis system, coupled with a membrane permeability-dependent dual-dye approach.
Abstract
Neutrophils are myeloid-lineage cells that form a crucial part of the innate immune system. The past decade has revealed additional key roles that neutrophils play in the pathogenesis of cancer, autoimmune diseases, and various acute and chronic inflammatory conditions by contributing to the initiation and perpetuation of immune dysregulation through multiple mechanisms, including the formation of neutrophil extracellular traps (NETs), which are structures crucial in antimicrobial defense. Limitations in techniques to quantify NET formation in an unbiased, reproducible, and efficient way have restricted our ability to further understand the role of neutrophils in health and diseases. We describe an automated, real-time, high-throughput method to quantify neutrophils undergoing NET formation using a live cell imaging platform coupled with a membrane permeability-dependent dual-dye approach using two different DNA dyes to image intracellular and extracellular DNA. This methodology is able to help assess neutrophil physiology and test molecules that can target NET formation.
Introduction
Neutrophil extracellular traps (NETs) are web-like chromatin structures extruded from neutrophils in response to various inflammatory stimuli. NETs are composed of DNA, histones, and various anti-microbial proteins/peptides, which trap and kill infectious pathogens and invoke inflammatory responses1.
While NETs are beneficial for host defense against pathogens, they have gathered attention as a potential driver of various autoimmune diseases2, thrombosis3, metabolic diseases4, and metastatic growth of cancers5. As such, inhibition of NET formation is a potential therapeutic option for these diseases. However, despite some promising NETs-targeting molecules in development6, there is still no approved therapy that specifically affects this mechanism. This is, at least partially, attributable to the lack of objective, unbiased, reproducible, and high throughput quantification methods for NET formation.
We established and reported a new method utilizing a dual-color live-cell imaging platform7,8. Time-lapse images of neutrophils stained with membrane-permeable nuclear dye and membrane-impermeable DNA dye are analyzed by the software, and the numbers of pre- and post-NET-forming neutrophils are counted at multiple time points. Since the integrity of the plasma membrane is lost during NET formation by the regulation of PKCα-mediated Lamin B and CDK4/6-mediated Lamin A/C disassembly9, NET-forming neutrophils are stained by membrane-impermeable DNA dye while healthy neutrophils are not. This method overcomes the problems of previously reported techniques to quantify NET formation and provides unbiased, high-throughput, reproducible, and accurate NET quantification in an automated manner.
Protocol
Neutrophils from healthy human subjects were obtained after informed consent was provided under National Institutes of Health (NIH) Institutional Review Board (IRB) approved protocol. The protocol follows guidelines of NIH human research ethics committee.
1. Staining of the neutrophils and preparation of assay plate
- Take peripheral blood with appropriate written informed consent following the guideline of each institute and isolate neutrophils using any desired method. For example, the Ficoll-dextran method10,11 is a commonly used method for isolation of neutrophils from human peripheral blood.
NOTE: Although the method discussed here uses human neutrophils, mouse neutrophils may also be analyzed using a similar protocol. - Resuspend neutrophils in Roswell Park Memorial Institute (RPMI; see Table of Materials) 1640 medium at 2.0 x 106 neutrophils/mL and place neutrophil suspension into 1.5 mL centrifuge tube.
NOTE: We usually do not add fetal bovine serum (FBS) to the culture media because albumin in FBS can reduce NET formation12 and heat-stable nuclease in FBS can degrade NETs13. - Add membrane-permeable red DNA dye (see Table of Materials) at 1 µL per 1.5 mL of neutrophil suspension.
- Incubate at room temperature for 5 min in the dark. Centrifuge at 2500 x g for 5 min at room temperature and remove supernatant.
- Resuspend neutrophils in 1 mL of RPMI, then centrifuge at 2500 x g for 5 min at room temperature and remove supernatant. Repeat 2x (wash total 3x).
- Resuspend neutrophils in 1 mL of RPMI and count using a cell counter. Dilute neutrophil suspension to 1.5 x 105 neutrophils/mL.
- Add 4 µL of 1:100-pre-diluted membrane-impermeable green DNA dye (see Table of Materials) per 1 mL of neutrophil suspension.
- Put 100 µL of neutrophil suspension per well into a clear tissue-culture treated 96-well plate (see Table of Materials). Set each condition in triplicate.
NOTE: We have previously shown7 that there is no difference in NET formation and quantification with this method if neutrophils are placed in plates with or without poly-L-lysine coating. Therefore, the use of tissue culture treated plate is sufficient for this method. - Add 100 µL of RPMI containing stimulus reagents with or without inhibitor, or any other reagent of interest in respective wells. Always use positive control wells by adding 500 nM phorbol 12-myristate 13-acetate (PMA) or 2.5 µM calcium ionophore (A23187), 30 µM AKT inhibitor was used here.
- Place plate in the live cell analysis system (see Table of Materials) that is housed in a 5% CO2 incubator.
NOTE: Condensation may appear on the top and bottom of the plate a few minutes after this step. This may inhibit proper imaging. Wipe and remove condensation thoroughly before the start of the scanning. Place the plate in the machine, start the software, input scanning protocol, and then wipe the plate just before the first scan.
2. Scanning plate to visualize NET-forming neutrophils
- Start the software (see Table of Materials for software details). Launch Add Vessel by pressing + at the top left corner.
- Choose Scan on Schedule. Choose New.
NOTE: Once this is created, run the same scanning protocol by choosing Copy Previous and selecting stored protocol on the next screen. - Choose Standard. Set scan settings as: Cell-by-Cell options: None; Image channels: Phase, Green (Acquisition Time: 200 ms), Red (Acquisition Time: 400 ms); Objective: 20x.
- Choose the plate being used from the list. Select vessel location where to put the plate on the tray of the live cell imaging system.
- Select wells where samples are present and decide how many images to take per well. Based on the number of images per well, generate an estimated scan duration for the plate. Usually, 4 images per well is sufficient; however, this may vary depending on the conditions and frequency of scans.
- Input the information from each well (e.g., cell type and compound) by clicking Create Plate Map to provide a name for the study.
NOTE: The plate map can be prepared and saved in advance using the plate map editor in the software. The saved plate map data can be imported during this step. If desired, entering plate map information can be skipped and performed later. Data can also be obtained without entering information of plate layout. However, it is recommended to input plate information to make the subsequent analysis easier. - On the next screen, select Basic Analyzer as the analysis type, and choose Analysis Protocol from drop-down list, which shows previously used analysis definitions (not scanning protocols). Spectral unmixing for both green and red can be left at 0.0 %.
NOTE: This step may be skipped, if desired. - Schedule scan. For the experiment here, scan at every 15-20 min for 8 h . Set the starting time of the scan by dragging the white and gray bar on top of the screen.
NOTE: Avoid scanning too frequently, otherwise the machine may not work well due to overheating. Time of scanning should not exceed 12 h per 24 h. You may see an alert if scanning is too frequent. - Check the scan setting on the next screen and press Add to Schedule to start scanning. Wait until the initial set of images are scanned to confirm if everything is working well.
- Sometimes cells may not be properly focused. In that case, check if condensation is present (and wipe accordingly), plate is correctly set on the tray, or the position of the plate is correctly specified, etc. If cells are still floating and have not settled down to the bottom of the well, wait for about 5 min before scanning.
3. Setting the analysis definition to quantify NETs
- Open the study (vessel) to be analyzed on View tab. Press Launch Analysis on the left of the screen. Choose Create New Analysis Definition.
- If an analysis has been performed previously, use the same analysis definition with minor alterations by selecting Copy Existing Analysis Definition. In this case, go to step 3.3. If using the analysis without any alteration select Use Existing Analysis Definition; this is not recommended because the level of fluorescence may vary between assays on different days, and some minor corrections are usually necessary.
- Select Basic Analyzer. Use all image channels: Phase, Green, Red, and Overlap.
NOTE: Although we usually use green and red object masks to count NETs, overlap object mask is useful when debris matters. In such cases, the number of overlap object count will be used as numerators instead of green object count (see step 3.9). If overlap object count is used instead of green object count, all red signals must be counted correctly. Adjust the setting of red signal to count all red objects with low red signal in images taken in later timepoints but not to count debris. - Choose 6 - 8 representative sample images for training. For the experiment here, include the following images:
Images taken between 0 to 20 min to optimize the green signal setting to compensate for subtle green signals that can be generated in non-NET-forming neutrophils
Images of maximum NETs with positive control, taken between 3-6 h (time varies depending on the stimulation used) to optimize the green signal setting to define NET-forming neutrophils
Images taken around the 1 h timepoint to count the number of total cells (stained with red dye) since the nuclear red signal is the strongest around 1 h timepoint (when all the cells have settled to the bottom of the well) and gradually decreases due to cell death.
Images containing debris, to exclude them from being counted. - Set the analysis definition. Red objects and green objects correspond with nuclei of all neutrophils and those that are forming NETs, respectively. Start with the following example of and press Preview Current or Preview All, and modulate each parameter to optimize results:
For Green: Segmentation- Background subtraction: Top-Hat; Radius: 100 µM; Threshold: 0.3 GCU; Edge split: On; Edge sensitivity: -20; Cleanup -Hole fill: 100 µm2; Adjust size 0 pixels; Filters- Area: min 20 µm2, max 500 µm2; Eccentricity: max 0.97; Mean Intensity: min 1.00
For Red: Segmentation- Background subtraction: Top-Hat; Radius: 10 µM; Threshold: 1.0 GCU; Edge split: ON; Edge sensitivity: -50; Cleanup-Hole fill: 50 µm2; Adjust size 0 pixels; Filters- Area: min 20 µm2, max 400 µm2; Mean Intensity: min 1.5.- If excessive green signal appears in neutrophils which should not be forming NETs (e.g., unstimulated neutrophils at 0 min that have lobulated nuclei), it may be due to overflow of the red signal detected in the green channel. In such case, return to step 3.1, open Image Layers on the left of the screen, and remove the red signals from the green channel by using Spectral Unmixing.
- Another option is to set one well for single staining with nuclear red dye to clarify how much of the red signal should be removed from green channel. On the other hand, usually green signal bleeding into the red channel does not affect the analyses.
NOTE: It does not matter if the sensitivity to red signal is low and not all red signals are captured in the images that are taken at later timepoints (e.g., 6 h timepoint). The maximum number of red object count in each image is regarded as total number of neutrophils in the image and will be used as denominator at step 3.9. Usually, nuclear red signals peak around 1 h timepoint and gradually diminish due to cell death (Figure 1B). Therefore, adjust the setting of red signal to correctly count red objects in the images with maximum red signals.
- Select scan times and wells to be analyzed. Usually, all time points and wells should be analyzed. Provide a label to the analysis definition.
- Check the summary and launch the analysis. It takes a few hours for completion of analysis (the duration depends on the number of timepoints and wells). Once launched, the name of the analysis definition will be shown under the name of the study in the View tab, and the complete date will be shown when it is done.
- After it is completed, open the analyzed study by double clicking the name of the analysis definition. Open Layers on the left of the screen and check if each cell is correctly marked. If it is not correctly marked, return to step 3.1 and repeat the subsequent steps.
- Click Graph Metrics on the left of the screen to export the data. Select Green Count, Red Count or Overlap Count (Per Image), and then choose the timepoints and wells to be exported. For select grouping, select Plate Map Replicates if all wells are correctly specified when scanning is done.
- Export the data. Calculate the percentage of NET-forming cells for each condition/timepoint by the following equation using an appropriate software.
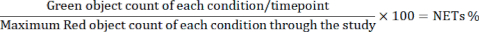
NOTE: The reason why the denominator is maximum red object count is that the number of Red object peaks at around 1 h timepoint and gradually decreases due to the cell death.
Results
This method provides phase contrast, red fluorescent (membrane-permeable dye) and green fluorescent (membrane-impermeable dye) images taken at each timepoint. Along with the NET-forming process, morphological changes are observed in phase contrast and red fluorescent images, and once the membrane is breached, green fluorescence can be observed (Figure 1). In this assay, NET-forming neutrophils are generally round, instead of forming web-like structure. This is because the resolution of the m...
Discussion
Current methods to quantify NETs ex vivo have several drawbacks that limit our ability to study neutrophils, NETs, and potential therapeutic targets in an unbiased and high-throughput way10,14. For example, direct counting of NET-forming cells after immunofluorescent staining, considered the gold standard for quantification of NETs, is low-throughput and dependent on operator's subjective view. A plate assay detecting the fluorescence of membrane-imp...
Disclosures
Authors have no competing financial interests.
Acknowledgements
We thank the Light Imaging Section in the Office of Science and Technology at the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (ZIA AR041199).
Materials
| Name | Company | Catalog Number | Comments |
| AKT inhibitor | Calbiochem | 124028 | |
| Clear 96-well plate | Corning | 3596 | |
| Live cell analysis system | Sartorius | N/A | Incucyte Software (v2019B) |
| Membrane-impermeable DNA green dye | Thermo Fisher Scientific | S7020 | |
| Nuclear red dye | Enzo | ENZ-52406 | Neutrophil pellet becomes bluish after staining. |
| RPMI | Thermo Fisher Scientific | 11835030 | Phenol red containig RPMI can be used. |
References
- Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303 (5663), 1532-1535 (2004).
- Wigerblad, G., Kaplan, M. J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat Rev Immunol. 23 (5), 274-288 (2022).
- Wagner, D. D., Heger, L. A. Thromboinflammation: From atherosclerosis to COVID-19. Arterioscler Thromb Vasc Biol. 42 (9), 1103-1112 (2022).
- Njeim, R., et al. NETosis contributes to the pathogenesis of diabetes and its complications. J Mol Endocrinol. 65 (4), R65-R76 (2020).
- De Meo, M. L., Spicer, J. D. The role of neutrophil extracellular traps in cancer progression and metastasis. Semin Immunol. 57, 101595 (2021).
- Nakabo, S., Romo-Tena, J., Kaplan, M. J. Neutrophils as drivers of immune dysregulation in autoimmune diseases with skin manifestations. J Invest Dermatol. 142 (3 Pt B), 823-833 (2022).
- Gupta, S., Chan, D. W., Zaal, K. J., Kaplan, M. J. A high-throughput real-time imaging technique to quantify NETosis and distinguish mechanisms of cell death in human neutrophils. J Immunol. 200 (2), 869-879 (2018).
- Nakabo, S., Kaplan, M. J., Gupta, S. Quantification of neutrophils undergoing NET formation and distinguishing mechanisms of neutrophil cell death by use of a high-throughput method. Methods Mol Biol. 2543, 129-140 (2022).
- Singh, J., et al. Moonlighting chromatin: when DNA escapes nuclear control. Cell Death Differ. 30 (4), 861-875 (2023).
- Carmona-Rivera, C., Kaplan, M. J. Induction and quantification of NETosis. Curr Protoc Immunol. 115, 14.41.11-14.41.14 (2016).
- Hsu, A. Y., Peng, Z., Luo, H., Loison, F. Isolation of human neutrophils from whole blood and buffy coats. J Vis Exp. (175), 62837 (2021).
- Neubert, E., et al. Serum and serum albumin inhibit in vitro formation of neutrophil extracellular traps (NETs). Front Immunol. 10, 12 (2019).
- von Kockritz-Blickwede, M., Chow, O. A., Nizet, V. Fetal calf serum contains heat-stable nucleases that degrade neutrophil extracellular traps. Blood. 114 (25), 5245-5246 (2009).
- Zhao, W., Fogg, D. K., Kaplan, M. J. A novel image-based quantitative method for the characterization of NETosis. J Immunol Methods. 423, 104-110 (2015).
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 18 (2), 134-147 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved