Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Monitorización de la evolución mecánica del tejido durante el cierre del tubo neural de un embrión de pollo
En este artículo
Resumen
Este protocolo se desarrolló para monitorizar longitudinalmente las propiedades mecánicas del tejido de la placa neural durante la neurulación del embrión de pollo. Se basa en la integración de un microscopio Brillouin y un sistema de incubación en el escenario, lo que permite obtener imágenes mecánicas en vivo del tejido de la placa neural en embriones de pollo cultivados ex ovo .
Resumen
El cierre del tubo neural (NTC) es un proceso crítico durante el desarrollo embrionario. El fallo en este proceso puede dar lugar a defectos del tubo neural, causando malformaciones congénitas o incluso la mortalidad. La NTC involucra una serie de mecanismos a nivel genético, molecular y mecánico. Si bien la regulación mecánica se ha convertido en un tema cada vez más atractivo en los últimos años, sigue siendo en gran medida inexplorado debido a la falta de tecnología adecuada para realizar pruebas mecánicas de tejido embrionario 3D in situ. En respuesta, hemos desarrollado un protocolo para cuantificar las propiedades mecánicas del tejido embrionario de pollo de una manera no invasiva y sin contacto. Esto se logra mediante la integración de un microscopio confocal de Brillouin con un sistema de incubación en el escenario. Para sondear la mecánica de los tejidos, se recolecta un embrión precultivado y se transfiere a una incubadora en el escenario para el cultivo ex ovo . Simultáneamente, las imágenes mecánicas del tejido de la placa neural son adquiridas por el microscopio de Brillouin en diferentes momentos durante el desarrollo. Este protocolo incluye descripciones detalladas de la preparación de las muestras, la implementación de experimentos de microscopía de Brillouin y el posprocesamiento y análisis de datos. Siguiendo este protocolo, los investigadores pueden estudiar longitudinalmente la evolución mecánica del tejido embrionario durante el desarrollo.
Introducción
Los defectos del tubo neural (DTN) son defectos congénitos graves del sistema nervioso central causados por fallos en el cierre del tubo neural (NTC) duranteel desarrollo embrionario. La etiología de las enfermedades tropicales desatendidas es compleja. Los estudios han demostrado que el NTC implica una secuencia de procesos morfogenéticos, que incluyen la extensión convergente, la flexión de la placa neural (por ejemplo, la constricción apical), la elevación del pliegue neural y, finalmente, la adhesión del pliegue neural. Estos procesos están regulados por múltiples mecanismos moleculares y genéticos 2,3, y cualquier mal funcionamiento en estos procesos puede dar lugar a defectos del tubo neural 4,5,6. Dado que cada vez hay más pruebas que sugieren que las señales mecánicas también desempeñan un papel crucial durante la NTC 3,7,8,9,10,11, y se han encontrado relaciones entre los genes y las señales mecánicas 12,13,14, se hace imperativo investigar la biomecánica tisular durante la neurulación.
Se han desarrollado varias técnicas para medir las propiedades mecánicas de los tejidos embrionarios, entre las que se encuentran la ablación con láser (LA)15, la disección y relajación de tejidos (TDR)16,17, la aspiración con micropipetas (MA)18, la nanoindentación basada en microscopía de fuerza atómica (AFM)19, los microindentadores (MI) y las microplacas (MP)20, la microrreología (RM) con pinzas ópticas/magnéticas 21,22,23y sensores basados en gotas24. Los métodos existentes pueden medir propiedades mecánicas con resoluciones espaciales que van desde escalas subcelulares hasta tisulares. Sin embargo, la mayoría de estos métodos son invasivos porque requieren contacto con la muestra (p. ej., MA, AFM, MI y MP), inyección de material externo (p. ej., RM y sensores basados en gotas) o disección de tejidos (p. ej., LA y TDR). Como resultado, es un desafío para los métodos existentes monitorear la evolución mecánica del tejido de la placa neural in situ25. Recientemente, la elastografía de coherencia óptica reverberante se ha mostrado prometedora para el mapeo mecánico sin contacto con alta resolución espacial26.
La microscopía confocal de Brillouin es una modalidad óptica emergente que permite la cuantificación sin contacto de la biomecánica tisular con resolución subcelular 27,28,29,30. La microscopía de Brillouin se basa en el principio de dispersión espontánea de la luz de Brillouin, que es la interacción entre la luz láser incidente y la onda acústica inducida por las fluctuaciones térmicas dentro del material. En consecuencia, la luz dispersa experimenta un cambio de frecuencia, conocido como desplazamiento de Brillouin ωR, siguiendo la ecuación31:
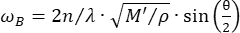 (1)
(1)
Aquí, 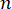 es el índice de refracción del material, λ es la longitud de onda de la luz incidente, M' es el módulo longitudinal, ρ es la densidad de masa y θ es el ángulo entre la luz incidente y la luz dispersa. Para el mismo tipo de materiales biológicos, la relación entre el índice de refracción y la densidad
es el índice de refracción del material, λ es la longitud de onda de la luz incidente, M' es el módulo longitudinal, ρ es la densidad de masa y θ es el ángulo entre la luz incidente y la luz dispersa. Para el mismo tipo de materiales biológicos, la relación entre el índice de refracción y la densidad 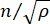 es aproximadamente constante 28,32,33,34,35,36. Por lo tanto, el desplazamiento de Brillouin se puede utilizar directamente para estimar los cambios mecánicos relativos en los procesos fisiológicos. La factibilidad de la microscopía de Brillouin ha sido validada en diversas muestras biológicas 29,37,38. Recientemente, se demostró la obtención de imágenes mecánicas de lapso de tiempo de un embrión de pollito vivo mediante la combinación de un microscopio Brillouin con un sistema de incubación en el escenario39. Este protocolo proporciona descripciones detalladas de la preparación de la muestra, la implementación del experimento y el posprocesamiento y análisis de los datos. Esperamos que este esfuerzo facilite la adopción generalizada de la tecnología Brillouin sin contacto para estudiar la regulación biomecánica en el desarrollo embrionario y los defectos congénitos.
es aproximadamente constante 28,32,33,34,35,36. Por lo tanto, el desplazamiento de Brillouin se puede utilizar directamente para estimar los cambios mecánicos relativos en los procesos fisiológicos. La factibilidad de la microscopía de Brillouin ha sido validada en diversas muestras biológicas 29,37,38. Recientemente, se demostró la obtención de imágenes mecánicas de lapso de tiempo de un embrión de pollito vivo mediante la combinación de un microscopio Brillouin con un sistema de incubación en el escenario39. Este protocolo proporciona descripciones detalladas de la preparación de la muestra, la implementación del experimento y el posprocesamiento y análisis de los datos. Esperamos que este esfuerzo facilite la adopción generalizada de la tecnología Brillouin sin contacto para estudiar la regulación biomecánica en el desarrollo embrionario y los defectos congénitos.
Protocolo
El protocolo ha sido aprobado por el Comité Institucional de Cuidado y Uso de Animales de la Universidad Estatal de Wayne.
1. Preparación experimental
- Use una solución de etanol al 70% para limpiar y esterilizar las tijeras y pinzas. Además, prepare pipetas desechables y una jeringa.
- Prepare un medio de lavado añadiendo 3,595 g de NaCl a 495 ml de agua desionizada. A continuación, añadir 5 ml de penicilina-estreptomicina (5 U/mL) al medio. Llene una placa de Petri de 100 mm con el medio de lavado y caliéntela a 37 °C.
- Prepare platos de cultivo de acuerdo con la Figura 1, que ilustra la configuración general.
- Prepara un trozo de papel de filtro, córtalo en forma rectangular de aproximadamente 17 mm × 20 mm y retira las cuatro esquinas. Cree un centro hueco en el papel de filtro, de aproximadamente 7 mm × 10 mm de tamaño para asegurar el embrión (Figura 1, izquierda).
NOTA: La recolección de embriones se describe en el paso 2. - Fije un anillo comercial disponible (Φ = 1 pulgada, consulte la Tabla de materiales) con una capa de película flexible (es decir, película de parafina), asegurándose de que la película flexible también tenga un centro hueco. Este anillo con la película se utilizará para sujetar el papel de filtro con el embrión.
- Finalmente, coloque el anillo preparado en una placa de Petri de 35 mm (Figura 1, derecha).
NOTA: El papel de filtro sirve para asegurar y sujetar el embrión extraído colocando el papel de filtro sobre la membrana y dejando el embrión en la zona hueca central (que se detallará en el paso 2). Las cuatro esquinas se cortaron para adaptarse al tamaño del anillo exterior (no es necesario si ya está instalado). Se utiliza una placa de cultivo con fondo de vidrio de 35 mm para una mejor propagación del rayo láser. La placa tiene un pocillo interior (Φ = 23 mm), que se puede llenar con albúmina para el cultivo ex ovo . El diámetro del anillo debe ser mayor que el diámetro del pozo interior para que el pozo pueda cubrirse con la película flexible del anillo (Figura 1, recuadro). El tamaño del papel de filtro no está restringido y se puede modificar según el tamaño del anillo elegido y la placa de cultivo. Alternativamente, el fondo del plato se puede recubrir previamente con una capa de agarosa (grosor: ~ 1 mm). Al eliminar la agarosa del centro de la capa con una cuchilla, se puede crear un pocillo con una forma similar al centro hueco de la película flexible para cargar la albúmina. Un punto crítico es que los cuatro lados del papel de filtro hueco deben tener suficiente ancho (> 5 mm) para asegurar la adhesión del embrión.
- Prepara un trozo de papel de filtro, córtalo en forma rectangular de aproximadamente 17 mm × 20 mm y retira las cuatro esquinas. Cree un centro hueco en el papel de filtro, de aproximadamente 7 mm × 10 mm de tamaño para asegurar el embrión (Figura 1, izquierda).
2. Extracción y cultivo ex ovo del embrión de pollo
NOTA: Este paso es una modificación de los informes publicados anteriormente40,41.
- Después del precultivo, saque el huevo de la incubadora y colóquelo en su eje largo en la bandeja del cartón de huevos. Limpiar la cáscara de huevo con etanol al 70%, asegurándose de cubrir toda la superficie, y luego dejar reposar durante 15 min.
- Sostén el huevo en su eje corto y rómpelo por la parte inferior. Abra el huevo sobre una placa de Petri limpia de 100 mm para extraer su contenido, como se ilustra en la Figura 2. Asegúrese de no girar el huevo mientras lo sostiene y lo rompe.
- Con una pipeta, transfiera y recoja aproximadamente 10 ml de la albúmina delgada (es decir, la albúmina líquida42) en un tubo de 15 ml para su uso en cultivo ex ovo . Llene el pocillo central de la placa de cultivo con la albúmina delgada recolectada (aproximadamente 0,9 ml), como se muestra en la Figura 3. El proceso de llenado debe ser lento, evitando la formación de burbujas en el interior del pocillo. Asegúrese de que la placa de cultivo con albúmina se caliente a 37 °C.
NOTA: Este plato se utilizará para cultivar el embrión ex ovo. Se utilizarán nuevas placas de cultivo llenas de medio de lavado durante la obtención de imágenes de Brillouin (consulte el paso 3). - Con papel de seda, retire suavemente la albúmina gruesa (es decir, la albúmina viscosa) adherida al embrión separando cuidadosamente la albúmina de la membrana vitelina, como se muestra en la Figura 4. Evite el contacto directo con la membrana vitelina.
- Después de eliminar toda la albúmina gruesa, pegue con cuidado el papel de filtro a la membrana vitelina. Asegúrese de que el eje del cuerpo del embrión esté alineado con el eje largo del rectángulo central en el papel de filtro. Usa unas tijeras para cortar la membrana que rodea el papel de filtro.
- Con unas pinzas, tire suavemente del papel de filtro aislado para separarlo de la yema en dirección oblicua. Dale la vuelta al papel de filtro para colocar el embrión con la parte dorsal hacia abajo (para la configuración del microscopio invertido). Sumerja con cuidado todo el papel de filtro de un lado del eje largo en la placa de Petri de 100 mm con el medio de lavado de forma oblicua.
- Lave la yema restante rociando suavemente el medio de lavado paralelo al papel de filtro con una pipeta limpia. Evite rociar directamente el medio de lavado sobre la membrana.
- Después de limpiar toda la yema, retire con cuidado el papel de filtro del medio de lavado y use papel de seda para absorber el exceso de medio de los bordes. A continuación, coloque el papel de filtro con el embrión en la placa de cultivo, asegurándose de que la cara dorsal del embrión esté hacia abajo, como se ilustra en la Figura 5. Para mantener la humedad, coloque un papel de seda humedecido en el plato.
- Transfiera la placa de cultivo a la incubadora en el escenario para el cultivo ex ovo .
3. Medición de Brillouin del embrión
- Prepare otro juego de platos de cultivo y llénelos con medio de lavado. Asegúrese de que el medio de lavado esté calentado a 37 °C.
- Cuando el embrión alcance la etapa de desarrollo deseada, transfiera el papel de filtro con el embrión a la placa de cultivo llena de medio de lavado. Coloque la placa de cultivo en la incubadora del microscopio Brillouin (consulte la tabla de materiales).
- Antes de realizar la medición de Brillouin, guarde una imagen de campo claro de todo el embrión como referencia. La configuración detallada del microscopio de Brillouin ha sido descrita previamente30 y se resume en la sección de Resultados (Figura 6).
- Mida la señal de Brillouin del agua y el metanol, que se utilizará en el paso 4 para el proceso de calibración.
- Ajuste la potencia del láser incidente y el tiempo de exposición de la cámara del dispositivo de carga acoplada multiplicadora de electrones (EMCCD, consulte la Tabla de materiales) para lograr al menos 10,000 recuentos de señal de Brillouin. Usando la imagen de campo claro como guía, configure el rango de escaneo y el tamaño del paso. Adquiera una imagen de Brillouin de la región de interés escaneando el embrión utilizando una etapa de traslación 2D.
NOTA: El rango de escaneo horizontal se puede determinar en función de la imagen de campo claro, y el rango de escaneo vertical (es decir, de profundidad) se puede determinar en función de la intensidad de la señal obtenida de un escaneo rápido y grueso. Para evitar cualquier fotodaño al embrión, limite la potencia incidente a 25 mW y ajuste el tiempo de exposición de la cámara EMCCD a 50 ms. Elija un tamaño de paso de 2 μm en la dirección horizontal y de 1 μm en la dirección vertical para equilibrar la calidad de la imagen y el tiempo de adquisición. - Después de completar el escaneo, retire con cuidado el papel de filtro con el embrión y use papel de seda para absorber el exceso de medio de lavado. A continuación, vuelva a colocar el papel de filtro en la placa de cultivo llena de albúmina fina para un cultivo continuo en la incubadora del escenario.
- Repita los pasos 3.2-3.5 a intervalos de tiempo regulares (p. ej., 1.5 h) para capturar imágenes de Brillouin de lapso de tiempo a medida que el embrión se desarrolla.
- Reconstruye la imagen 2D de Brillouin siguiendo el paso 4.
4. Reconstrucción de la imagen de Brillouin
- Calibrar el espectrómetro de Brillouin utilizando señales de agua y metanol de Brillouin para calcular el rango espectral libre (FSR) y la relación de conversión de píxel a frecuencia (PR) del espectrómetro30. Los valores calibrados de FSR y PR se utilizarán para calcular el desplazamiento de Brillouin del embrión en cada píxel.
NOTA: La Figura 7A muestra un espectro de Brillouin sin procesar capturado por la cámara EMCCD. Sumando verticalmente el espectro y luego haciendo un ajuste lorentziano, se puede obtener la distancia de pico Δd de los dos puntos (Figura 7B). Al obtener la distancia máxima del agua Δdagua y el metanol Δdmetanol, la FSR y la PR se pueden calcular sobre la base de las ecuaciones30: PR = 2· (ωmetanol-ω agua)/(Δdagua- Δdmetanol), y FSR = 2·ωagua + PR·Δdagua, con el conocido desplazamiento de Brillouin del agua ωagua = 6,01 GHz y metanol ωmetanol = 4,49 GHz a 660 nm. - Obtener la distancia máxima de la señal de Brillouin en cada píxel de la muestra. Calcule el desplazamiento de Brillouin en función de la FSR y la PR calibradas: ωmuestra = 0,5 (FSR - PR xΔd muestra)30, donde ωmuestra es el desplazamiento de Brillouin de la muestra, yΔd muestra es la distancia máxima de la señal de Brillouin.
- Reconstruya la imagen 2D de Brillouin basándose en los desplazamientos de Brillouin de todos los píxeles.
NOTA: Para realizar un análisis local, se puede seleccionar una región de interés (por ejemplo, la placa neuronal) de la imagen de Brillouin adquirida y cuantificar sus propiedades mecánicas. Un enfoque común es calcular el desplazamiento promedio de Brillouin de la región seleccionada.
Resultados
La figura 6 muestra el esquema del microscopio Brillouin. El sistema emplea un láser de 660 nm como fuente de luz. Se coloca un aislador justo después del cabezal láser para rechazar cualquier luz reflejada, y se utiliza un filtro de densidad neutra (ND) para ajustar la potencia del láser. Un par de lentes, L1 y L2, con distancias focales de f1 = 16 mm y f2 = 100 mm, respectivamente, se utilizan para expandir el rayo láser. Se emplea una placa de media onda (HWP) y un polarizador lineal...
Discusión
El desarrollo temprano del embrión puede verse fácilmente afectado por alteraciones externas. Por lo tanto, se requiere la máxima precaución durante la extracción y transferencia de muestras. Un problema potencial es el desprendimiento del embrión del papel de filtro, lo que puede conducir a la contracción de la membrana vitelina y dar lugar a un artefacto inclinado de la placa neural en las imágenes de Brillouin. Además, este encogimiento puede detener el desarrollo del embrión. Se debe prestar atención a var...
Divulgaciones
Los autores declaran que no tienen ningún conflicto de intereses.
Agradecimientos
Este trabajo cuenta con el apoyo del Instituto Nacional de Salud Infantil y Desarrollo Humano Eunice Kennedy Shriver, Institutos Nacionales de Salud (K25HD097288, R21HD112663).
Materiales
| Name | Company | Catalog Number | Comments |
| 100 mm Petri dish | Fisherbrand | FB0875713 | |
| 2D motorized stage | Prior Scientific | H117E2 | |
| 35 mm Petri dish | World Precision Instruments | FD35-100 | |
| Brillouin Microscope with on-stage incubator | N/A | N/A | This is a custom-built Brillouin Microscope system based on Ref. 30 |
| Chicken eggs | University of Connecticut | N/A | |
| CMOS camera | Thorlabs | CS2100M-USB | |
| EMCCD camera | Andor | iXon | |
| Ethanol | Decon Laboratories, Inc. | #2701 | |
| Filter paper | Whatman | 1004-070 | |
| Incubator for in ovo culture | GQF Manufacturing Company Inc. | GQF 1502 | |
| Ring | Thorlabs | SM1RR | |
| Microscope body | Olympus | IX73 | |
| NaCl | Sigma-Aldrich | S9888 | |
| On-stage incubator | Oko labs | OKO-H301-PRIOR-H117 | |
| Parafilm | Bemis | PM-996 | |
| Penicillin-Streptomycin | Gibco | 15070-063 | |
| Pipettes | Fisherbrand | 13-711-6M | |
| Scissors | Artman instruments | N/A | 3pc Micro Scissors 5 |
| Syringe | BD | 305482 | |
| Tissue paper | Kimwipes | N/A | |
| Tube | Corning | 430052 | |
| Tweezers | DR Instruments | N/A | Microdissection Forceps Set |
Referencias
- Greene, N. D. E., Copp, A. J. Neural tube defects. Annual Review of Neuroscience. 37 (1), 221-242 (2014).
- Suzuki, M., Morita, H., Ueno, N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Development, Growth & Differentiation. 54 (3), 266-276 (2012).
- Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D. E., Copp, A. J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development. 144 (4), 552-566 (2017).
- Juriloff, D. M., Harris, M. J. Mouse models for neural tube closure defects. Human Molecular Genetics. 9 (6), 993-1000 (2000).
- Copp, A. J., Greene, N. D. E. Genetics and development of neural tube defects. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 220 (2), 217-230 (2010).
- Wang, M., De Marco, P., Capra, V., Kibar, Z. Update on the role of the non-canonical wnt/planar cell polarity pathway in neural tube defects. Cells. 8 (10), 1198 (2019).
- Galea, G. L., et al. Biomechanical coupling facilitates spinal neural tube closure in mouse embryos. Proceedings of the National Academy of Sciences. 114 (26), E5177-E5186 (2017).
- Moon, L. D., Xiong, F. Mechanics of neural tube morphogenesis. Seminars in Cell & Developmental Biology. 130, 56-69 (2022).
- Christodoulou, N., Skourides, P. A. Distinct spatiotemporal contribution of morphogenetic events and mechanical tissue coupling during xenopus neural tube closure. Development. 149 (13), (2022).
- De Goederen, V., Vetter, R., Mcdole, K., Iber, D. Hinge point emergence in mammalian spinal neurulation. Proceedings of the National Academy of Sciences. 119 (20), 2117075119 (2022).
- Christodoulou, N., Skourides, P. A. Somitic mesoderm morphogenesis is necessary for neural tube closure during xenopus development. Frontiers in Cell and Developmental Biology. 10, 1091629 (2023).
- Nikolopoulou, E., et al. Spinal neural tube closure depends on regulation of surface ectoderm identity and biomechanics by grhl2. Nature Communications. 10 (1), 2487 (2019).
- Nychyk, O., et al. Vangl2-environment interaction causes severe neural tube defects, without abnormal neuroepithelial convergent extension. Disease Models & Mechanisms. 15 (1), 049194 (2022).
- Li, B., Brusman, L., Dahlka, J., Niswander, L. A. Tmem132a ensures mouse caudal neural tube closure and regulates integrin-based mesodermal migration. Development. 149 (17), (2022).
- Zulueta-Coarasa, T., Fernandez-Gonzalez, R. Laser ablation to investigate cell and tissue mechanics in vivo. Integrative Mechanobiology: Micro-and Nano Techniques in Cell Mechanobiology. , 128-147 (2015).
- Wiebe, C., Brodland, G. W. Tensile properties of embryonic epithelia measured using a novel instrument. Journal of Biomechanics. 38 (10), 2087-2094 (2005).
- Luu, O., David, R., Ninomiya, H., Winklbauer, R. Large-scale mechanical properties of xenopus embryonic epithelium. Proceedings of the National Academy of Sciences. 108 (10), 4000-4005 (2011).
- Maître, J. L., Niwayama, R., Turlier, H., Nédélec, F., Hiiragi, T. Pulsatile cell-autonomous contractility drives compaction in the mouse embryo. Nature Cell Biology. 17 (7), 849-855 (2015).
- Krieg, M., et al. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biology. 10 (4), 429-436 (2008).
- Zamir, E. A., Srinivasan, V., Perucchio, R., Taber, L. A. Mechanical asymmetry in the embryonic chick heart during looping. Annals of Biomedical Engineering. 31, 1327-1336 (2003).
- Bambardekar, K., Clément, R., Blanc, O., Chardès, C., Lenne, P. F. Direct laser manipulation reveals the mechanics of cell contacts in vivo. Proceedings of the National Academy of Sciences. 112 (5), 1416-1421 (2015).
- Savin, T., et al. On the growth and form of the gut. Nature. 476 (7358), 57-62 (2011).
- Almonacid, M., et al. Active diffusion positions the nucleus in mouse oocytes. Nature Cell Biology. 17 (4), 470-479 (2015).
- Campàs, O., et al. Quantifying cell-generated mechanical forces within living embryonic tissues. Nature Methods. 11 (2), 183-189 (2014).
- Campàs, O. A toolbox to explore the mechanics of living embryonic tissues. Seminars in Cell & Developmental Biology. 55, 119-130 (2016).
- Christian, Z. D., et al. High-resolution 3D biomechanical mapping of embryos with reverberant optical coherence elastography (Rev-OCE). Proceedings of SPIE. , 123670 (2023).
- Scarcelli, G., Yun, S. H. J. N. P. Confocal brillouin microscopy for three-dimensional mechanical imaging. Nature Photonics. 1 (1), 39-43 (2008).
- Scarcelli, G., et al. Noncontact three-dimensional mapping of intracellular hydromechanical properties by brillouin microscopy. Nature Methods. 12 (12), 1132-1134 (2015).
- Prevedel, R., Diz-Muñoz, A., Ruocco, G., Antonacci, G. Brillouin microscopy: An emerging tool for mechanobiology. Nature Methods. 16 (10), 969-977 (2019).
- Zhang, J., Scarcelli, G. Mapping mechanical properties of biological materials via an add-on brillouin module to confocal microscopes. Nature Protocols. 16 (2), 1251-1275 (2021).
- Boyd, R. W. . Nonlinear optics. , (2020).
- Scarcelli, G., Kim, P., Yun, S. H. In vivo measurement of age-related stiffening in the crystalline lens by brillouin optical microscopy. Biophysical Journal. 101 (6), 1539-1545 (2011).
- Scarcelli, G., Pineda, R., Yun, S. H. Brillouin optical microscopy for corneal biomechanics. Investigative Ophthalmology & Visual Science. 53 (1), 185-190 (2012).
- Antonacci, G., Braakman, S. Biomechanics of subcellular structures by non-invasive brillouin microscopy. Scientific Reports. 6 (1), 37217 (2016).
- Zhang, J., et al. Tissue biomechanics during cranial neural tube closure measured by brillouin microscopy and optical coherence tomography. Birth Defects Research. 111 (14), 991-998 (2019).
- Zhang, J., et al. Nuclear mechanics within intact cells is regulated by cytoskeletal network and internal nanostructures. Small. 16 (18), 1907688 (2020).
- Elsayad, K., Polakova, S., Gregan, J. Probing mechanical properties in biology using brillouin microscopy. Trends in Cell Biology. 29 (8), 608-611 (2019).
- Poon, C., Chou, J., Cortie, M., Kabakova, I. Brillouin imaging for studies of micromechanics in biology and biomedicine: From current state-of-the-art to future clinical translation. Journal of Physics: Photonics. 3 (1), 012002 (2020).
- Handler, C., Scarcelli, G., Zhang, J. Time-lapse mechanical imaging of neural tube closure in live embryo using brillouin microscopy. Scientific Reports. 13 (1), 263 (2023).
- Chapman, S. C., Collignon, J., Schoenwolf, G. C., Lumsden, A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental dynamics: an official publication of the American Association of Anatomists. 220 (3), 284-289 (2001).
- Schmitz, M., Nelemans, B. K. A., Smit, T. H. A submerged filter paper sandwich for long-term ex ovo time-lapse imaging of early chick embryos. Journal of Visualized Experiments. (118), e54636 (2016).
- Nys, Y., Guyot, N., Nys, Y., Bain, M., VanImmerseel, F. . Improving the safety and quality of eggs and egg products, vol 1: Egg chemistry, production and consumption. , 83-132 (2011).
- Berghaus, K. V., Yun, S. H., Scarcelli, G. High speed sub-ghz spectrometer for brillouin scattering analysis. Journal of Visualized Experiments. (106), e53468 (2015).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. Journal of Morphology. 88 (1), 49-92 (1951).
- Schlüßler, R., et al. Mechanical mapping of spinal cord growth and repair in living zebrafish larvae by brillouin imaging. Biophysical Journal. 115 (5), 911-923 (2018).
- Williams, R. M., Sauka-Spengler, T. Ex ovo electroporation of early chicken embryos. STAR Protocols. 2 (2), 100424 (2021).
- Chapman, S. C., Collignon, J., Schoenwolf, G. C., Lumsden, A. Improved method for chick whole-embryo culture using a filter paper carrier. Developmental Dynamics. 220 (3), 284-289 (2001).
- Edrei, E., Scarcelli, G. Adaptive optics in spectroscopy and densely labeled-fluorescence applications. Optics Express. 26 (26), 33865-33877 (2018).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoExplorar más artículos
This article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados