A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Optimizing Photoneuromodulation Techniques to Evaluate the Role of Green Light-Emitting Diodes in Pain Management
* These authors contributed equally
In This Article
Summary
Recent developments in pain research highlight the potential of photoneuromodulation using green light-emitting diodes (GLED) as a non-pharmacological treatment. GLED modulates pain pathways, offering effective pain relief. This article aims to standardize and refine GLED exposure protocols, improving consistency across studies and advancing the clinical application of this therapy.
Abstract
Despite extensive research and the identification of numerous analgesic targets, the range of pharmacological treatments available for pain remains limited. However, a potential paradigm shift could introduce a new wave of non-pharmacological pain treatments with remarkable safety, efficacy, and tolerability. One promising area of investigation is photoneuromodulation using green light-emitting diodes (GLED, 525 nm), which have shown potential in alleviating pain in both acute and chronic conditions, leading to numerous preclinical and clinical studies exploring the efficacy of this therapy. These research projects have demonstrated how exposure to GLED enhances the activity of the endogenous opioid system in the brain and spinal cord after M-cone activation in the retina. The findings suggest that GLED may alleviate pain by modulating the descending pain pathway. In light of the compelling effects of GLED, the proliferation of photoneuromodulation investigations underscores the importance of establishing consistency in well-defined and standardized exposure protocols for preclinical and clinical trials. In preclinical studies, beneficial effects have been observed following a minimum of 2 days of exposure, with protocols involving 8 h of light at 100 lux during the 12 h light phase. In clinical trials, exposure protocols are tailored to the specific pathology under investigation. Exposure for 15 min has proven favorable in the modulation of acute post-surgical pain. For modulation of chronic pain, patients are instructed to use GLED at home for 1 to 2 h a day over 10 weeks. This article details preclinical and clinical protocols to improve reproducibility and consistency in the different studies evaluating photoneuromodulation benefits. By establishing these standardized protocols, this work aims to advance the clinical translation of GLED phototherapy as a viable non-pharmacological treatment for pain.
Introduction
Pharmacological treatments, particularly opioids, continue to be heavily relied upon for managing both acute and chronic pain conditions1. The effectiveness of pain management can be significantly affected by the frequency and severity of side effects associated with opioid use2. For this reason, a substantial amount of patients under opioid treatment do not achieve successful pain management3. Hence, pain physicians and the patient community are increasingly seeking non-pharmacological treatments that avoid the side effects associated with traditional pain medications. Photoneuromodulation has emerged as a promising solution and a safe therapy for managing pain.
Photoneuromodulation (PNM) is a non-invasive technique that uses light-emitting diodes (LED) to regulate biological processes4. Phototherapy was established thousands of years ago using sunlight, or heliotherapy, to treat skin conditions5. Subsequently, the concept of light influencing biological tissues has broadened, leading to the development of the photoneuromodulation term. PNM research is now expanding worldwide and has shown its effectiveness in a variety of clinical applications, including pain management6,7,8,9, improving sleep quality in patients with Alzheimer's disease10, and controlling depression11.
There is a growing emphasis on preclinical research and clinical trials aimed at investigating the mechanisms and therapeutic potential of photoneuromodulation for pain management. Among these approaches, green light-emitting diode therapy (GLED), using a 525 nm wavelength stimulation, has shown promising efficacy in reducing various types of pain, including migraines, fibromyalgia, and post-surgical pain12,13,14,15,16. Clinical trials have demonstrated that green light therapy consistently benefits patients suffering from migraine across multiple studies12,17,18, by reducing both headache pain and photophobia intensity during active migraine attacks19, as well as decreasing the frequency and duration of migraine episodes12. Preclinical studies also demonstrated that exposure to GLED can reverse thermal and mechanical hypersensitivity in a nerve injury model of neuropathic pain20. Further, preclinical studies have explored the mechanisms through which GLED influences pain perception and sensory thresholds13,21,22,23,24. These studies highlight the involvement of M-cones and the subsequent modulation of the ventral lateral geniculate nucleus (vLGN), which increases the activity of enkephalinergic neurons projecting to the dorsal raphe nucleus (DRN)22. Additional research has also emphasized the critical role of the rostral ventromedial medulla (RVM)21, a key regulator of descending pain modulation. Collectively, these findings suggest that GLED alters pain perception by modulating visual circuits that act on the descending pain pathways20,25. However, further research is required to facilitate its translation into clinical use.
In this article, we detail a comprehensive methodology for implementing GLED-based PNM, aiming to provide a reproducible framework for both experimental and clinical use. We describe the design and operation of GLED exposure, outline standardized application protocols, and discuss key considerations for ensuring efficacy and reproducibility. Additionally, we provide a detailed protocol for assessing the activity of both ascending and descending pain pathways, enabling a deeper investigation into their roles in modulating GLED-induced analgesia. By sharing this approach, we aim to advance research in non-pharmacological pain management and contribute to developing accessible, effective, and safer therapies.
Protocol
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Arizona and conform to the guidelines for using laboratory animals of the National Institutes of Health. Pathogen-free, adult Sprague Dawley rats (weight at testing: 275-330 g) were housed in standard vivarium rat cages (3 rats per cage) in climate-controlled rooms on a 12-hour light/dark cycle and were allowed ad lib access to food and water. All behavioral experiments were conducted by experimenters blinded to the treatment conditions. All human procedures received approval from the University of Arizona Institutional Review Board (IRB) under protocol number (STUDY00000370). This study is registered with ClinicalTrials.gov under NCT05295225.
1. Light exposure protocol in animals
- Optimizing light exposure and preparing animal housing
- Obtain visible spectrum light-emitting diodes (LED) flex strips with the following characteristics: (i) Green LEDs (Table of Materials), wavelength of 525 nm, power of 8 W, voltage of 120 V, and a 120° beam angle. (ii) White LEDs (Table of Materials), power of 9.6 W, voltage of 120 V, and a 120° beam angle.
- Ensure the accuracy of the light spectrum with a spectrometer (for this study, we used the Biomedical Device Prototyping Service from the BIO5 Institute at the University of Arizona).
NOTE: Green LED should have a Center Wavelength of 525 ± 10 nm (the brightest point of emission), a Full-Width Half Maximum (FWHM) that spans less than 40 nm, and a Record Intensity Drop (where the light intensity drops to 50% of the maximum) which should be at 500 ± 5 nm and 530 ± 5 nm21. White LED FWHM should span more than 90 nm. Multiple sources of white light can be used. Experimenters must ensure the low intensity of 525 nm wavelength and a large span of the spectrum, covering multiple wavelengths21. - Use a lux meter (Table of Materials) to measure and optimize light intensity. Cover LEDs with black tape (Table of Materials) as needed to obtain the desired intensity.
NOTE: It is crucial to measure and validate light intensity, as different intensities can affect sensory thresholds in varying ways (Figure 1). If the intensity is not accurate, cover or uncover some of the LEDs until the desired intensity is reached. - Secure the LED strips to the upper edges of wire shelves in a dark room (Table of Materials) to ensure each shelf is equipped with a dedicated light exposure source. Place timers (Table of Materials) on the LED strips to expose the cages for 8 h per day from 6 am to 2 pm.
- Install transparent static cages on the shelves. Fully enclose the shelves with dark sheets on all sides (Table of Materials) to optimize exposure while minimizing light interference.
- Use a lux meter to obtain the final optimization of light intensity inside the cage by covering or uncovering some of the LEDs (100 lux at the center of each cage) (Supplementary Figure 1).
- Obtention of baseline sensory behavior and start of exposure
- Upon arrival, allow the rats to acclimate to the animal facility for 1 week. After this acclimation period, habituate the rats to the experimenter's handling for at least 7 days.
- Categorize the animals into three experimental groups based on their treatment conditions: (1) green light exposure (GLED) combined with surgical intervention, (2) white light exposure (WLED) combined with surgical intervention, and (3) WLED control group with sham surgery.
- Acclimate the rats in clear plexiglass boxes on a wire mesh (Table of Materials) for 1 h prior to testing, in the same room as the testing area, with the experimenter preferably present.
- To assess preoperative thresholds, measure paw withdrawal thresholds using the Dixon up-and-down method with von Frey filaments26 on the left hind paw (Table of Materials), starting with the 4.31 filament (19.6 mN) to establish the baseline paw withdrawal threshold.
NOTE: Mechanical sensitivity should be assessed using the "up-and-down" method by determining the withdrawal threshold. If the animal does not respond to the 4.31 filament (19.6 mN), use the thicker 4.56 filament (39.2 mN) (a response is noted visually as withdrawal, shake, or licking of the affected paw). If the animal responds to the 4.31 filament, use the thinner 4.08 (9.8 mN) filament.- Apply each filament perpendicular to the plantar surface of the hind paw while the animals are positioned in suspended wire mesh cages.
- Adjust filament pressure, either increasing or decreasing, based on the previous filament size.
- Continue using either progressively thicker or thinner filaments, depending on whether the animal had positive or negative subsequent responses, respectively.
- Record both negative and positive responses on the datasheet provided in Supplementary Table 1.
NOTE: Each filament should be applied one at a time in a sequential manner. After the first positive response, test the same paw 4 more times with different filaments. To ensure reliable results across all animals, the experimenter must avoid applying the filament to the footpads.
- Acclimate the rats in clear plexiglass boxes on the Hargreaves apparatus (Table of Materials) for 1 h prior to testing in the same room as the testing area to measure thermal sensitivity baseline using the Hargreaves test.
NOTE: The Hargreaves test requires the rats to remain still for a few seconds. If the rats are still overly active after the initial 1 h habituation period, extend the acclimation time as needed. Ensure the rats are calm and stationary but alert enough to avoid falling asleep. Before conducting the Hargreaves test (Table of Materials), it is crucial to set the infrared light intensity to establish a baseline for measuring pain sensitivity. The goal is for the withdrawal latency to average around 20 s for baseline, which provides enough sensitivity to detect changes in pain response, such as hyper/hypoalgesia. Upon paw withdrawal, a motion detector stops both the stimulus and the timer. To prevent tissue damage, a maximum cutoff of 33.5 s is applied. - To assess preoperative behavioral thresholds, place the infrared laser under the center of the animal's left hind paw (using the guiding lines of the Infrared emitter).
- Start the heat stimulus to measure the time it takes for the animal to withdraw its paw in response to the heat (withdrawal latency).
NOTE: If the average withdrawal latency is not close to 20 s, adjust the intensity and repeat the test until the desired average is determined. The intensity is then kept constant for the remainder of the experiment. In this study, we used a stimulus intensity of 30 (50 W). If the test needs to be repeated, allow 5 min intervals before repeating the test on the same animal. - Record the withdrawal latency on the datasheet provided in Supplementary Table 2.
NOTE: To avoid affecting the temperature of the heat stimulus, clean up any urine during the trials.
- Start the heat stimulus to measure the time it takes for the animal to withdraw its paw in response to the heat (withdrawal latency).
- Following the acquisition of baseline sensory behaviors, house the animals in static cages for light exposure with continuous access to food and water for 4 days before the surgery (8 h per day, from 6 am to 2 pm).
- Induction of pain model and post-surgical evaluation of sensory thresholds
- After exposure concludes at 2 pm on day 4, perform an incision surgery following the Brennan model on the left hind paw to induce postoperative pain27,28.
- Continue the exposure on day 5. After the exposure concludes at 2 pm on day 5, conduct the von Frey and Hargreaves tests, as previously described, to evaluate thermal and mechanical hypersensitivity 1 day post-surgery.
- Continue the exposure on day 6. After the exposure concludes at 2 pm on day 6, conduct the von Frey and Hargreaves tests, as previously described, to evaluate thermal and mechanical hypersensitivity 2 days post-surgery.
NOTE: Exclude any animal from the study if it reaches the endpoint criteria established by the Institutional Animal Care and Use Committee. - Perform euthanasia on the animals following the protocols outlined by the Institutional Animal Care and Use Committee after completing the behavioral testing.
- Animal data analysis
- Input the mechanical withdrawal patterns of answers into Allodynia Software (National Instruments, LabView 2015) to evaluate mechanical sensory thresholds29.
NOTE: The software uses the nonparametric Dixon method, as detailed by Chaplan et al.30. - Report the withdrawal latencies in a spreadsheet for subsequent statistical analysis of thermal allodynia.
- Generate a plot displaying the mean sensitivity (thresholds or latencies) as a function of time.
- Input the mechanical withdrawal patterns of answers into Allodynia Software (National Instruments, LabView 2015) to evaluate mechanical sensory thresholds29.
2. Light exposure protocol in humans
- Setting up the exposure room for human subject evaluation
- Begin with preparing the exposure room, ensuring it is completely free of any external light sources to eliminate potential interference.
NOTE: This step is essential to isolate the effects of the LED lights. - Position the LED lights at a distance of 3-6 feet from the subjects' chair.
NOTE: This range is based on previous clinical trials to ensure consistent light intensity and effective exposure. - Install the 2 m LED strips, ensuring the desired light intensity range of 90-100 lux using a luxmeter (Table of Materials and Supplementary Figure 2).
- Optimize light intensity by repositioning the light strips or covering some of the LEDs, ensuring that subjects receive 90-100 lux in the primary area where they are seated.
- Begin with preparing the exposure room, ensuring it is completely free of any external light sources to eliminate potential interference.
- Familiarization steps for testing mechanical temporal summation evaluation
- Before conducting any evaluation, ensure proper hand hygiene by thoroughly washing hands, followed by donning gloves.
- Clean and sanitize the calibrated von Frey filament (6.65 mN, equivalent to 300 g of force) to ensure sterility before evaluating mechanical sensitivity.
- Request permission to expose the trapezius muscle for the upcoming assessment.
- Inform the patient that the von Frey filament will be applied on the non-dominant side of the trapezius muscle until the filament bends.
- Instruct the patient to rate their pain on a scale of 0 to 10, where 0 represents no pain, and 10 represents the worst pain imaginable immediately after the operator says "Now".
NOTE: Ensure the patient provides a prompt and accurate response when the operator says "now". - Apply the filament 3 times at three different points (1 inch apart) on the non-dominant trapezius muscle, allowing a 5 s interval between each application.
- Inform the subject that von Frey filament will be applied 10 times on the same spot once per 1 s and ask the subject to rate the pain of the last application.
NOTE: Use the same spot on the trapezius muscle for all 10 applications. - Measure the temporal summation effect by applying the filament 10 times on the non-dominant trapezius and ask the subject to rate the pain at the end of the stimuli.
- Testing for mechanical temporal summation
- After the familiarization steps, allow a 3 min break and inform the patient that the von Frey filament will be applied to the dominant side of the trapezius muscle.
- Clean and sanitize the calibrated von Frey filament.
- Instruct the patient to rate their pain on a scale of 0 to 10 immediately after the operator says "Now".
- Apply the filament 3 times at 3 different points (1 inch apart) on the dominant trapezius muscle, allowing a 5 s interval between each application. Record the patient response on the record sheet (Supplementary Table 3).
- Inform the subject that the von Frey filament will be applied 10 times on the same spot once per 1 s and ask the subject to rate the pain of the last application.
- Measure the temporal summation effect by applying the filament 10 times on the dominant trapezius. Record the subject's response on the record sheet (Supplementary Table 3).
- Repeat the process two times with 3 min intervals and record subjects' responses on the record sheet (Supplementary Table 3).
- Familiarization steps for conditioned pain modulation evaluation
- Prepare 12 °C cold-water bath (Supplementary Figure 3).
NOTE: Verify the temperature before the test using the thermometer (Table of Materials). Have extra ice if needed to adjust the temperature during the testing period. - Clean and sanitize the algometer.
- Use Medoc software for evaluation of CPM (Table of Materials).
- Select the AlgoMed option (Supplementary Figure 4). On the home screen, find and click the Algometer Device to activate it.
- Select the correct patient from the list and pick the test program.
- Select the site for applying the device. For this familiarization step, choose the non-dominant trapezius muscle from the body diagram (Supplementary Figure 5).
NOTE: After clicking go to test, one will be directed to the testing screen. Before applying any pressure, the software will require one to perform a pretest, where the device is not applying pressure yet. This pretest must be completed each time a new test is started. - Start a test and 5 s timeout.
- Inform the patient that the device measures the amount of pressure applied, and it will be used to apply pressure to the non-dominant side of the trapezius muscle. Ask the patient to say "stop" as soon as they begin to feel any pain.
- Click start. The system will impose a 5 s wait before beginning to apply the device.
- Apply the algometer on the trapezius by increasing force at a rate of 30 kPa/s (Supplementary Figure 6).
- Record the patient response on the record sheet (Supplementary Table 3).
NOTE: For this study, the maximum pressure applied is 650 kPa. This study specifically assesses the onset of pain, not evaluate pain tolerance. - Inform the participants that they will use an ice water bath for the assessment.
- Check the water temperature using the thermometer (Table of Materials) to be around 12 °C. Set a timer for 10 s.
- Instruct the participant to immerse their dominant hand in the water up to the wrist, ensuring the hand is relaxed and fingers are spread apart.
- Ask the participant to rate their pain on a scale from 0 to 10 when the operator says "now" by the end of 10 s.
- Ask for the pain rating at the end of the 10 s and record the patient response on the record sheet (Supplementary Table 3).
- Prepare 12 °C cold-water bath (Supplementary Figure 3).
- Testing conditioned pain modulation
- Check the water temperature using the thermometer (Table of Materials).
- Clean and sanitize the algometer.
- After a 3 min break, start the evaluation of conditioned pain modulation (CPM) by requesting permission to expose the dominant trapezius muscle for the upcoming assessment.
- Ask the patient to say "stop" as soon as they begin to feel any pain. Click start on the software.
- Apply the algometer on the trapezius by increasing the force at a rate of 30 kPa/s and record the patient response on the record sheet (Supplementary Table 3).
- Repeat the application 2 more times on different sites of the dominant trapezius muscle, with a 3 min interval between each.
- Ensure the algometer is thoroughly cleaned and sanitized before each application to prevent slipping caused by the accumulation of skin oils from subjects.
- Record the patient response on the record sheet (Supplementary Table 3). Wait 5 min before moving to the conditioned stimulus testing.
- Click start. The system will impose a 5 s wait before beginning to apply the device.
- Clean and sanitize the algometer.
- Instruct the patient to immerse their non-dominant hand up to the wrist in the ice water bath.
- Apply the algometer on the trapezius by increasing the force at a rate of 30 kPa/s and record the patient response on the record sheet (Supplementary Table 3).
- Repeat the application two more times on different sites of trapezius muscle, with a 5 min interval between each.
NOTE: Discontinue the study if a previously undiscovered medical condition is identified after initial screening, particularly if the condition has the potential to interfere with the study. - Following the acquisition of the baseline pre-exposure values, start exposing the patient to the light condition assigned to them.
- Instruct volunteers to avoid staring directly at the light source.
NOTE: Instead, they should allow the light to enter their peripheral vision as they would with any ambient light source in their home (Supplementary Figure 7). - Expose subjects to LED light for 1.5 h.
NOTE: For prolonged light treatment, subjects self-administer exposure at home for up to 10 weeks. Surveys, such as the Fibromyalgia Impact Questionnaire (FIQ), the HIT-6 (Headache Impact Test), the PSQI (Pittsburgh Sleep Quality Index), and the EQ-5D-5L (to assess overall health), along with pain questionnaires, can be used to monitor the treatment's effects over time. - During the exposure, encourage volunteers to engage in activities that do not require additional light sources, such as reading or writing.
NOTE: Intensities of 4-100 lux provide sufficient illumination. - Discourage sleeping during the exposure period to ensure full adherence to the protocol.
- After light exposure therapy, repeat all the measurements completed at the baseline.
- Human data analysis
- For mechanical temporal summation, calculate the average of the 3 baseline measures and the average of the 3 repeated stimuli.
- Calculate the mechanical temporal summation percentage by dividing the average pain rating after the repetitive stimuli by the average baseline pain rating and multiplying the result by 100 to express it as a percentage.
NOTE: A percentage greater than 100% indicates an increase in pain perception with repeated stimuli, suggesting the presence of temporal summation. - For mechanical conditioned pain modulation (CPM), calculate the average of the last 2 baseline pain thresholds (in kPa) obtained during the unconditioned stimuli (without cold bath).
- Calculate the average of the last 2 pain threshold values (in kPa) during the conditioning stimulus.
- Use the following equation to calculate CPM:
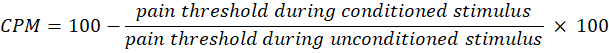
NOTE: A CPM value of 0 indicates no change in pain perception, a negative value indicates pain sensory inhibition during the conditioned stimulus, and a positive value indicates pain facilitation during the conditioned stimulus. - Evaluate the changes in both temporal summation and CPM before and after light exposure to assess how the therapy affects the activity of the ascending and descending pain pathways, respectively.
Results
Greenlight exposure increases paw withdrawal latencies in a dose-dependent manner
Figure 1A demonstrates that exposure to green light-emitting diodes (GLED) at various intensities (4, 50, 100, and 200 lux) significantly increased paw withdrawal latencies in a naïve rat model over a 7-day exposure period, indicating an antinociceptive effect of GLED. Baseline latencies before light exposure were comparable across groups. Starting fr...
Discussion
Recent studies have explored the mechanisms underlying green light (GLED) analgesia13,21,22,23,24. However, further standardization of the methodology is needed to enhance its translation into clinical practice. The dose-dependent antinociceptive effects observed in preclinical models highlight the importance of optimizing exposure parameters to maximize thera...
Disclosures
Dr. Ibrahim has disclosed an outside interest in Luxxon Therapeutics to the University of Arizona. Conflicts of interest resulting from this interest are being managed by The University of Arizona in accordance with its policies. All other authors have no conflict of interest to report. None of the authors of the manuscript received any remuneration, reimbursement, or honorarium in any other manner. The authors are not affiliated with any vendor or pharmaceutical company associated with this study. None of this research, manuscript, or abstract has been previously presented and is not being considered for publication by any other journal.
Acknowledgements
This research was supported by the Comprehensive Center for Pain and Addiction-University of Arizona (M.M.I., L.F.M.), the Anesthesiology Department at the University of Arizona (L.F.M.), and the Medical Scientist Training Program (MSTP) at the University of Arizona, College of Medicine, Tucson.
Materials
| Name | Company | Catalog Number | Comments |
| 24 h Mechanical mini timer for LED strips | bn-link | BND-60/U47 | https://www.bn-link.com/products/bn-link-indoor-24-hour-mechanical-outlet-timer-3-prong-2-pack?variant=42704897245237¤cy= USD&utm_medium=product_sync& utm_source=google&utm_content= sag_organic& utm_campaign= sag_organic&gad_source=1& gclid=Cj0KCQjwurS3BhCGARI sADdUH50dy8sYj4Ku2ZmM14-3Yp3iajSY 4TgRze8UvSuyhq81-h 1E6GChOXgaAhwYEALw_wcB |
| AC 5050 SMD LED Tape Rope Strip Lighting | LED Supply Co | LS-AC50-GR | https://www.ledsupply.com/led-strips/ac-power-5050-led-strips Green Strip Lighting for all exposure rooms 120V AC, 60Hz |
| AC 5050 SMD LED Tape Rope Strip Lighting | LED Supply Co | LS-AC50-WH | https://www.ledsupply.com/led-strips/ac-power-5050-led-strips White Strip Lighting for all exposure rooms 120V AC, 60Hz |
| Allodynia Software | National Instruments, LabView 2015 | https://www.ni.com/en-us/shop/product/labview.html | |
| Amazon Basics Lightweight Super Soft Easy Care Microfiber 4-Piece Bed Sheet Set with 14-Inch Deep Pockets, Queen, Black, Solid | Amazon Basics Store | Amazon.com: Amazon Basics Lightweight Super Soft Easy Care Microfiber 4-Piece Bed Sheet Set with 14-Inch Deep Pockets, Queen, Black, Solid : Amazon Basics: Home & Kitchen | |
| Computerized Pressure Pain Algometer | Medoc advanced medical systems | ID 00186 | https://www.medoc-web.com/algomed |
| Digital Lux Meter | Edmund Optics | 52270 | https://www.edmundoptics.com/ |
| Elevated metal mesh stand for Von Frey | Bioseb | BIO-STD2-EVF | https://www.bioseb.com/en/pain-mechanical-allodynia_hyperalgesia/1689-elevated-metal_mesh-stand-30-cm-height-to-fit-up_to-2-pvf-cages.html |
| Fisherbrand Thermometers | Fischer Scientific | 13-201-577 | https://www.fishersci.com/shop/products/fisherbrand-10-30-ground-joint-thermometers-6/13201927 |
| Medline Autoclavable Plastic Washbasins | Truway Health | 42141606 | https://truwayhealth.com/medline-autoclavable-plastic-washbasins/?cmp_id=21122060336&adg_id= &kwd=&device=c& gad_source=1&gclid= CjwKCAjw0aS3BhA3EiwAKaD2ZTHY8_ 7W__ gXC7Wf3Kv3jJa6KQrNI-4JrdYqKM9IO v8moeW6ylEpzRoCnZ8QAvD_BwE |
| Modular holder cages for rats and mice | Bioseb | BIO-PVF | https://bioseb.com/en/pain-mechanical-allodynia-hyperalgesia/1206-modular-holder-cages-for-rats-and-mice.html |
| Plantar Test for Thermal Stimulation - Hargreaves Apparatus | Ugo Basile | 37570 | https://ugobasile.com/products/categories/pain-and-inflammation/plantar-test-for-thermal-stimulation includes semi-transparent glass panel and individual animal enclosures for 6 rats/12 mice |
| Scotch 700 Electrical Tape, 3/4 in. x 66 ft. x 0.007 in. | 3M | https://www.3m.com/3M/en_US/p/d/cbgnawus1596/ | |
| Touch Test Sensory Evaluators (von Frey Filaments) | North Coast Medical and Rehabilitation Products | NC12775-99 | https://www.ncmedical.com/products/touch-test-sensory-evaluators_1278.html |
| Touch Test Sensory Evaluators (von Frey Filaments) | North Coast Medical and Rehabilitation Products | NC12775-20 | https://www.ncmedical.com/products/touch-test-sensory-evaluators_1278.html |
| TRINITY EcoStorage 5-Tier , 48 x 24 x 72, Commercial Wire Shelving | Trinity | 952471 | https://trinityii.com/ecostorage-5-tier-48x24x72-wire-shelving-nsf-with-wheels-chrome/ |
References
- Alorfi, N. M. Pharmacological methods of Narrative review of medication used. Int J Gen Med. 16, 3247-3256 (2023).
- Cherny, N., et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. 19 (9), 2542-2554 (2001).
- Hanks, G. W., et al. Morphine in cancer pain: modes of administration. Expert Working Group of the European Association for Palliative Care. BMJ. 312 (7034), 823-826 (1996).
- de Freitas, L. F., Hamblin, M. R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 22 (3), 7000417 (2016).
- Pathak, M. A., Fitzpatrick, T. B. The evolution of photochemotherapy with psoralens and UVA (PUVA): 2000 BC to 1992 AD. J Photochem Photobiol B. 14 (1), 3-22 (1992).
- Kemper, K. J. ."Let there be light." Research on phototherapy, light therapy, and photobiomodulation for healing - Alternative therapy becomes mainstream. Complement Ther Med. 41, A1-A6 (2018).
- Olesen, J., et al. Headache Classification Committee of the International Headache Society(IHS), The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38 (1), 1-211 (2018).
- Santiago, R., Gomes, S., Ozsarfati, J., Zitney, M. Photobiomodulation for modulation of neuropathic pain and improvement of scar tissue. Scars Burn Heal. 8, 20595131221134052 (2022).
- González-Muñoz, A., et al. Efficacy of photobiomodulation therapy in the treatment of pain and inflammation: A literature review. Healthcare (Basel). 11 (7), 938 (2023).
- Figueiro, M. G., et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer's disease and related dementia living in long-term care facilities. Clin Interv Aging. 9, 1527-1537 (2014).
- Eastman, C. I., Young, M. A., Fogg, L. F., Liu, L., Meaden, P. M. Bright light treatment of winter depression: A placebo-controlled trial. Arch Gen Psychiatry. 55 (10), 883-889 (1998).
- Martin, L. F., et al. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia. 41 (2), 135-147 (2021).
- Martin, L. F., et al. light antinociceptive and reversal of thermal and mechanical hypersensitivity effects rely on endogenous opioid system stimulation. J Pain. 22 (12), 1646-1656 (2021).
- Nelli, A., Wright, M. C., Gulur, P. Green light-based analgesia - novel non-pharmacological approach to fibromyalgia pain: A pilot study. Pain Physician. 26 (4), 403-410 (2023).
- Martin, L., et al. light exposure improves pain and quality of life in fibromyalgia patients: A preliminary one-way crossover clinical trial. Pain Med. 22 (1), 118-130 (2021).
- Qaiser, H., Uzair, M., Arshad, M., Zafar, A., Bashir, S. Evaluating the potential of green light exposure on nociception-A mini review. CNS Neurol Disord Drug Targets. 23 (6), 675-679 (2024).
- Lipton, R. B., et al. Narrow band green light effects on headache, photophobia, sleep, and anxiety among migraine patients: an open-label study conducted online using daily headache diary. Front Neurol. 14, 1282236 (2023).
- Posternack, C., Kupchak, P., Capriolo, A. I., Katz, B. J. Targeting the intrinsically photosensitive retinal ganglion cell to reduce headache pain and light sensitivity in migraine: A randomized double-blind trial. J Clin Neurosci. 113, 22-31 (2023).
- Noseda, R., et al. Migraine photophobia originating in cone-driven retinal pathways. Brain. 139 (7), 1971-1986 (2016).
- Ibrahim, M. M., et al. Long-lasting antinociceptive effects of green light in acute and chronic pain in rats. Pain. 158 (2), 347-360 (2017).
- Martin, L. F., et al. light exposure elicits anti-inflammation, endogenous opioid release and dampens synaptic potentiation to relieve post-surgical pain. J Pain. 24 (3), 509-529 (2023).
- Tang, Y. L., et al. Green light analgesia in mice is mediated by visual activation of enkephalinergic neurons in the ventrolateral geniculate nucleus. Sci Transl Med. 14 (674), eabq6474 (2022).
- Cao, P., et al. light induces antinociception via visual-somatosensory circuits. Cell Rep. 42 (4), 112290 (2023).
- Wu, X. Q., et al. Glutamatergic and GABAergic neurons in the vLGN mediate the nociceptive effects of green and red light on neuropathic pain. Neurobiol Dis. 183, 106164 (2023).
- Sprenger, C., Eichler, I. C., Eichler, L., Zöllner, C., Büchel, C. Altered signaling in the descending pain-modulatory system after short-term infusion of the µ-opioid agonist remifentanil. J Neurosci. 38 (10), 2454-2470 (2018).
- Zahn, P. K., Brennan, T. J. Primary and secondary hyperalgesia in a rat model for human postoperative pain. Anesthesiology. 90 (3), 863-872 (1999).
- Brennan, T. J., Zahn, P. K., Pogatzki-Zahn, E. M. Mechanisms of incisional pain. Anesthesiol Clin North Am. 23 (1), 1-20 (2005).
- Martin, L., et al. Conotoxin contulakin-G engages a neurotensin receptor 2/R-type calcium channel (Cav2.3) pathway to mediate spinal antinociception. Pain. 163 (9), 1751-1762 (2022).
- Korah, H. E., et al. Partial sciatic nerve ligation: A mouse model of chronic neuropathic pain to study the antinociceptive effect of novel therapies. J VIs Exp. (188), e64555 (2022).
- Chaplan, S. R., Bach, F. W., Pogrel, J. W., Chung, J. M., Yaksh, T. L. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 53 (1), 55-63 (1994).
- Lockwood, S., Dickenson, A. H. What goes up must come down: insights from studies on descending controls acting on spinal pain processing. J Neural Transm (Vienna). 127 (4), 541-549 (2020).
- Staud, R., Robinson, M. E., Price, D. D. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 8 (11), 893-901 (2007).
- Ventura, L., et al. light exposure reduces primary hyperalgesia and proinflammatory cytokines in a rodent model of knee osteoarthritis: Shedding light on sex differences. Biomedicines. 12 (9), 2005 (2024).
- Cheng, K., Martin, L. F., Slepian, M. J., Patwardhan, A. M., Ibrahim, M. M. Mechanisms and pathways of pain photobiomodulation: A narrative review. J Pain. 22 (7), 763-777 (2021).
- Cheng, K., Martin, L. F., Calligaro, H., Patwardhan, A., Ibrahim, M. M. Case report: Green light exposure relieves chronic headache pain in a colorblind patient. Clin Med Insights Case Rep. 15, 11795476221125164 (2022).
- Takemura, Y., et al. Effects of green color exposure on stress, anxiety, and pain during peripheral intravenous cannulation in dental patients requiring sedation. Int J Environ Res Public Health. 18 (11), 5939 (2021).
- Berkley, K. J., Hubscher, C. H. Are there separate central nervous system pathways for touch and pain. Nat Med. 1 (8), 766-773 (1995).
- Bannister, K., Kucharczyk, M. W., Graven-Nielsen, T., Porreca, F. Introducing descending control of nociception: a measure of diffuse noxious inhibitory controls in conscious animals. Pain. 162 (7), 1957-1959 (2021).
- Millan, M. J. Descending control of pain. Prog Neurobiol. 66 (6), 355-474 (2002).
- Ossipov, M. H., Morimura, K., Porreca, F. Descending pain modulation and chronification of pain. Curr Opin Support Palliat Care. 8 (2), 143-151 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved