Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
In Vivo Multimodal Imaging and Analysis of Mouse Laser-Induced Choroidal Neovascularization Model
W tym Artykule
Podsumowanie
Here, we present the usefulness of longitudinal in vivo imaging in the follow-up of morphological changes of laser-induced choroidal neovascularization in mice.
Streszczenie
Laser-induced choroidal neovascularization (CNV) is a well-established model to mimic the wet form of age-related macular degeneration (AMD). In this protocol, we aim to guide the reader not simply through the technical considerations of generating laser-induced lesions to trigger neovascular processes, but rather focus on the powerful information that can be obtained from multimodal longitudinal in vivo imaging throughout the follow-up period.
The laser-induced mouse CNV model was generated by a diode laser administration. Multimodal in vivo imaging techniques were used to monitor CNV induction, progression and regression. First, spectral domain optical coherence tomography (SD-OCT) was performed immediately after the lasering to verify a break of Bruch's membrane. Subsequent in vivo imaging using fluorescein angiography (FA) confirmed successful damage of Bruch's membrane from serial images acquired at the choroidal level. Longitudinal follow-up of CNV proliferation and regression on days 5, 10, and 14 after the lasering was performed using both SD-OCT and FA. Simple and reliable grading of leaky CNV leasions from FA images is presented. Automated segmentation for measurement of total retinal thickness, combined with manual caliber application for measurement of retinal thickness at CNV sites, allow unbiased evaluation of the presence of edema. Finally, histological verification of CNV is performed using isolectin GS-IB4 staining on choroidal flatmounts. The staining is thresholded, and the isolectin-positive area is calculated with ImageJ.
This protocol is especially useful in therapeutics studies requiring high-throughput-like screening of CNV pathology as it allows fast, multimodal, and reliable classification of CNV pathology and retinal edema. In addition, high resolution SD-OCT enables the recording of other pathological hallmarks, such as the accumulation of subretinal or intraretinal fluid. However, this method does not provide a possibility to automate CNV volume analysis from SD-OCT images, which has to be performed manually.
Wprowadzenie
The first successful attempt to mimic the pathology of human CNV in rodents was demonstrated almost three decades ago with a krypton laser in Long-Evans rats1. Thereafter, a krypton laser was used to break Bruch's membrane in the most popular mouse strain, C57BL/6J2,3,4. The success rate of CNV induction was verified with FA and histological stains. A rapid development of noninvasive imaging modalities, such as OCT, fostered the growth of the field of rodent preclinical models. The ability to monitor morphological changes in the retina at multiple time points in the same eye significantly contributes to the reduction of animal use, and increases efficiency in experimental studies. Histological evaluation of CNV lesions is rather straightforward, and requires labeling of abnormal vascular growth around the site of laser administration, image acquisition, and area/volume estimation using an image analysis software. In contrast, in vivo imaging modalities introduce more complex analyses of CNV pathology and its interpretation.
Here we present a simple and relatively fast method to grade induction, progression, and regression of CNV using FA, SD-OCT, and the automated segmentation method in the mouse laser-induced CNV model.
Protokół
All animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the EC Directive 86/609/EEC for animal experiments, using protocols approved and monitored by the Animal Experiment Board of Finland.
1. Laser-induced mouse CNV model 5
- Inspect the eyes of the animal macroscopically for any abnormalities.
- Weigh the mouse.
- Calculate and prepare an appropriate amount of anesthetics to use, based on the weight of the animal, e.g. a mixture of medetomidine (1 mg/kg), ketamine (75 mg/kg), and distilled water (0.9% NaCl solution) at a ratio of 1:1.5:2.5, or ketamine (40-75 mg/kg), xylazine (5 mg/kg), and distilled water (0.9% NaCl solution) at a ratio of 1:2.5:1; for a 20 g mouse, inject 0.1 mL of mixture.
- Inject anesthetic intraperitoneally.
- Place mouse back into the cage and wait until animal is anesthetized. Confirm the mouse is properly anesthetized by lack of a pedal reflex.
- Ensure the use of laser safety personal protective equipment.
- Turn on a slit lamp and a 532 nm diode laser.
- Remove the mouse from the cage and place on the heating pad.
- Apply one drop of Tropicamide for pupillary dilation. Wait for 3-5 min for full (3 mm) pupillary dilation.
- Place the mouse on the stage of the slit lamp.
- Place one drop of opthalmic liquid gel on a coverslip to applanate the cornea.
- Orient the mouse eye with the optic nerve head in the center.
- Set the laser power to 100 mW, the duration to 100 ms, and the spot size to 50 µm.
- Focus the laser beam on the retinal pigment epithelium (RPE).
- Make three laser shots into one eye by avoiding retinal blood vessels ideally at the 4, 8, and 12 o'clock positions around the optic nerve, respectively. Inspect the fundus of the eye after all laser shots for absence of retinal bleeding. The contralateral eye serves as a non-lasered control.
- Discard the coverslip and place mouse back on the heating pad.
- Apply one drop of PEG gel drops on both eyes.
2. SD-OCT 6,7
- Place the mouse into the rodent alignment stage, and immobilize the head.
- Align the lens of the SD-OCT system (e.g., Bioptigen/Leica Envisu R2200) to face the eye for in vivo imaging using X- and Y-stage controllers.
- Perform SD-OCT scans to verify breaks of Bruch's membrane: once the SD-OCT scans the whole eye, manually move the reference line on the lasered sites. Breaks of Bruch's membrane should be clearly visible in lasered areas (see Figure 1).
3. Fluorescein angiography 7,8,9
- Remove mouse with the holder, and place it on the FA system (e.g., Heidelberg Spectralis HRA2).
- Focus at laser burn areas of the fundus of the eye using infrared reflectance mode with the head of the optic nerve in the middle of the viewing window.
- Inject 0.1 mL of 5% fluorescein sodium salt for a 20 g mouse subcutaneously or intraperitoneally.
- Focus on the choroidal level.
- Take an image from the choroidal focus level.
- Re-focus at the retinal level and take an image.
- Wait for 30 s and repeat steps 3.4-3.6.
- Remove the mouse from the holder and place it on the heating pad.
- Reverse anesthesia by α2-antagonist for medetomidine, atipamezole (0.5 mg/kg, i.p.), or wait for animal recovery from anesthesia.
- Repeat in vivo SD-OCT and FA imaging in anesthetized animals on the follow-up days 5, 10, and 14.
4. CNV Grading
- Grade the damage of Bruch's membrane from OCT images and choroidal FA images taken immediately after the lasering on day 0 as following:
0 - Bruch's membrane was not damaged
1 - successful damage of Bruch's membrane - Grade the presence of CNV from lasered spots that had leakage as observed by comparing dynamics of fluorescein signal in a series of retinal FA images as following:
0 - normal appearance of retina
0.5 - faint staining of leakage
1.0 - leaky CNV areas
Note: Use the OCT imaging for additional confirmation of CNV or in questionable FA where the presence of intraretinal fluid in OCT images would suggest CNV grading.
5. Retinal Thickness Measurements
- Use an automated segmentation software for retinal thickness measurements. Ensure that the total retinal thickness is considered, as the thickness of all layers from nerve fiber layer to RPE (healthy measurement sites), or to an imaginary line connecting RPE around the site of damage (lasered sites) (see also Figure 7).
Wyniki
A bubble or subretinal bleeding immediately after lasering is not always visible. Therefore, SD-OCT is particularly important to verify damage of Bruch's membrane. Figure 1 shows an example of OCT imaging at different time points after laser administration.
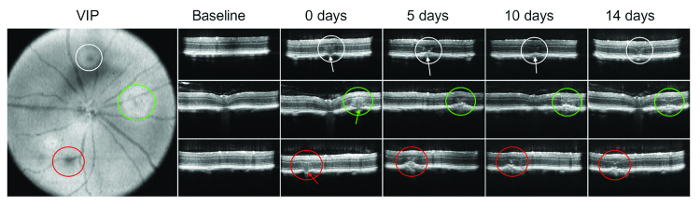
Figure 1: OCT...
Dyskusje
Multimodal imaging offers valuable tools for CNV pathology evaluation. Here we presented an imaging protocol consisting of FA, SD-OCT, and automatic segmentation for the quick, reproducible, and reliable evaluation of CNV pathology. A break of Bruch's membrane after laser administration was confirmed. In addition, the use of SD-OCT at this stage also allowed immediate visualization of possible intraretinal and subretinal hemorrhages, which may confound the interpretation of results. Retinal leaks were graded based on the...
Ujawnienia
The author Symantas Ragauskas, Ph.D. is an employee (research scientist) and shareholder of Experimentica Ltd. that offers contract research services employing the preclinical CNV model used in this Article.
The author Eva Kielczewski is an employee (research applications engineer, OCT) of Leica Microsystems that produces SD-OCT systems used in this Article.
The author Joseph Vance is an employee (NA OCT Sales Director) of Leica Microsystems that produces SD-OCT systems used in this Article. Joseph Vance is also president and managing director of Spective, LLC.
The author Simon Kaja, Ph.D. is consultant Chief Scientific Officer and shareholder of Experimentica Ltd., a preclinical contract research organization that offers contract research services, incl. the preclinical CNV model used in this article. Simon Kaja, Ph.D. is also CEO of K&P Scientific, LLC, a life sciences consulting firm, and serves as the Dr. John P. and Therese E. Mulcahy Endowed Professor in Ophthalmology at Loyola University Chicago, Stritch School of Medicine. The terms of this arrangement have been reviewed and approved by Loyola University Chicago in accordance with its conflict of interest policy.
The author Giedrius Kalesnykas, Ph.D. is an employee (CEO) and shareholder of Experimentica Ltd. that offers contract research services employing the preclinical CNV model used in this Article.
Podziękowania
The authors would like to thank Yuliya Naumchuk (Loyola University Chicago) and Agne Žiniauskaitė (Experimentica Ltd.) for excellent technical and videographic support. Dr. Kaja’s research program is supported by the Dr. John P. and Therese E. Mulcahy Endowed Professorship in Ophthalmology at Loyola University Chicago.
Materiały
| Name | Company | Catalog Number | Comments |
| Medetomidine (commercial name Domitor) | Orion | Vnr 01 56 02 | Anesthesia |
| Ketamine | Intervet | Vnr 51 14 85 | Anesthesia |
| 0,9% NaCl | B Braun | 357 0340 | Anesthesia |
| Xylazine (commercial name Rompun vet) | Bayer | vnr 14 89 99 | Anesthesia |
| Tropicamide | Santen | Vnr 04 12 36 | Mydriatic agent |
| Viscotears | Alcon | Vnr 44 54 81 | Lubricant |
| Systane | Alcon | - | Lubricant |
| 5% Fluorescein sodium salt | Sigma Aldrich | F6377-100G | Fluoresent agent |
| Atipamezole (commercial name Antisedan) | Orion | Vnr 47 19 53 | Anesthesia |
Odniesienia
- Dobi, E. T., Puliafito, C. A., Destro, M. A new model of experimental choroidal neovascularization in the rat. Arch. Ophthalmol. Chic. Ill 1960. 107, 264-269 (1989).
- Tobe, T., et al. Evolution of neovascularization in mice with overexpression of vascular endothelial growth factor in photoreceptors. Invest. Ophthalmol. Vis. Sci. 39, 180-188 (1998).
- Seo, M. S., et al. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am. J. Pathol. 154, 1743-1753 (1999).
- Grossniklaus, H. E., Kang, S. J., Berglin, L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 29, 500-519 (2010).
- Shah, R. S., Soetikno, B. T., Lajko, M., Fawzi, A. A. A Mouse Model for Laser-induced Choroidal Neovascularization. J Vis Exp. (106), e53502 (2015).
- Giani, A., et al. In vivo evaluation of laser-induced choroidal neovascularization using spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 52, 3880-3887 (2011).
- Gong, Y., et al. Optimization of an Image-Guided Laser-Induced Choroidal Neovascularization Model in Mice. PloS One. 10, e0132643 (2015).
- Sheets, K. G., et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol. Vis. 16, 320-329 (2010).
- Hoerster, R., et al. In-vivo and ex-vivo characterization of laser-induced choroidal neovascularization variability in mice. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 250, 1579-1586 (2012).
- Sulaiman, R. S., et al. A Simple Optical Coherence Tomography Quantification Method for Choroidal Neovascularization. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. 31, 447-454 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone