需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
测量水润谷胱甘肽诱导饲养响应
摘要
Here we describe a simple assay for the quantification of the feeding response in hydra induced by the reduced form of glutathione. This assay relies on measuring the distance between the apical end of the tentacle and mouth of hydra.
摘要
Hydra is among the most primitive organisms possessing a nervous system and chemosensation for detecting reduced glutathione (GSH) for capturing the prey. The movement of prey organisms causes mechanosensory discharge of the stinging cells called nematocysts from hydra, which are inserted into the prey. The feeding response in hydra, which includes curling of the tentacles to bring the prey towards the mouth, opening of the mouth and consequent engulfing of the prey, is triggered by GSH present in the fluid released from the injured prey. To be able to identify the molecular mechanism of the feeding response in hydra which is unknown to date, it is necessary to establish an assay to measure the feeding response. Here, we describe a simple method for the quantitation of the feeding response in which the distance between the apical end of the tentacle and mouth of hydra is measured and the ratio of such distance before and after the addition of GSH is determined. The ratio, called the relative tentacle spread, was found to give a measure of the feeding response. This assay was validated using a starvation model in which starved hydra show an enhanced feeding response in comparison with daily fed hydra.
引言
Hydra is the most primitive organism possessing a nervous system and chemosensation for detecting reduced glutathione (GSH) for capturing the prey1. It feeds on a variety of animals such as nematode, crustacea, insect larvae, tadpoles and newly hatched fish1. The movement of these prey organisms causes mechanosensory discharge of the stinging capsules called nematocysts from hydra, which are inserted into the prey2. GSH present in the fluid released from the injured prey subsequently activates the feeding response in hydra which includes curling of the tentacles to bring the prey towards the mouth, opening of the mouth, and consequent engulfing of the prey. Multiple molecules, such as dopamine3, glutamate4, GABA, glycine5, NMDA receptors6, and allatotropin7, have been shown to be involved in the feeding response in hydra. It has also been shown that the chemosensory response induced by GSH is modulated by the feeding status of the animal such that starved hydra exhibited enhanced feeding response1. Such an increase in the GSH sensitivity is biologically relevant since under starvation hydra need to find its prey at higher sensitivity.
Although the feeding response induced by GSH can be clearly observed under microscope, the methods typically used for measuring the feeding response observations are non-quantitative. In most of the cases, the time during which the mouth of the hydra remains open was taken as a measure of the feeding response8,9; whereas in another case, quantitation was based on the number of hydra out of a population showing the feeding response10. However, observing the mouth opening time of the hydra polyps is cumbersome and subject to variation induced by uncontrollable parameters such as the direction of the mouth orientation during observations. Similarly, since the feeding response is a quantitative parameter, population-based approaches are subject to variations/errors caused by the opinion or observational bias of the individual observer. To circumvent these issues we have developed a method for the relative quantification of the feeding response in hydra (Hydra vulgaris Ind-Pune11) based on the distance of the apical end of the tentacle from the mouth of the hydra polyp.
Access restricted. Please log in or start a trial to view this content.
研究方案
在饲养响应1.水润文化与测量
- 通过每天用卤虫喂养它们并保持它们在培养基(1毫摩尔Tris-HCl缓冲液,pH值7.6,1 mM氯化钠,1mM的氯化钙 ,0.1mM的氯化钾,和0.1mM 硫酸镁 )包含在保持在培养水螅息肉玻璃碗,在18℃下进行12小时光照,12小时黑暗周期如前所述12。
- 用于测量馈响应,传送一个成熟的具有5-6个触须到24孔板的单个孔水螅息肉。通过倾斜地取出从井的残余介质中,然后立即加入500μl的新鲜培养基。
- 准备在九头蛇媒体9微米的谷胱甘肽的解决方案。由于谷胱甘肽溶液是容易氧化,通常使用为每个实验新鲜制备的谷胱甘肽溶液。
- 板转移到具有用于图像记录规定一个显微镜的成像平台。使用深色背景,这样的行为Ò˚F水螅水螅可以清晰成像对对比背景。
- 房间用于观察和成像水螅自由的行为与波动强度,气流和噪音的灯。这些干扰也可导致水螅息肉显示触手收缩 - 甚至在没有谷胱甘肽。
- 让息肉5分钟放松。
- 确保息肉位于沿井,使得行为可以清楚地拍摄的中心区域。如果息肉是在井的边缘,使之在中心通过使用移液管冲洗介质,并再次使其放松。
- 捕捉在松弛状态下九头蛇的图像。这将是零时间点的观察。
- 快速添加9微米谷胱甘肽溶液以达到在该井的3μM的终浓度。根据实验和响应由水螅示的目的,测试一系列不同浓度的谷胱甘肽和噗的瑟所需的适当的浓度。
- 添加谷胱甘肽的解决方案,采集图像每隔15-30秒4-5分钟后后,立即启动定时器。时间推移成像过程中不要改变倍率设置。
- 添加谷胱甘肽的溶液轻轻地和均匀的流动,使得在井的动物的位置将被最低限度地干扰在视显微镜的领域。然而,如果息肉添加谷胱甘肽溶液后广泛移动时,移动板轻轻地使息肉中的视图用于图像捕获的字段。
- 在对照实验中,使用的介质缺乏谷胱甘肽,同时保持所有其他参数相同。
- 要确保在第一天的一半执行所有上述实验步骤: - :00之前,以避免昼夜节律对馈送响应的程度的可能影响。
- 打开每个使用GNU图像处理程序(GIMP)拍摄的图像中。
- 使用"测量"工具,可从菜单>工具>测量来确定每个触角和hypostome的顶端末端之间的距离。如果嘴开口中的任何图像的观察,确定了开口的中心与所述触手的尖端之间的距离。请参考这个距离作为触手蔓延。
- 之前,谷胱甘肽曝光后计算平均价差触须每个息肉。计算的平均触手蔓延在零时间点,以该比率在每个随后的时间点。这个比例将被称为相对触手蔓延。
- 重复测量,至少20息肉。
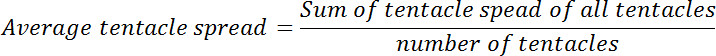

2.方法验证使用饥饿型号
- 对于饥饿,转移几水螅珊瑚虫为一个单独的玻璃碗里,不要喂它们5天。每日与卤虫饲料的几个息肉,对照组以同样大小的碗。改变介质从每日两实验的碗,以避免在培养基中的真菌生长。
- 在实验当天,喂水螅的对照组与卤虫1小时,并从培养基中除去所有未吃掉的和死的卤虫后使用这些水螅用于后续实验。
- 衡量饥饿水润与对照组按上面的步骤1.描述,以避免任何偏见的方法九头蛇比较饲养的反应,由于观测时间,每次交替的饥饿的测量和控制水螅珊瑚虫。
Access restricted. Please log in or start a trial to view this content.
结果
谷胱甘肽导致水螅表现出对嘴吞噬猎物的目的触手卷曲。触手卷曲等带来的触角更接近hypostome根尖末端。这将导致减少的触手蔓延,或的触手与hypostome( 图1)顶端末端之间的直线距离。相对触手蔓延,或平均触手之比之前流传,并添加谷胱甘肽后,均在多个息肉减少随着时间的推移。相对触手蔓延除了缺乏谷胱甘肽培养基之后,但是,只减少瞬时和达到单元值在大约一分钟内(
Access restricted. Please log in or start a trial to view this content.
讨论
在水螅摄食行为代表了最原始的化学感受系统的后生动物之一。虽然谷胱甘肽(GSH)后刺丝囊辅助捕获猎物释放的甲壳类流体存在检测前不久1,无论是GSHR蛋白质也不是假定的编码基因/ S进行了表征,从九头蛇是最新的。一些尝试已经进行了表征谷胱甘肽结合蛋白在水螅8,14,15,但是,这些推测的受体蛋白质的身份仍不清楚并且很少有其他分子组分,它有可能向馈送反应中,已?...
Access restricted. Please log in or start a trial to view this content.
披露声明
The authors declare no competing financial interests.
致谢
Authors are thankful to K. P. Madhu, Nita Beliappa and staff of the Media Centre of Indian Institute of Science Education and Research, Pune for their help in the video production. The work was supported by funding under the Centre of Excellence program of Department of Biotechnology, Government of India to SG and postdoctoral fellowship by Department of Science and Technology, Government of India to RK.
Access restricted. Please log in or start a trial to view this content.
材料
| Name | Company | Catalog Number | Comments |
| Cooled Incubator | Panasonic | MIR-254-PE | |
| Microscope | Leica | S8AP0 | |
| Camera for the microscope | Leica | EC3 | |
| Reduced glutathione | Sigma | G4251 | Stored at 4 °C. Bring the bottle to room temperature before opening to avoid oxidation |
| Image editing program | GIMP | Version 2.8 |
参考文献
- Loomis, W. F. Glutathione control of the specific feeding reactions of hydra. Ann. Ny. Acad. Sci. 62, 209-228 (1955).
- Beckmann, A., Ozbek, S. The Nematocyst: a molecular map of the Cnidarian stinging organelle. Int. J. Dev. Biol. 56, 577-582 (2012).
- Venturini, G., Carolei, A. Dopaminergic receptors in Hydra. Pharmacological and biochemical observations. Comp. Biochem. Phys. C. 102, 39-43 (1992).
- Kass-Simon, G., Scappaticci, A. A. Glutamatergic and GABAnergic control in the tentacle effector systems of Hydra vulgaris. Hydrobiologia. 530-531, 67-71 (2004).
- Pierobon, P., Tino, A., Minei, R., Marino, G. Different roles of GABA and glycine in the modulation of chemosensory responses in Hydra vulgaris (Cnidaria, Hydrozoa). Hydrobiology. 178, 59-66 (2004).
- Pierobon, P., Sogliano, C., Minei, R., Tino, A., Porcu, P., Marino, G., Tortiglione, C., Concas, A. Putative NMDA receptors in Hydra: a biochemical and functional study. Eur. J. Neurosci. 20, 2598-2604 (2004).
- Alzugaray, M. E., Adami, M. L., Diambra, L. A., Hernandez-Martinez, S., Damborenea, C., Noriega, F. G., Ronderos, J. R. Allatotropin: An ancestral myotropic neuropeptide involved in feeding. PLoS ONE. 8, (2013).
- Bellis, S. L., Laux, D. C., Rhoads, D. E. Affinity purification of Hydra glutathione binding proteins. FEBS Lett. 354, 320-324 (1994).
- Lenhoff, H. M. The discovery of the GSH receptor in Hydra and its evolutionary significance. Glutathione in the Nervous System. Shaw, C. A. , Taylor&Francis. Bristol. 25-43 (1998).
- Venturini, G. The hydra GSH receptor. Pharmacological and radioligand binding studies. Comp. Biochem. Phys. C. 87, 321-324 (1987).
- Reddy, P. C., Barve, A., Ghaskadbi, S. Description and phylogenetic characterization of common hydra from India. Curr. Sci. 101, 736-738 (2011).
- Horibata, Y., et al. Unique catabolic pathway of glycosphingolipids in a hydrozoan, Hydra magnipapillata. Involving endoglycoceramidase. J. Biol. Chem. 279, 33379-33389 (2004).
- Koizumi, O., Maeda, N. Rise of feeding threshold in satiated Hydra. J. Comp. Physiol. 142, 75-80 (1981).
- Bellis, S. L., Kass-Simon, G., Rhoads, D. E. Partial characterization and detergent solubilization of the putative glutathione chemoreceptor from hydra. Biochemistry. 31, 9838-9843 (1992).
- Morita, H., Hanai, K. Taste receptor proteins in invertebrates - with special reference to glutathione receptor of hydra. Chem. Senses. 12, 245-250 (1987).
- Colasanti, M., Venturini, G., Merante, A., Musci, G., Lauro, G. M. Nitric oxide involvement in Hydra vulgaris very primitive olfactory- like system. Journal of Neurosci. 17, 493-499 (1997).
- Kuhn, A., Tsiairis, C. D., Williamson, M., Kalbacher, H., Grimmelikhuijzen, C. J., Holstein, T. W., Gründer, S. Three homologous subunits form a high affinity peptide-gated ion channel in Hydra. J. Biol. Chem. 285, 11958-11965 (2010).
- Wang, M., Yao, Y., Kuang, D., Hampson, D. R. Activation of family C G-protein-coupled receptors by the tripeptide glutathione. J. Biol. Chem. 281, 8864-8870 (2006).
- Ruggieri, R. D., Pierobon, P., Kass-Simon, G. Pacemaker activity in hydra is modulated by glycine receptor ligands. Comp. Biochem. Phys. C. 138, 193-202 (2004).
- Ramazani, R. B., Krishnan, H. R., Bergeson, S. E., Atkinson, N. S. Computer automated movement detection for the analysis of behavior. J. Neurosci. Meth. 162, 171-179 (2007).
Access restricted. Please log in or start a trial to view this content.
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。