需要订阅 JoVE 才能查看此. 登录或开始免费试用。
Method Article
在体内小鼠激光诱导脉络膜新生血管模型的多模态成像与分析
摘要
在这里, 我们介绍了纵向的在体内成像在激光诱导的小鼠脉络膜新生血管形态学变化中的作用。
摘要
激光诱导脉络膜新生血管 (CNV) 是模拟年龄相关性黄斑变性 (AMD) 的湿型的一个良好建立的模型。在本议定书中, 我们的目标是引导读者不只是通过技术考虑产生激光诱导的损害, 以触发新生血管的过程, 而是侧重于强大的信息, 可以获得从多式联运纵向在体内的整个随访期内的成像。
激光诱导的小鼠 CNV 模型是由二极管激光管理产生的。多模态在体内成像技术被用来监测 CNV 的诱导, 进展和回归。首先, 在激光后立即进行光谱域光学相干层析成像 (SD OCT) 来验证赫膜的断裂。随后使用荧光素血管造影 (FA) 进行的活体成像证实了赫膜从脉络膜水平获得的序列图像的成功损伤。CNV 增殖和回归的纵向后续在天 5, 10 和14以后使用了 SD OCT 和 FA 激光执行。提出了从 FA 图像中 CNV leasions 的简单可靠的分级方法。自动分割的测量总视网膜厚度, 结合手动口径应用测量视网膜厚度的 CNV 地点, 允许无偏见的评价存在水肿。最后, 利用 isolectin GS-IB4 染色对脉络膜 flatmounts 进行了 CNV 的组织学验证。染色是值, isolectin 阳性面积计算 ImageJ。
该协议特别适用于需要高通量样的 CNV 病理筛查的治疗研究, 因为它可以快速、多式、可靠地分类 CNV 病理和视网膜水肿。此外, 高分辨率 SD OCT 可以记录其他病理特征, 如视网膜或 intraretinal 液的积累。但是, 此方法不提供从 SD OCT 图像中自动进行 CNV 卷分析的可能性, 必须手动执行。
引言
第一次成功的尝试模仿人类 CNV 在啮齿目动物的病理证实了近三年前与氪激光在长埃文斯鼠1。此后, 氪激光被用来打破赫的细胞膜在最流行的鼠标应变, C57BL/6J2,3,4。用 FA 和组织学染色验证了 CNV 诱导的成功率。快速发展的无创成像模式, 如 OCT, 促进了啮齿动物的临床前模型领域的增长。在同一眼的多个时间点, 监测视网膜形态变化的能力大大降低了动物的使用, 提高了实验研究的效率。组织学评价 CNV 病变是相当直接的, 并要求标签的异常血管生长周围的激光管理, 图像采集, 和面积/体积估计使用图像分析软件。相比之下,在体内成像模式引入了对 CNV 病理及其解释的更复杂的分析。
在这里, 我们提出了一个简单的和相对快速的方法来评分的诱导, 进展, 和回归的 CNV 使用 FA, SD OCT, 和自动分割方法的小鼠激光诱导 CNV 模型。
研究方案
所有的动物都是按照帕特声明, 为使用动物在眼科和视力研究和欧共体指令 86/609/EEC 动物实验, 使用的协议批准和监测的动物实验委员会芬兰。
1. 激光诱导小鼠 CNV 模型5
- 从宏观上检查动物的眼睛是否有异常。
- 称量鼠标。
- 计算和准备适当数量的麻醉剂使用, 根据动物的重量,例如medetomidine (1 毫克/千克), 氯胺酮 (75 毫克/千克) 和蒸馏水 (0.9% NaCl 溶液) 的比例为 1:1. 5:2. 5, 或氯胺酮 (40-75 毫克/千克),嗪 (5 毫克/千克), 蒸馏水 (0.9% 氯化钠溶液) 的比率为 1:2. 5:1;对于20克老鼠, 注入0.1 毫升的混合物。
- 注射麻醉腹腔。
- 把老鼠放回笼子里, 等到动物被麻醉。确认鼠标没有踏板反射就能正确麻醉。
- 确保使用激光安全个人防护设备。
- 打开一个狭缝灯和 532 nm 二极管激光器。
- 将鼠标从笼子里取出, 放在暖气垫上。
- 应用一滴卡进行瞳孔扩张。等待3-5 分钟的全 (3 毫米) 瞳孔扩张。
- 将鼠标放在狭缝灯的舞台上。
- 将一滴眼科液体凝胶放在片上, 扁平角膜。
- 用中心的视神经头定位鼠标的眼睛。
- 将激光功率设置为100兆瓦, 持续时间为100毫秒, 光斑尺寸为50µm。
- 将激光束聚焦于视网膜色素上皮 (RPE)。
- 通过避免视网膜血管理想地在 4, 8, 和12点周围的视神经, 分别做三激光射击入一只眼睛。在所有激光照射后检查眼底, 因为没有视网膜出血。对侧眼作为非激光的控制。
- 丢弃片并将鼠标放回加热垫上。
- 用一滴 PEG 凝胶滴在两只眼睛上。
2. SD-OCT 6,7
- 将鼠标放置到啮齿动物的对齐阶段, 并固定头部。
- 将 SD-OCT 系统 (例如, Bioptigen/莱卡 Envisu R2200) 的镜头对准, 以使用 X 和 Y 级控制器对活体进行成像。
- 执行 sd oct 扫描以验证赫膜的断裂: 一旦 sd oct 扫描整个眼睛, 在激光站点上手动移动参考线。赫膜的断裂应在激光区域中清晰可见 (请参见图 1)。
3. 荧光素血管造影7,8,9
- 取出鼠标与持有人, 并将其放在 FA 系统 (例如, 海德堡 Spectralis HRA2)。
- 聚焦在眼睛眼底的激光烧伤区域, 用红外线反射模式与视神经的头部在观察窗口中间。
- 注射0.1 毫升的5% 荧光素钠盐为20克鼠皮下或腹腔。
- 关注脉络膜水平。
- 从脉络膜的焦点水平进行图像。
- 重新聚焦在视网膜水平上, 并采取图像。
- 等待三十年代, 重复步骤 3.4-3.6。
- 将鼠标从支架上取出, 并将其放在加热垫上。
- 反向麻醉的2拮抗剂的 medetomidine, 唑 (0.5 毫克/千克, ip), 或等待动物恢复从麻醉。
- 在后续的天数5、10和14中, 在麻醉动物中重复在体内 SD OCT 和 FA 成像。
4. CNV 分级
- 赫在0天激光后立即采取的 OCT 图像和脉络膜 FA 图像的损伤等级如下:
0-赫的膜未损坏
1-赫膜的成功损坏 - 通过比较一系列视网膜 FA 图像中荧光素信号的动态观察, 发现有渗漏的激光斑点 CNV 的存在, 如下所示:
0-视网膜的正常出现
0.5-渗漏的微弱的染色
1.0-漏的 CNV 区域
注: 使用 oct 成像, 以进一步确认 CNV 或可疑 FA 中存在的 intraretinal 流体在 OCT 图像将建议 CNV 评分。
5. 视网膜厚度测量
- 使用自动分割软件进行视网膜厚度测量。确保视网膜总厚度被认为是从神经纤维层到 rpe (健康测量点) 的所有层的厚度, 或者是连接 rpe (激光站点) 周围的假想线 (也请参见图 7)。
结果
激光后立即出现气泡或视网膜出血并不总是可见的。因此, SD OCT 对于验证赫膜的损伤尤为重要。图 1显示了激光管理后在不同时间点的 OCT 成像的示例。
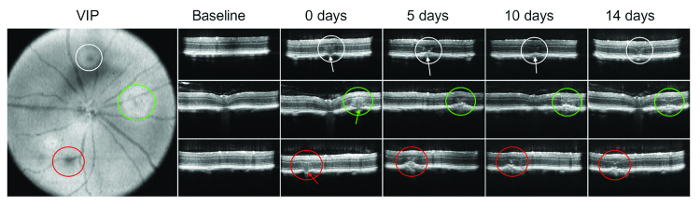
图 1: 眼睛眼底的 OCT (VIP 图像) 显示在白色、绿色和红色圆圈?...
讨论
多模态成像为 CNV 病理评价提供了有价值的工具。在这里, 我们提出了一个成像协议, 包括 FA, SD OCT, 自动分割的快速, 重现性, 可靠的评价 CNV 病理。经激光管理后, 赫膜断裂。此外, 在这个阶段使用 SD OCT 也可以立即可视化可能的 intraretinal 和视网膜出血, 这可能混淆结果的解释。根据 FA 图像的荧光素信号对视网膜渗漏进行分级。使用 SD OCT 提供了更详细的描述 CNV 病理。此外, 纵向 SD OCT 分析在不同?...
披露声明
作者 Symantas Ragauskas, 博士是 Experimentica Ltd. 的一名雇员 (研究科学家) 和股东, 提供合同研究服务, 采用了本文所用的临床前 CNV 模型。
作者 Eva Kielczewski 是一个员工 (研究应用工程师, oct) 的徕卡系统, 生产的 SD oct 在本文中使用。
作者约瑟夫万斯是一个雇员 (NA oct 销售总监) 的徕卡微系统, 生产的 SD oct 体系在本文中使用。约瑟夫. 万斯也是 Spective 的总裁兼董事总经理。
作者西蒙卡洛特尼斯卡娅博士是 Experimentica Ltd. 的顾问首席科学官和股东, 这是一家提供合同研究服务的临床前合同研究机构, incl. 了本文所用的临床前 CNV 模型。西蒙卡洛特尼斯卡娅, 博士也是 & p 科学, LLC, 生命科学咨询公司的首席执行官, 并担任 Dr. 约翰 p 和苔蕾丝 e. 马尔卡希在 Stritch 医学院芝加哥大学的眼科教授。这项安排的条款已根据其利益冲突政策, 由美国芝加哥大学学院审查和批准。
作者 Giedrius Kalesnykas, 博士是 Experimentica Ltd. 的一名雇员 (CEO) 和股东, 提供合同研究服务, 采用本文所用的前 CNV 模型。
致谢
作者要感谢 Yuliya Naumchuk (芝加哥大学) 和 Agne žiniauskaitė (Experimentica Ltd.) 的优秀技术和录像支持。Dr. 卡洛特尼斯卡娅的研究项目是由 Dr. 的约翰 P 和苔蕾丝马尔卡希在美国芝加哥大学的眼科教授的支持。
材料
| Name | Company | Catalog Number | Comments |
| Medetomidine (commercial name Domitor) | Orion | Vnr 01 56 02 | Anesthesia |
| Ketamine | Intervet | Vnr 51 14 85 | Anesthesia |
| 0,9% NaCl | B Braun | 357 0340 | Anesthesia |
| Xylazine (commercial name Rompun vet) | Bayer | vnr 14 89 99 | Anesthesia |
| Tropicamide | Santen | Vnr 04 12 36 | Mydriatic agent |
| Viscotears | Alcon | Vnr 44 54 81 | Lubricant |
| Systane | Alcon | - | Lubricant |
| 5% Fluorescein sodium salt | Sigma Aldrich | F6377-100G | Fluoresent agent |
| Atipamezole (commercial name Antisedan) | Orion | Vnr 47 19 53 | Anesthesia |
参考文献
- Dobi, E. T., Puliafito, C. A., Destro, M. A new model of experimental choroidal neovascularization in the rat. Arch. Ophthalmol. Chic. Ill 1960. 107, 264-269 (1989).
- Tobe, T., et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest. Ophthalmol. Vis. Sci. 39, 180-188 (1998).
- Seo, M. S., et al. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am. J. Pathol. 154, 1743-1753 (1999).
- Grossniklaus, H. E., Kang, S. J., Berglin, L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 29, 500-519 (2010).
- Shah, R. S., Soetikno, B. T., Lajko, M., Fawzi, A. A. A Mouse Model for Laser-induced Choroidal Neovascularization. J Vis Exp. (106), e53502 (2015).
- Giani, A., et al. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52, 3880-3887 (2011).
- Gong, Y., et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PloS One. 10, e0132643 (2015).
- Sheets, K. G., et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol. Vis. 16, 320-329 (2010).
- Hoerster, R., et al. In-vivo and ex-vivo characterization of laser-induced choroidal neovascularization variability in mice. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 250, 1579-1586 (2012).
- Sulaiman, R. S., et al. A Simple Optical Coherence Tomography Quantification Method for Choroidal Neovascularization. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. 31, 447-454 (2015).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。