A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Performing an In Vitro Genome-Wide CRISPR Knockout Screen in Chimeric Antigen Receptor T Cells
In This Article
Summary
The article describes a protocol for the application of an in vitro model for exhaustion to complete a genome-wide CRISPR knockout screen in healthy donor chimeric antigen receptor T cells.
Abstract
Chimeric antigen receptor T (CART) cell therapy is an innovative form of targeted immunotherapy that has revolutionized the treatment of cancer. However, the durable response remains limited. Recent studies have shown that the epigenetic landscape of preinfusion CART cell products can influence response to therapy, and gene editing has been proposed as a solution. However, more work needs to be done to determine the optimal gene editing strategy. Genome-wide CRISPR screens have become popular tools to both investigate mechanisms of resistance and optimize gene editing strategies. Yet their application to primary cells presents many challenges. Here we describe a method to complete a genome-wide CRISPR knockout screen in CART cells from healthy donors. As a proof-of-concept model, we designed this method to investigate the development of exhaustion in CART cells targeting the CD19 antigen. However, we believe that this approach can be used to address a variety of mechanisms of resistance to therapy in different CAR constructs and tumor models.
Introduction
Chimeric antigen receptor T (CART) cell therapy has shown impressive success in the treatment of B-cell malignancies; however, the durable response is limited to 30-40%1,2,3,4,5. While researchers have developed and tested several approaches to address mechanisms of resistance to CART cell therapy, including the optimization of CAR design, gene editing, and combination therapies, the development of resistance remains largely unknown. Recently, there has been increasing evidence that the baseline gene expression profile of preinfusion CART cell products is an important determinant of both the toxicity and efficacy of CART therapy6,7,8,9. As such, gene editing during CART cell production has become a popular approach to improve CART cell therapy success.
For instance, our lab previously showed that using clustered regularly interspaced short palindromic repeats (CRISPR) Cas9 technology to knock out the gene granulocyte-macrophage colony-stimulating factor (GM-CSF) improves CART cell activity and reduces signs of CART-associated toxicity10,11,12. Additionally, CRISPR-engineered cell therapy has been tested in clinical trials and has shown efficacy and safety13. Together, this indicates that gene editing with CRISPR technology can not only enhance our understanding of CART cell biology but also generate translatable CART cell products.
Genome-wide CRISPR knockout screens have become increasingly popular tools in cancer biology research to understand mechanisms of resistance to therapeutics. In this technique, tens of thousands of guide RNAs (gRNAs) are delivered to pools of cells to encourage the entry of one gRNA per cell14. Then, cells undergo pressure-inducing conditions where cells transduced with gRNAs targeting essential genes die and cells transduced with gRNAs targeting inhibitory genes survive and proliferate. By using next-generation sequencing, we can determine how gRNA representation changes throughout the CRISPR screen14.
However, the scale and selection time required for genome-wide gene perturbations can be challenging to accomplish in primary cells, like CART cells. As such, groups have utilized targeted CRISPR screens to further understand the mechanisms of therapeutic resistance15,16. Targeted screens are often easier to complete in primary cells because they have smaller libraries that require fewer cells to achieve adequate library representation. While these studies have improved our understanding of mechanisms of resistance to CART cell therapy, targeted screens introduce a bias due to the manual selection of gene targets. This article seeks to describe a method to complete an in vitro genome-wide CRISPR knockout screen in CART cells from healthy donors. As such, this high-throughput approach allows for efficient, unbiased identification of key pathways and genes that can be edited to improve therapeutic responses17,18,19.
In particular, the protocol described in this article is designed to increase the field's understanding of CART cell exhaustion by completing the genome-wide CRISPR knockout screen with an in vitro model for exhaustion. Exhaustion is a dysfunctional CART cell fate that has been implicated in non-response to CART cell therapy20,21,22. This cellular fate is known to be epigenetically regulated, and it is characterized by a decrease in CART cell proliferation, a decrease in effector cytokine production, and an increase in the expression of inhibitory receptors23. In prior literature, gene editing has been able to prevent the development of exhaustion by either up- or downregulating key genes24,25,26. Given both the decrease in proliferative ability as CART cells become exhausted and the evidence that gene editing can prevent its occurrence, we modeled our in vitro genome-wide CRISPR knockout screen on this cellular fate. However, this protocol could be amended in the future to explore other mechanisms of resistance to CART cell therapy.
Protocol
Importantly, the protocol outlined below follows guidelines from and has received approval from the Mayo Clinic's Institutional Review Board (IRB 18-005745) and the Institutional Biosafety Committee (IBC HIP00000252.43). All cell culture work, including lentiviral production, should be carried out in a cell culture hood with appropriate personal protective equipment. In particular, lentiviral work should be conducted under biosafety level 2 (BSL-2) precautions, including the use of 10% bleach to disinfect items before disposal.
1. Amplification of the CRISPR Library
- Amplify the CRISPR library according to the manufacturer's recommendations. Briefly, electroporate the CRISPR library into four aliquots of electrocompetent cells by completing the following:
- Add 2 µL of 50 ng/µL CRISPR library plasmid to 25 µL of electrocompetent cells in a 1.0 mm cuvette.
- Electroporate the samples using the following settings: 10 µF, 600 Ohms, 1,800 volts, and a time constant between 3.5 ms and 4.5 ms.
- Recover in 975 µL of recovery media and transfer to a tube with an additional 1 mL of recovery media.
- Rotate all aliquots at 250 RPM for 1 h at 37 °C.
- Plate the transformations:
- Pool all aliquots of electroporated cells and mix well.
- Plate all electroporated cells by spreading 400 µL of the transformation mix onto prewarmed 10 cm Luria broth (LB) plates with 100 µg/mL carbenicillin. Place the plate in an incubator at 32 °C for 14 h.
- Harvest the colonies:
- Pipette 1 mL of LB media + 100 µg/mL carbenicillin onto each plate. Scrape the colonies off each plate and collect them into a 50 mL conical tube.
- Add another 1 mL of LB media + 100 µg/mL carbenicillin to each plate to wash and ensure complete recovery.
- Perform a maxi-prep by following a maxi-prep kit's instructions.
- Pool the resulting plasmid DNA.
2. Next-generation sequencing to verify baseline gRNA representation in the CRISPR library
NOTE: NGS primers for this CRISPR library have been designed in a previous publication27. Using a different reverse primer for each sample will barcode each sample and allow for the pooling of samples during sequencing.
- Prepare the samples for PCR with the following reaction mixture (total volume = 50 µL): high-fidelity PCR master mix, 2x: 25 µL; pooled template DNA, 0.5 µg: 1 µL; NGS library forward primer, 10 µM: 1.25 µL; NGS library reverse primer, 10 µM: 1.25 µL; DNase-free water: 21.5 µL.
- Perform PCR with the following cycling conditions: cycle 1: denature at 98 °C for 3 min; cycles 2-23: denature at 98 °C for 10 s, anneal at 63 °C for 10 s, extend at 72 °C for 25 s; cycle 24: extend at 72 °C for 2 min.
- Purify the PCR reaction by pooling the PCR reactions for each sample and purifying the PCR product with a PCR purification kit according to the manufacturer's instructions.
- Run 3 µg of the PCR product for each sample on a 2% (w/v) agarose gel.
- Remove the PCR product (~260-270 bp) from the gel by extracting the DNA from the gel by using a gel extraction kit according to the manufacturer's instructions. Aim for at least 500 ng of DNA.
- Store the extracted samples at -20 °C.
- Perform next-generation sequencing (NGS) with the samples and aim for 100 reads per gRNA27.
- Analyze the sequencing results with the MAGeCK-VISPR pipeline and ensure greater than 99.5% of the gRNAs in the library are represented28.
3. Production of CRISPR library and CAR expression lentiviruses
- Prepare lentivirus as previously described10,29.
- Day 1: Start with T175 flasks of 80-90% confluent 293T cells that have been growing in D10 media composed of 10% fetal bovine serum (FBS) (v/v), 1% penicillin-streptomycin-glutamine (PSG) (v/v), and Dulbecco's modified eagle's medium. Before starting, warm transfection reagents and DNA to room temperature and quantify the DNA.
- For each 293T flask, label two 50 mL conical tubes A and B. Add 4.5 mL of reduced serum medium to tube A and to tube B.
- In a sterile 1.5 mL microcentrifuge tube, mix the following DNA and allow to sit for 1 min: 18 µg of pCMVR8.74 (gag, pol, tat, and rev), 7 µg of pMD2.G (VSV-G), and 15 µg of lentiviral expression vector (CRISPR library A or CAR19-28ζ vectors).
- Add 129 µL of transfection reagent to tube A. Mix tube A well by finger vortexing.
- Add the DNA mixture to tube B. Mix tube B well by finger vortexing.
- Add 111 µL of neutral co-lipid reagent to tube B. Mix tube B well by finger vortexing.
- Add the contents of tube A to tube B and mix well by finger vortexing. Incubate tube B for 30 min at room temperature.
- Towards the end of the 30 min incubation period, prepare the 293T cells by aspirating the existing media. Add 16 mL of R10 media composed of 10% FBS (v/v), 1% PSG (v/v), and Roswell Park Memorial Institute (RPMI) 1640.
- After the 30 min incubation period, add the contents of tube B (approximately 9 mL) dropwise to the flask of 293T cells. Lay the flask down and gently rotate it before incubating it at 37 ˚C, 5% CO2.
- On Day 2 (approximately 24 h after transfection), collect lentivirus as follows:
NOTE: Prechill all equipment and materials and perform the following on ice to keep the viral supernatant cold.- Transfer the supernatant from the transfected 293T flasks to prechilled 50 mL conical tubes.
- Add 30 mL of prewarmed R10 media to the 293T flasks.
- Pellet cellular debris by centrifuging the 50 mL conical tubes at 900 × g for 10 min at 4 ˚C in closed rotors.
- After centrifuging, filter the supernatant with a 0.45 µm filter. Spin the filtrate in an ultracentrifuge at 112,700 × g at 4 ˚C for 2 h.
- After the 2 h spin, aspirate the supernatant until approximately 5 mL is remaining. Resuspend the viral particles by gently pipetting up and down 10x. Store the viral suspension at 4 ˚C overnight.
- On Day 3, collect and store lentivirus as follows:
- Transfer the supernatant from the transfected 293T flasks to prechilled 50 mL conical tubes.
- Add 30 mL of 10% bleach to the 293T flasks.
- Pellet the cellular debris in the supernatant by centrifuging the 50 mL conical tubes at 900 × g for 10 min at 4 ˚C in closed rotors.
- After centrifuging, filter the supernatant with a 0.45 µm filter. Spin the filtrate in an ultracentrifuge at 112,700 × g at 4 ˚C for 2 h.
- Following the 2 h centrifuge, aspirate the supernatant until approximately 2 mL is remaining, and resuspend by gently pipetting up and down 10x. Store the viral supernatant in 300-500 µL aliquots and keep at -80 ˚C.
4. Calculation of viral titer for CAR-expressing lentivirus
NOTE: CAR lentivirus was titered as previously described10,12.
- On Day 1, activate and plate T-cells .
- Isolate 1 × 106 T-cells from healthy donor PBMCs using a negative selection kit.
- Count the T-cells.
- Wash enough CD3+/CD28+ beads to stimulate the T-cells at a 3:1 bead:cell ratio. For example, to stimulate 1 × 106 T-cells, wash 3 × 106 beads. To do so, wash the beads by completing the following 3x:
- Use magnetic separation to remove the beads from suspension.
- Remove the supernatant.
- Add 1000 µL of T-cell media (TCM) composed of 10% human serum (v/v), 1% PSG (v/v), and hematopoietic cell medium.
NOTE: TCM should be sterilized prior to use by filtering with a 0.45 µm sterile vacuum filter and then with a 0.22 µm sterile vacuum filter. - Add beads to the T-cell suspension at a 3:1 bead:cell ratio.
- Resuspend the T-cells to 1 × 106 cells/mL in TCM.
- Add 100 µL of activated T-cells to 10 wells of a 96-well plate. Incubate the plate at 37 ˚C, 5% CO2.
- On Day 2, add a dilution of CAR lentivirus by creating a dilution plate as follows:
- Add 100 µL of TCM to seven wells of a 96-well plate.
- To the first well, add 50 µL of the virus of interest; mix well.
- Serially dilute the virus further by adding 50 µL from well 1 to well 2; mix well.
- Add 50 µL from well 2 to well 3 and continue this pattern until well 7.
- After mixing well 7, remove 50 µL. Ensure that all wells have a total volume of 100 µL.
- Add 50µL from each well of the dilution plate to the first seven wells of the T-cell plate from Day 1. Mix well.
- To the last three wells of the T-cell plate, add 50 µL of TCM; mix well. Store the plate in an incubator at 37 ˚C, 5% CO2.
- On Day 3, feed the cells by adding 100 µL of TCM to all wells of the T-cell plate; mix well.
- On Day 4, perform flow cytometry to detect the percentage of cells expressing CAR with an anti-kappa chain antibody, a surrogate marker for CAR expression, by completing the following:
- Spin down the T-cell plate at 1,000 × g for 3 min and decant.
- Wash the plate 3x with anti-kappa chain antibody buffer (composed of 4% bovine serum albumin in PBS (w/v)) by adding 200 µL of anti-kappa chain antibody buffer, spinning down the plate at 1,000 × g for 3 min, and decanting.
- Add a biotin anti-kappa chain antibody to each well. Incubate for 45 min on a rocker at 4 ˚C.
- Wash the plate 3x with anti-kappa chain antibody buffer as in step 4.4.2.
- Stain the cells for flow cytometry with a fluorochrome-conjugated streptavidin antibody and a live/dead antibody. Incubate the samples for 15 min at room temperature in the dark.
- Wash the samples once in flow buffer (composed of 2% FBS (v/v) and 1% sodium azide (v/v) in PBS) by adding 200 µL of flow buffer, spinning down the plate at 1,000 × g for 3 min, and decanting.
- Resuspend in 100 µL of flow buffer and run on the cytometer.
- Perform negative gating with the three untransduced wells.
5. Calculation of viral titer for CRISPR library lentivirus
NOTE: This protocol describes a minimum number of T-cells for titrating the CRISPR library lentivirus, but it can be scaled up to accommodate more T-cells and thus higher volumes of virus.
- On Day 0, activate the T-cells by adding 18 × 106 CD3+/CD28+ beads (3:1 bead:cell ratio) to 6 × 106 T-cells and bringing the final volume to 6 mL with TCM. Aliquot 0.5 mL of the T-cell suspension into 12 wells of a 48-well plate. Store in an incubator at 37 ˚C, 5% CO2.
- Twenty-four hours later, on Day 1, add CAR virus at a multiplicity of infection (MOI) of 3 to every well. Mix well by pipetting up and down several times. Store in an incubator at 37 ˚C, 5% CO2.
- Forty-eight hours later, on Day 2, add a series of CRISPR library virus volumes to the activated CART cells by adding one of the following volumes of CRISPR virus to each well (0 µL, 0 µL, 6.25 µL, 6.25 µL, 12.5 µL, 12.5 µL, 25 µL, 25 µL, 50 µL, 50 µL, 100 µL, or 100 µL). Mix well by pipetting up and down several times. Store in an incubator at 37 ˚C, 5% CO2.
- On Day 3, add 1 µg of puromycin per mL of media to half of the wells, including one well that received each of the following CRISPR virus volumes (0 µL, 6.25 µL, 12.5 µL, 25 µL, 50 µL, and 100 µL). The other wells will not be treated with puromycin.
- From Days 4 - 8, count the T-cells daily and adjust the final cell density to 1 × 106 cells/mL with 1 µg/mL puromycin in TCM for the puromycin-treatment wells and with TCM for the untreated wells.
- On Day 8, count the number of live cells per well and calculate the transduction units/mL of viral supernatant by using the following equation:
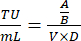
Where A: Live cell count in virally transduced well; B: Live cell count in no-antibiotic-selection control well; V: Virus volume used for infection in each well; D: Dilution Fold. - Using the TU/mL well calculated from a well showing approximately 20% transduction efficiency, calculate the volume of virus needed to transduce 1 × 106 cells at an MOI of 0.3 by using the following formula:
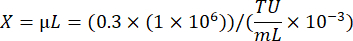
6. CART cell production phase of CRISPR screen (Days 0 - 8)
NOTE: The protocol that follows describes the completion of the CRISPR screen in one biological replicate. However, this protocol has previously been validated with three biological replicates and we recommend the use of at least three biological replicates for others completing this protocol30.
- Day 0: Isolate and stimulate T-cells from healthy donor blood as previously described10.
- Isolate peripheral blood mononuclear cells (PBMCs) from de-identified healthy donor blood.
- Add 15 mL of a density gradient medium to a density gradient separation tube.
- Add 1:2 diluted blood to the top of the density gradient separation tube. Centrifuge at 1,200 × g for 10 min at room temperature.
NOTE: Blood should be diluted with isolation buffer (2% FBS (v/v) in phosphate-buffered saline [PBS]). - Poor off the top layer into a new 50 mL conical tube and double the volume with isolation buffer. Centrifuge the sample at 300 × g for 8 min at 4 °C. Aspirate the supernatant.
- Resuspend the cell pellet with isolation buffer and count the PBMCs.
- Isolate T-cells from PBMCs using a negative selection kit. Follow the manufacturer's instructions for the kit.
NOTE: Assume a 10-20% recovery of T-cells from PBMCs. - Stimulate T-cells with CD3+/CD28+ beads.
- Count the T-cells.
- Aliquot enough CD3+/CD28+ beads to stimulate the T-cells at a 3:1 bead:cell ratio. For example, to stimulate 110 × 106 T-cells, aliquot 330 × 106 beads. Wash the beads 3x by first separating the beads out of suspension with magnetic separation and then adding 1,000 µL of TCM.
- Add the beads to the T-cell suspension. Resuspend the T-cells to 1 × 106 cells/mL in TCM.
- Aliquot the T-cells in 6-well plates for best transduction efficiency. Add 5 × 106 T-cells to each well. Incubate the cells at 37 °C, 5% CO2 for 24 h.
- Isolate peripheral blood mononuclear cells (PBMCs) from de-identified healthy donor blood.
- Day 1: Under BSL-2+ conditions, thaw enough CAR lentivirus to transduce T-cells at a multiplicity of infection of 3.0. Gently break up the T-cell rosettes before adding the appropriate amount of pooled lentivirus to transduce 5 × 106 T-cells at an MOI of 3.0. Incubate the cells at 37 ˚C, 5% CO2.
NOTE: Pool lentivirus from multiple productions into one tube before transducing T-cells. This ensures that all T-cells receive the same treatment. Additionally, lentivirus should only be thawed once. - Day 2: Under BSL-2+ conditions, thaw enough CRISPR lentivirus to transduce T-cells at an MOI of 0.3. Gently break up the T-cell rosettes and resuspend the T-cells before adding the appropriate amount of pooled lentivirus to transduce 5 × 106 T-cells at an MOI of 0.3. Incubate the cells at 37 ˚C, 5% CO2.
NOTE: Pool lentivirus from multiple productions into one tube before transducing T-cells. This ensures that all T-cells receive the same treatment. - Days 3 - 5: Resuspend the CART cells to break up rosettes and count the cells. Bring the final cell concentration to 1 × 106 cells/mL in TCM with 1 µg/mL puromycin. Incubate the cells at 37 ˚C, 5% CO2.
- Day 6: Resuspend the CART cells to break up rosettes, remove the CD3+/CD28+ beads through magnetic separation, and count the CART cells. Add TCM with 1 µg/mL puromycin to bring the final cell concentration to 1 × 106 cells/mL and incubate the cells at 37 ˚C, 5% CO2. Determine CAR expression with flow cytometry as described in step 4.4.
7. gRNA selection phase of CRISPR screen (Days 8 - 22)
- Day 8: Preserve samples for next-generation sequencing (NGS) and start the co-culture.
- Wash the CART cells 3x in TCM by centrifuging the samples at 300 × g for 5 min at 4 ˚C, aspirating the supernatant, and resuspending the samples in 20 mL of TCM.
- Count the CART cells.
- Preserve samples for NGS by completing the following:
- Aliquot enough CART cells in a 50 mL conical tube to maintain a gRNA representation of 500 cells per gRNA (e.g., aliquot approximately 33 × 106 CART cells if using a CRISPR library with 65,383 gRNAs). Centrifuge the sample at 300 × g for 5 min at 4 ˚C.
- Resuspend the cell pellet in 1 mL of PBS and transfer to a 2 mL microcentrifuge tube. Wash the 50 mL conical tube with 1 mL of PBS and transfer the wash to the 2 mL microcentrifuge tube.
- Centrifuge the samples at 500 × g for 5 min at 4 ˚C. Remove the supernatant. Store the cell pellets at -20 ˚C.
- Start the co-culture by adding CART cells to CD19+ JeKo-1 cells at a 1:1 ratio. For example, add 80 × 106 CART cells to 80 × 106 JeKo-1 cells. Bring the total volume of the co-culture to 160 mL with TCM. Store the samples in an incubator at 37 ˚C, 5% CO2.
NOTE: We recommend starting with at least 80 × 106 CART cells to maintain library representation and to have enough cells to preserve the samples at Day 15 and Day 22.
- Day 10: To restimulate the CART cells, collect the co-cultures into 50 mL conical tubes and centrifuge the samples at 300 × g for 5 min at 4 ˚C. Resuspend each sample in 80 mL of TCM, and add 80 × 106 JeKo-1 cells in 80 mL of TCM. Store the samples in an incubator at 37 ˚C, 5% CO2.
- Days 12 and 14: Restimulate CART cells as in step 7.2.
- Day 15: Preserve the samples for NGS and restimulate the CART cells.
- Isolate CART cells from co-culture by first counting the cells and then combining a positive selection kit for CD4+ T-cells with a positive selection kit for CD8+ T-cells to isolate T-cells from the CART-JeKo-1 co-cultures. Count the T-cells.
- Preserve the samples for NGS by aliquoting 33 × 106 CART cells in a 50 mL conical tube and centrifuging the sample at 300 × g for 5 min at 4 ˚C. Resuspend the sample in 1 mL of PBS and transfer it to a 2 mL microcentrifuge tube. Wash the 50 mL conical tube with 1 mL of PBS and transfer the wash to the 2 mL microcentrifuge tube. Centrifuge the samples at 500 × g for 5 min at 4 ˚C; remove the supernatant; and store the cell pellets at -20 ˚C.
- Set up co-cultures to continue the in vitro model for exhaustion by adding 80 × 106 CART cells to 80 × 106 JeKo-1 cells. Then, bring the total volume of the co-culture to 160 mL with TCM. Store the samples in an incubator at 37 ˚C, 5% CO2.
- Days 17, 19, and 21: Restimulate CART cells as in step 7.2.
- Day 22: Preserve the samples for NGS.
- Isolate CART cells from the co-culture by first counting the cells. Then, combine a positive selection kit for CD4+ T-cells with a positive selection kit for CD8+ T-cells to isolate T-cells from the CART-JeKo-1 co-cultures. Count the T-cells.
- To preserve samples for NGS, repeat step 7.4.2.
8. Preparation of genomic DNA for next-generation sequencing
NOTE: To maintain adequate coverage, genomic DNA (gDNA) should be isolated from at least 33 × 106 CART cells. Additionally, the entirety of the gDNA from 33 × 106 CART cells should be prepared for sequencing.
- Isolate gDNA from frozen cell pellets by using a gDNA isolation kit.
- Purify the gDNA through ethanol precipitation.
- Prechill 100% EtOH to -20 °C.
- Aliquot eluted DNA equally into several microcentrifuge tubes (200-250 µL). Add the following to each aliquot: two volumes of 100% EtOH (500 µL for 250 µL aliquot or 400 µL for 200 µL); 0.1 volume of 3 M sodium acetate (25 µL for 250 µL aliquot or 20 µL for 200 µL); and 1 µL of 20 mg/mL glycogen. Incubate the samples overnight at -20 °C.
- On the second day, spin the samples at 13,000 × g for 20 min to pellet the precipitated DNA. Remove the supernatant. Wash the pellets with 1 mL of 70% EtOH by spinning at 7,500 × g for 10 min.
- Remove residual EtOH completely. Allow the pellets to dry for 30-45 min.
- Resuspend the pellets in 50 µL of sterile water.
- Prepare gDNA for NGS.
NOTE: NGS primers for this CRISPR library that amplify gRNAs with Illumina adapter sequences have been designed in a previous publication27. Using a different reverse primer for each sample will barcode each sample and allow for the pooling of samples during sequencing.- Prepare the samples for PCR with the following reaction mixture (total volume = 50 µL): high-fidelity PCR master mix, 2x: 25 µL; pooled template DNA, 0.5 µg: 1 µL; NGS library forward primer, 10 µM: 1.25 µL; NGS library reverse primer, 10 µM: 1.25 µL; DNase-free water: 21.5 µL.
- Perform PCR with the following cycling conditions: cycle 1: denature at 98 °C for 3 min; cycles 2-23: Denature 98 °C for 10 s, anneal at 63 °C for 10 s, extend at 72 °C for 25 s; cycle 24: extend at 72 °C for 2 min.
- Pool the PCR reactions for each sample and purify the PCR product with a PCR purification kit according to the manufacturer's instructions.
- Run 3 µg of the PCR product for each sample on a 2% (w/v) agarose gel.
- Remove the PCR product (~260-270 bp) from the gel by extracting the DNA from the gel by using a gel extraction kit according to the manufacturer's instructions. Store the extracted samples at -20 °C.
- Perform next-generation sequencing (NGS) with the samples. Aim for more than 500 reads per gRNA in the library.
9. Analysis of sequencing results
- To analyze NGS, use the MAGeCK-VISPR platform28. Use the standard settings for the maximum likelihood estimation (MAGeCK-MLE) algorithm. To prepare the output files for analysis:
- Run FASTQC to evaluate sequence file quality31.
- Merge paired-end sequencing files for each sample with bbmerge32.
- Analyze the samples in the MAGeCK-VISPR platform with the MAGeCK-MLE algorithm. For the MAGeCK-MLE analysis, normalize all gRNA counts to the list of 1,000 non-targeting gRNAs.
- Assess sequencing file quality by evaluating GC content, base quality, sequencing reads, and mapped reads.
- Assess the success of the selective pressure phase of the CRISPR screen by evaluating changes in the Gini index, principal component analysis (PCA) clustering, and pathways associated with the list of negatively selected genes32.
- Interrogate the top hits by conducting literature searches and performing pathway analysis.
Results
To interrogate genes and pathways that can be edited to improve CART cell activity in an unbiased manner, we designed an in vitro genome-wide CRISPR knockout screen (Figure 1). This screen has two phases: a CART cell production phase and a selective pressure phase. In the CART cell production phase, at least 110 × 106 T-cells are first isolated from healthy donor PBMCs and activated with CD3+/CD28+ beads. The following day, on Day 1
Discussion
Gene editing has become a powerful tool in both understanding the mechanisms of resistance to therapies as well as designing novel CART cell therapies to improve the longevity and activity of CART cells16,17,26. While some gene editing strategies have shown improvements in CART cell activity in both preclinical models and clinical trials, there is still work to be done to optimize gene editing strategies. To address this need, r...
Disclosures
SSK is an inventor on patents in the field of CAR immunotherapy that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis), Humanigen (through Mayo Clinic), Mettaforge (through Mayo Clinic), and MustangBio (through Mayo Clinic), and Chymal therapeutics (through Mayo Clinic). CS, CMR, and SSK are inventors on patents that are licensed to Immix Biopharma. SSK receives research funding from Kite, Gilead, Juno, BMS, Novartis, Humanigen, MorphoSys, Tolero, Sunesis/Viracta, LifEngine Animal Health Laboratories Inc., and Lentigen. SSK has participated in advisory meetings with Kite/Gilead, Calibr, Luminary Therapeutics, Humanigen, Juno/BMS, Capstan Bio, and Novartis. SSK has served on the data safety and monitoring board with Humanigen and Carisma. SSK has severed a consultant for Torque, Calibr, Novartis, Capstan Bio, BMS, Carisma, and Humanigen. CMS and SSK are inventors of intellectual property that resulted from this protocol.
Acknowledgements
This study was partly funded by the Mayo Clinic Center for Individualized Medicine (SSK), Mayo Clinic Comprehensive Cancer Center (SSK), Mayo Clinic Center for Regenerative Biotherapeutics (SSK), National Institutes of Health K12CA090628 (SSK) and R37CA266344-01 (SSK), Department of Defense grant CA201127 (SSK), Predolin Foundation (SSK), and Minnesota Partnership for Biotechnology and Medical Genomics (SSK). CMS is supported by the Mayo Clinic Graduate School of Biomedical Sciences. CRISPR screen schematic (Figure 1) was created with BioRender.com (Siegler, L. (2022) https://BioRender.com/k71r054).
Materials
| Name | Company | Catalog Number | Comments |
| 293T cells | ATCC | CRL-3216 | Cells used for lentivirus production |
| Biotin ProteinL Antibody | GenScript | M00097 | anti-kappa chain antibody for CAR detection |
| Bovine Serum Albumin | Millipore Sigma | A7906 | |
| Carbenicillin disodium salt | Millipore Sigma | C1389-1G | Carbenicillin antibiotic |
| CD4 Isolation Beads | Miltenyi Biotec | 130-045-101 | |
| CD8 Isolation Beads | Miltenyi Biotec | 130-045-201 | |
| CTS (Cell Therapy Systems) Dynabeads CD3/CD28 | Gibco | 40203D | |
| Cytoflex | Beckman Coulter | NC2279958 | |
| DNase-Free Water | Invitrogen | AM9937 | |
| Dulbecco's modified eagle's medium (DMEM) | Corning | 10-017-CV | |
| Dulbecco's Phosphate-Buffered Saline | Gibco | 14190-144 | |
| EasySep Human T Cell Isolation Kit | STEMCELL Technologies | 17951RF | Negative isolation kit |
| Endura Electrocompetent Cells | Biosearch Technologies | 60242-1 | Electrocompetent cells with recovery medium |
| Ethanol | Millipore Sigma | E7023 | |
| Fetal bovine serum (FBS) | Corning | 35-010-CV | |
| GeCKO v2 CRISPR Knockout Pooled Library A | AddGene | 1000000048 | CRISPR library plasmid |
| Gene Pulser II | Bio-Rad | 165-2105 | Electroporator |
| Glycogen | Millipore Sigma | 10901393001 | |
| JeKo-1 | ATCC | CRL-3006 | CD19+ target cells |
| Lipofectamine 3000 Transfection Reagent | ThermoFisher Scientific | L3000075 | Transfection reagent kit with a transfection reagent (Lipofectamine 3000 Reagent) and a neutral co-lipid reagent (p3000) |
| LIVE/DEAD Aqua | Invitrogen | L34966 | |
| Lymphoprep | STEMCELL Technologies | 7851 | Density gradient medium |
| Machery-Nagel NucleoBond Xtra Maxi Kits | ThermoFisher Scientific | 12748412 | Maxi-prep kit |
| NEBNext High-Fidelity 2X PCR MasterMix | New England BioLabs | M0541S | High fidelity PCR mastermix |
| Opti-MEM I Reduced Serum Medium | Gibco | 31985-070 | Reduced serum medium |
| pCMVR8.74 | AddGene | 22036 | Lentiviral packaging plasmid |
| Pennicillin-streptomycin-glutamine (100X) | Life Technologies | 10378-016 | |
| pMD2.G | AddGene | 12259 | VSV-G envelope expressing plasmid |
| Pooled Human AB Serum | Innovative Research | ISERABHI | |
| Puromycin | Millipore Sigma | P8833 | |
| QIAquick Gel Extraction Kit | Qiagen | 28704 | Gek extraction kit |
| Qucik-DNA Midiprep Plus Kit | Zymo Research | D4075 | Kit used to isolate gDNA |
| RoboSep-S | STEMCELL Technologies | 21000 | Automated cell separator |
| Roswell Park Memorial Institute 1640 Medium (RPMI) | Gibco | 21870092 | |
| SepMate-50 | STEMCELL Technologies | 85450 | Density gradient separation tube |
| Sodium Acetate | Invitrogen | AM9740 | |
| Sodium Azide | Fisher Scientific | 71448-16 | |
| Streptavidin Antibody (PE) | BioLegend | 405203 | Secondary antibody used for CAR detection |
| T100 Thermal Cycler | Bio-Rad | 1861096 | |
| Ultracentrifuge (Optima XPN-80) | BeckmanCoulter | A99839 | |
| Vacuum Filter Systems, 0.22 µm | ThermoFisher Scientific | 567-0020 | |
| Vacuum Filter Systems, 0.45 µm | ThermoFisher Scientific | 165-0045 | |
| X-VIVO 15 Serum-Free Hematopoietic Cell Medium | Lonza | 04-418Q | Hematopoietic cell medium |
References
- Neelapu, S. S., et al. 5-year follow-up supports curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma (zuma-1). Blood. 141 (19), 2307-2315 (2023).
- Neelapu, S. S., et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 377 (26), 2531-2544 (2017).
- Locke, F. L., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (zuma-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 20 (1), 31-42 (2019).
- Maude, S. L., et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 378 (5), 439-448 (2018).
- Schuster, S. J., et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 380 (1), 45-56 (2018).
- Bai, Z., et al. Single-cell antigen-specific landscape of CAR T infusion product identifies determinants of CD19-positive relapse in patients with all. Sci Adv. 8 (23), eabj2820 (2022).
- Fraietta, J. A., et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 24, 563-571 (2018).
- Gennert, D. G., et al. Dynamic chromatin regulatory landscape of human CAR T cell exhaustion. Proc Natl Acad Sci USA. 118 (30), e2104758118 (2021).
- Deng, Q., et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med. 26 (12), 1878-1887 (2020).
- Sterner, R. M., Cox, M. J., Sakemura, R., Kenderian, S. S. Using CRISPR/CAS9 to knock out GM-CSF in CAR-T cells. J Vis Exp. (149), e59629 (2019).
- Sterner, R. M., et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 133 (7), 697-709 (2019).
- Cox, M. J., et al. GM-CSF disruption in CART cells modulates T cell activation and enhances CART cell anti-tumor activity. Leukemia. 36 (6), 1635-1645 (2022).
- Ottaviano, G., et al. Phase 1 clinical trial of CRISPR-engineered CAR19 universal T cells for treatment of children with refractory B cell leukemia. Sci Transl Med. 14 (668), eabq3010 (2022).
- Joung, J., et al. Genome-scale CRISPR-CAS9 knockout and transcriptional activation screening. Nat Protoc. 12, 828-863 (2017).
- Mccutcheon, S. R., et al. Transcriptional and epigenetic regulators of human CD8+ T cell function identified through orthogonal CRISPR screens. Nat Genet. 55, 2211-2223 (2023).
- Zhu, W., Kelly, C., Dagur, P., Dunbar, C. E., Cordes, S. CRISPR activation screen to optimize chimeric antigen receptor (CAR) T cell immunophenotype. Blood. 142 (Supplement 1), 4820-4820 (2023).
- Wang, D., et al. CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 11 (5), 1192-1211 (2021).
- Shifrut, E., et al. Genome-wide CRISPR screens in primary human T cells reveal key regulators of immune function. Cell. 175 (7), 1958-1971.e15 (2018).
- Belk, J. A., et al. Genome-wide crispr screens of T cell exhaustion identify chromatin remodeling factors that limit T cell persistence. Cancer Cell. 40 (7), 768-786.e7 (2022).
- Jiang, P., et al. Single-cell ATAC-seq maps the comprehensive and dynamic chromatin accessibility landscape of CAR-T cell dysfunction. Leukemia. 36 (11), 2656-2668 (2022).
- Rossi, J., et al. Preinfusion polyfunctional anti-CD19 chimeric antigen receptor T cells are associated with clinical outcomes in NHL. Blood. 132 (8), 804-814 (2018).
- Beider, K., et al. Molecular and functional signatures associated with CAR T cell exhaustion and impaired clinical response in patients with B cell malignancies. Cells. 11 (7), 1140 (2022).
- Wherry, E. J. T. cell exhaustion. Nat Immunol. 12, 492-499 (2011).
- Prinzing, B., et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci Transl Med. 13, eabh0272 (2021).
- Lynn, R. C., et al. C-jun overexpression in CAR T cells induces exhaustion resistance. Nature. 576, 293-300 (2019).
- Tang, N., et al. TGF-beta inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight. 5 (4), e133977 (2020).
- Joung, J., et al. Genome-scale CRISPR-CAS9 knockout and transcriptional activation screening. Nat Protoc. 12 (4), 828-863 (2017).
- Li, W., et al. Quality control, modeling, and visualization of CRISPR screens with MAGeCK-VISPR. Genome Biol. 16, 281 (2015).
- Sakemura, R., et al. Targeting cancer-associated fibroblasts in the bone marrow prevents resistance to CART-cell therapy in multiple myeloma. Blood. 139 (26), 3708-3721 (2022).
- Stewart, C. M., et al. IL-4 drives exhaustion of CD8+ CART cells. Nat Commun. 15 (1), 7921 (2024).
- . FastQC: A quality control tool for high throughput sequence data Available from: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010)
- . Bbmerge Available from: https://github.com/BioInfoTools/BBMap/blob/master/sh/bbmerge.sh (2019)
- Pattanayak, V., et al. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed CAS9 nuclease specificity. Nat Biotechnol. 31 (9), 839-843 (2013).
- Doench, J. G., et al. Rational design of highly active sgRNAs for CRISPR-CAS9-mediated gene inactivation. Nat Biotechnol. 32 (12), 1262-1267 (2014).
- Dong, M. B., et al. Systematic immunotherapy target discovery using genome-scale in vivo CRISPR screens in CD8 T cells. Cell. 178 (5), 1189-1204.e23 (2019).
- Zhou, P., et al. Single-cell CRISPR screens in vivo map T cell fate regulomes in cancer. Nature. 624 (7990), 154-163 (2023).
- Sutra Del Galy, A., et al. In vivo genome-wide CRISPR screens identify SOCS1 as intrinsic checkpoint of CD4+ TH1 cell response. Sci Immunol. 6 (66), eabe8219 (2021).
- Ramos, A., et al. Leukemia-intrinsic determinants of CAR-T response revealed by iterative in vivo genome-wide CRISPR screening. Nat Commun. 14 (1), 8048 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved