Se requiere una suscripción a JoVE para ver este contenido. Inicie sesión o comience su prueba gratuita.
Method Article
Electroporación basada en sustrato poroso con monitoreo de impedancia eléctrica transepitelial
En este artículo
Resumen
La electroporación de sustratos porosos (PSEP) combina una entrega consistente y de alto rendimiento con una alta viabilidad de celda. La introducción de las mediciones de impedancia eléctrica transepitelial (TEEI) proporciona información sobre los procesos intermedios de PSEP y permite la entrega sin etiquetas. Este artículo discute un método para realizar experimentos de entrega de PSEP y análisis de medición TEEI simultáneamente.
Resumen
La electroporación de sustrato poroso (PSEP) es un método emergente de electroporación que proporciona un alto rendimiento y una entrega constante. Al igual que muchos otros tipos de administración intracelular, la PSEP depende en gran medida de los marcadores fluorescentes y la microscopía fluorescente para determinar el éxito de la administración. Para obtener información sobre los pasos intermedios del proceso de electroporación, se desarrolló una plataforma PSEP con monitoreo integrado de impedancia eléctrica transepitelial (TEEI). Las células se cultivan en insertos disponibles comercialmente con membranas porosas. Después de un período de incubación de 12 h para permitir la formación de una monocapa celular completamente confluente, los insertos se colocan en medios de transfección ubicados en los pocillos del dispositivo PSEP. A continuación, las monocapas celulares se someten a una forma de onda definida por el usuario y la eficiencia de la entrega se confirma mediante microscopía fluorescente. Este flujo de trabajo se puede mejorar significativamente con mediciones TEEI entre microscopía pulsante y fluorescente para recopilar datos adicionales sobre el proceso PSEP, y estos datos adicionales de TEEI se correlacionan con métricas de entrega, como la eficiencia y la viabilidad de la entrega. Este artículo describe un protocolo para realizar PSEP con mediciones TEEI.
Introducción
La electroporación es una técnica en la que las células se exponen a un campo eléctrico, creando poros temporales en la membrana celular a través de los cualespueden pasar las cargas, incluidas las proteínas, el ARN y el ADN. La versión más utilizada es la electroporación a granel (BEP). La BEP se realiza llenando una cubeta con un electrolito que contiene millones de células, exponiendo el electrolito a alto voltaje y permitiendo que la carga entre en las células a través de la difusión oendocitosis. El BEP tiene muchas ventajas, como el alto rendimiento y los numerosos sistemas disponibles en el mercado. Sin embargo, existen limitaciones en la entrega de BEP. El posicionamiento inconsistente de la celda en relación con los electrodos y el blindaje del campo eléctrico de las celdas adyacentes causan una variabilidad significativa en la exposición al campo eléctrico durante BEP 3,4. El alto voltaje requerido para BEP también tiene un impacto negativo significativo en la viabilidad de la celda5. Desde su creación en 20116, ha habido un creciente interés en un método de electroporación llamado electroporación de sustrato poroso (PSEP), aunque a veces se le conoce con otros nombres, como electroporación localizada y nano o microelectroporación 1,7,8. A diferencia de la suspensión celular de BEP, la PSEP se lleva a cabo en células que se adhieren a un sustrato poroso. No solo se prefiere un estado adherente para la mayoría de las líneas celulares humanas9, sino que los poros del sustrato también se centran en la corriente eléctrica, localizando el potencial eléctrico transmembrana (TMP) en regiones específicas de la membrana celular10,11. Esta localización permite una reducción significativa en el voltaje aplicado, disminuyendo el daño y aumentando la viabilidad de la celda. Esta combinación de efectos ayuda a controlar el desarrollo de los poros de la membrana celular, lo que resulta en una entrega más consistente y eficiente 1,5,12.
Un estudio reciente introdujo un dispositivo PSEP con una guía de electrodos chapada en oro de seis pocillos para sostener insertos de membrana porosa disponibles comercialmente13 (Figura 1A,B), una práctica que fue introducida por primera vez por Vindis et al.14. El dispositivo puede aplicar pulsos y medir la impedancia eléctrica a través de la monocapa de la célula, conocida como impedancia eléctrica transepitelial (TEEI), en tiempo real13. La interfaz de usuario del dispositivo permite un control completo sobre la forma de onda y la polaridad de la electroporación. Es importante destacar que las mediciones de impedancia en tiempo real se pueden utilizar para predecir los resultados de la entrega sin la necesidad de reactivos costosos o marcadores fluorescentes, un concepto conocido como administración sin etiquetas15.
La plataforma PSEP consta de dos componentes eléctricos principales personalizados: el cuerpo principal del dispositivo, que alberga el generador de pulsos y el equipo de medición TEEI, y la guía de electrodos, donde se insertan los sustratos porosos y se produce la electroporación. Los diagramas para todos los componentes electrónicos personalizados e impresos en 3D se pueden encontrar en GitHub: https://github.com/YangLabUNL/PSEP-TEEI. Además de la electrónica personalizada, también se requiere una computadora para que la plataforma funcione correctamente. El software personalizado requiere MATLAB (versión 2021a o posterior) para ejecutarse y Microsoft Excel para almacenar y acceder a los datos para su análisis. El programa controla la electrónica personalizada y proporciona la interfaz gráfica de usuario (GUI) para ajustar la configuración. Estos programas también estuvieron disponibles en GitHub: https://github.com/YangLabUNL/PSEP-TEEI.
Los datos preliminares sugieren que este proceso es posible para diferentes tipos de células adherentes (Figura 1C), pero en este artículo solo se discutirá la preparación de células A431 utilizando parámetros que Brooks et al.13 encontraron óptimos para esta línea celular. Además, debido a que la carga de yoduro de propidio (PI) es citotóxica, se realizan dos experimentos, el primero con un medio de transfección de PI de alta concentración para cuantificar la eficiencia de la entrega, y el segundo con solo medios de cultivo celular para medir TEEI en escalas de tiempo más largas. Estos experimentos utilizan formas de onda de electroporación idénticas, lo que permite correlacionar los resultados (Figura 1D).
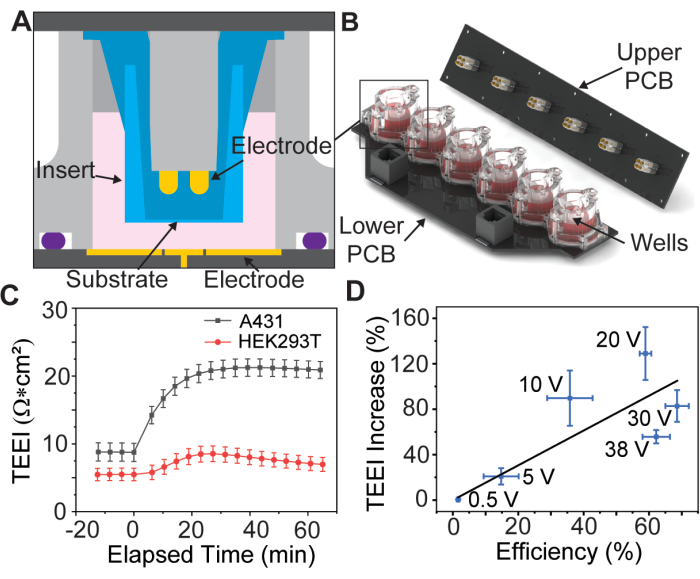
Figura 1: Diagrama de ensamblaje de la guía de electrodos y datos fundamentales. (A) Modelo CAD de un inserto dentro de un pocillo de la guía de electrodos. (B) Modelo CAD de la guía de electrodos. (C) Aumento de impedancia debido a PSEP para líneas celulares seleccionadas, n = 3 por línea celular. Barra de error: error estándar de la media. (D) Eficiencia de entrega vs. TEEI aumentar los datos de correlación. La eficiencia de la entrega se calculó dividiendo el número de células marcadas en las imágenes de PI y calceína de los experimentos de entrega por el número total de células identificadas con Hoechst. El recuento de células se determinó utilizando una canalización CellProfiler personalizada, n = 6 por voltaje. Barra de error: (eje x e y) error estándar de la media. Esta figura se reproduce de Brooks et al.13 con permiso. Haga clic aquí para ver una versión más grande de esta figura.
Protocolo
Los detalles de los reactivos y el equipo utilizado en el estudio se enumeran en la Tabla de Materiales.
1. Preparación de reactivos y cultivo celular
- Prepare el medio de cultivo celular añadiendo 50 mL de suero fetal bovino (FBS) y 5 mL de penicilina-estreptomicina a un recipiente de 500 mL de Medio Eagle Modificado de Dulbecco (DMEM). Producir once alícuotas de 50 mL para reducir el riesgo de contaminación y refrigerar a 4 °C.
- Cree 1 mL de 25 μg/mL de fibronectina plasmática humana en solución madre salina tamponada con fosfato (PBS) de acuerdo con las instrucciones del fabricante.
- Cree 15 mL de yoduro de propidio de 0,1 mg/mL en una solución madre de DMEM para permitir experimentos con diferentes concentraciones de carga.
- Cultivo de células A431 en un matraz T75 que contenga 12 mL de los medios de cultivo celular preparados. Las células se pasaron cada 1-2 días para mantener el 50% de confluencia.
2. Preparación de la muestra
- Recubrimiento de fibronectina
- Seleccione doce insertos y dos placas de 24 pocillos. Coloque los insertos en una placa de pocillo, creando dos filas de seis. Deje la segunda placa de pocillo a un lado hasta más tarde.
- Cree 1.300 μL de solución de fibronectina de 1 μg/mL mezclando 52 μL de solución madre de fibronectina y 1.248 μL de PBS en un tubo de 1,5 mL.
- Distribuya 100 μL de la solución de fibronectina en cada inserto. Incubar los insertos en la placa de pocillos a 37 °C durante 3 h.
- Ajuste de la concentración celular para optimizar la densidad celular
- Aproximadamente 1 h antes de que se complete la incubación de la fibronectina, retire el matraz T75 de células A431 de la incubadora para la extracción celular.
- Retire el medio del matraz con un aspirador y lávelo con 5 ml de PBS. Retire el PBS de la misma manera y agregue 3 mL de Tripsina. Incubar durante 3-4 minutos antes de golpear el costado del matraz para separar completamente las células.
- Añada 6 mL de medio de cultivo celular al matraz, mezclando vigorosamente con una pipeta para separar las células restantes, y transfiera el contenido a un tubo de centrífuga de 15 mL. Centrifugar a 100 x g y 20 °C durante 5 min.
- Retire los medios de cultivo celular y la tripsina del tubo de centrífuga con un aspirador, teniendo cuidado de no alterar la pastilla celular. Agregue 1 ml de medio al tubo de centrífuga y pipetee hacia adelante y hacia atrás (sin producir burbujas) para romper el gránulo de celdas y resuspender las celdas.
- Pipetear 10 μL de la suspensión celular, 40 μL de medio de cultivo celular y 50 μL de colorante azul de tripán en un tubo de 200 μL, usando una pipeta para mezclar bien.
- Retire 10 μL de la mezcla de colorante e inyéctlela en un hemocitómetro. Cuente las células utilizando la dilución al 10% de la mezcla de colorantes para estimar el recuento total de células en el tubo de centrífuga de 15 ml.
NOTA: Para este protocolo, suponga una concentración de 5.000.000 de células/mL. - Multiplique la densidad de siembra deseada por el área de superficie de la membrana del inserto, divida por las celdas contadas/mL en la suspensión y multiplique por 1,000 para calcular los microlitros requeridos de suspensión celular por inserto.
- Para encontrar la cantidad total de suspensión de celdas requerida, multiplique esta cifra por 10 (para asegurar suficientes celdas para 9 muestras, ya que 3 de los 12 insertos serán controles sin celdas) y redondee al número entero más cercano. En este caso, se requiere un total de 135 μL de la suspensión celular para este experimento.
- Cree 2.000 μL de solución celular ajustada mezclando los 135 μL de la suspensión celular previamente calculados con 1.865 μL de medios de cultivo celular en un tubo de centrífuga separado de 15 mL.
- Células de siembra
- Retire el exceso de fibronectina de cada inserto una vez que se complete la incubación de la fibronectina.
- Lave los insertos dos veces añadiendo 100 μL de agua destilada estéril a cada inserto. Retire el agua siguiendo el mismo orden en que se agregó para garantizar un tiempo de lavado constante entre insertos.
- Vuelva a lavar el inserto añadiendo 100 μL de medio de cultivo celular a cada inserto. Retire el papel siguiendo el mismo orden en el que se añadió para garantizar un tiempo de lavado constante entre los insertos.
- Insertos de muestra de células
- Células de siembra pipeteando 200 μL de solución celular ajustada en cada inserto. Para garantizar una confluencia constante entre los insertos, mezcle la solución celular en el tubo de centrífuga antes de la distribución y vuelva a mezclar dentro de cada inserto después de la distribución.
- Insertos de control negativo
- Pipetee 200 μL de medio de cultivo celular en cada inserto. Para mantener la coherencia con los insertos de muestras celulares, utilice la pipeta para mezclar los medios de cultivo celular dentro de cada inserto.
- Etiquetado e incubación
- Dibuje una línea que divida la segunda placa de pocillo en dos columnas de tres pozos de ancho (para condiciones ejecutadas por triplicado) utilizando un marcador permanente. Separe cada columna en filas. Etiquete cada región de la cuadrícula con los parámetros pertinentes.
- Agregue 1 mL de medio de cultivo celular a cada pocillo para recibir un inserto para el experimento. Transfiera los insertos de la placa de pocillos de preparación a su ubicación adecuada en la placa de pocillos experimentales etiquetada e incube a 37 °C durante al menos 12 h.
3. Procedimiento experimental
- Experimento de entrega
- Pipetear 1,5 mL de la solución de PI de 0,1 mg/mL en cada pocillo de la guía de electrodos. Coloque un inserto en cada pocillo de la guía de electrodos, colocando los pies del inserto en las ranuras de alineación para que el inserto quede al ras con la superficie superior del pocillo (Figura 1A, B).
- Atornille la placa de circuito impreso (PCB) del electrodo superior a la parte superior de los pocillos de la guía de electrodos y conecte la guía de electrodos al dispositivo PSEP.
- Coloque el mazo de electrodos en la incubadora a 37 °C durante al menos 1 h para permitir que la temperatura se equilibre.
- Haga clic en el menú desplegable junto a "Membrana" en la esquina superior izquierda de la GUI y haga clic en GBO de 400 nm. Repita este paso para "Electrolito", "Celdas", "Densidad de siembra de celdas" y "Duración de celdas", seleccionando DMEM, A431, 200 y 12, respectivamente.
NOTA: Estos valores son solo para fines de mantenimiento de registros y no afectan la función del dispositivo. Asegúrese de ajustar estos valores según sea necesario para un correcto seguimiento de los datos. - Escriba 1 en el campo de edición Duración del tiempo posterior al pulso (min) en el lado derecho de la GUI para cambiar el tiempo de medición posterior al pulso predeterminado a 1 minuto. Deje todas las demás configuraciones en el estado predeterminado.
NOTA: Los parámetros de pulso predeterminados crean una forma de onda cuadrada con 30 voltios, 20 Hz, 1 ms de duración y 200 pulsos. Los parámetros de medición TEEI predeterminados son 0,5 voltios y 100 Hz, 1.000 Hz, 10.000 Hz y 100.000 Hz. - Haga clic en el botón Ejecutar e ingrese los nombres apropiados para los pozos 1-3 y 4-6 cuando se le solicite. Haga clic en Aceptar para iniciar el experimento.
- Retire la guía de electrodos de la incubadora y transfiera los insertos a las ubicaciones originales en la placa de pocillos del experimento cuando el programa haya terminado de ejecutarse.
- Mezcle 2 μL de Hoechst 33342 y 5 μL de calceína AM con 123 μL de medios de cultivo celular en un tubo de 200 μL.
- Pipetee suavemente 10 μL de la solución de tinción en cada inserto post-pulso y vuelva a colocar los insertos en la incubadora durante 5 min.
- Transfiera la placa de pocillos al soporte de placas de un microscopio fluorescente con un objetivo de aumento de 5x. Imagen utilizando el campo claro y la fluorescencia de cada tinción. Centre el inserto sobre el objetivo antes de disparar la cámara.
NOTA: Las longitudes de onda de excitación para PI, calceína AM y Hoechst 33342 son 558 nm, 495 nm y 353 nm, respectivamente. Las longitudes de onda de emisión son 575 nm, 519 nm y 465 nm, respectivamente.
- Experimento de medición TEEI
- Pipetear 1,5 mL de los medios de cultivo celular en cada pocillo de la guía de electrodos. Coloque los insertos de muestra de celda en los pocillos 1-3 y los insertos de control en los pocillos 4-6, ajustando los pies del inserto en las ranuras de alineación para que el inserto quede al ras con la superficie superior del pocillo.
- Atornille la PCB del electrodo superior a la parte superior de los pocillos de la guía de electrodos y conecte la guía de electrodos al dispositivo PSEP.
- Coloque el mazo de electrodos en la incubadora a 37 °C durante al menos 1 h para permitir que la temperatura se equilibre.
- Haga clic en el menú desplegable junto a "Membrana" en la esquina superior izquierda de la GUI y haga clic en GBO de 400 nm. Repita este paso para "Electrolito", "Celdas", "Densidad de siembra de celdas" y "Duración de celdas", seleccionando DMEM, A431, 200 y 12, respectivamente.
NOTA: Estos valores son solo para fines de mantenimiento de registros y no afectan la función del dispositivo. Asegúrese de ajustar estos valores según sea necesario para un correcto seguimiento de los datos. - Deje todas las configuraciones restantes en el estado predeterminado.
NOTA: Los parámetros de pulso predeterminados crean una forma de onda cuadrada con 30 voltios, 20 Hz, 1 ms de duración y 200 pulsos. Los parámetros de medición TEEI predeterminados son 0,5 voltios y 100 Hz, 1.000 Hz, 10.000 Hz y 100.000 Hz. - Haga clic en el botón Ejecutar e ingrese los nombres apropiados para los pozos 1-3 y 4-6 cuando se le solicite. Haga clic en Aceptar para iniciar el experimento.
- Retire la guía de electrodos de la incubadora y transfiera los insertos a las ubicaciones originales en la placa de pocillos del experimento cuando el programa haya terminado de ejecutarse.
- Mezcle 2 μL de Hoechst 33342, 5 μL de calceína AM y 10 μL de PI con 113 μL de medio de cultivo celular en un tubo de reacción de 200 μL.
- Pipetee 10 μL de la solución de tinción en cada inserto post-pulso y vuelva a colocar los insertos en la incubadora durante 5 min.
- Transfiera la placa de pocillos al soporte de placas de un microscopio de imágenes fluorescente con una lente de objetivo 5x. Imagen utilizando el campo claro y la fluorescencia de cada tinción. Centre el inserto sobre el objetivo antes de disparar la cámara.
NOTA: Las longitudes de onda de excitación para PI, calceína AM y Hoechst 33342 son 558 nm, 495 nm y 353 nm, respectivamente. Las longitudes de onda de emisión son 575 nm, 519 nm y 465 nm, respectivamente.
4. Análisis de datos
- Análisis de datos de imagen con la canalización de CellProfiler
- Utilice el flujo de trabajo personalizado de CellProfiler que se proporciona en GitHub:https://github.com/YangLabUNL/PSEP-TEEI para procesar las imágenes del experimento de entrega y medición de TEEI.
- Análisis TEEI
- Haga clic en la pestaña Análisis en la GUI.
- Cambie el indicador de tipo de impedancia a TEEI en la parte inferior de la GUI.
- Haga clic en la flecha en el cuadro superior izquierdo para mostrar todos los nombres de experimentos en el archivo de datos. Seleccione todos los datos de la muestra de células del experimento de medición TEEI.
- Haga clic en la flecha del siguiente cuadro a la derecha para mostrar todos los nombres de los experimentos en el archivo de datos. Seleccione todos los datos de la plaquita de control de la medición TEEI.
- Haga clic en Ejecutar. Aparecerá una figura básica que contiene los datos de la muestra de celda seleccionada con la frecuencia de medición más baja.
- En el cuadro de opciones de muestra en el lado derecho de la GUI, haga clic en la flecha para mostrar todos los datos de inserción seleccionados. Los valores atípicos se pueden eliminar seleccionando los datos apropiados y haciendo clic en Eliminar a continuación.
NOTA: Cualquier dato que se haya eliminado del análisis con el último clic del botón Eliminar se puede recuperar con el botón Deshacer . - Haga clic en Listo para pasar a la siguiente figura cuando se muestren los datos deseados en la figura.
- Repita los pasos 4.2.6 y 4.2.7 para el resto de los datos de la muestra de células y los datos de control. Cuando se haya confirmado el conjunto de datos final haciendo clic en "Listo", aparecerá la figura de análisis completa.
- Guarde la figura de análisis.
Resultados
El protocolo dado establece un método para utilizar las mediciones TEEI para examinar los procesos intermedios de electroporación y hacer predicciones de entrega, específicamente para la línea de celdas A431 y la carga PI. Si bien la modificación de este protocolo se discute más adelante en el artículo, es importante tener en cuenta ahora que, si bien los valores específicos pueden cambiar, las tendencias generales en la respuesta siguen siendo consistentes. Por ejemplo, los dato...
Discusión
La Figura 2C demuestra que los aumentos de TEEI desde el mínimo y las disminuciones desde la línea de base se trazan para cada voltaje de forma de onda PSEP. El aumento del TEEI crea un arco parabólico, que alcanza un máximo de alrededor de 20 voltios antes de reducirse, mientras que la disminución del TEEI desde la línea de base aumenta exponencialmente a medida que aumenta el voltaje. La eficiencia de entrega y los porcentajes de muerte en la
Divulgaciones
Los autores declaran no tener ningún conflicto de intereses.
Agradecimientos
Agradecemos el apoyo financiero de la NSF (Premios 1826135, 1936065, 2143997), los Institutos Nacionales de Ciencias Médicas Generales de los NIH P20GM113126 (Centro de Comunicación Biomolecular Integrada de Nebraska) y P30GM127200 (Centro de Nanomedicina de Nebraska), la Iniciativa de Colaboración de Nebraska y el Apoyo de Bioingeniería Voelte-Keegan. El dispositivo fue fabricado en el Centro Central de Investigación de Nanoingeniería (NERCF), que está parcialmente financiado por la Iniciativa de Investigación de Nebraska.
Materiales
| Name | Company | Catalog Number | Comments |
| 15 mL Conical Centrifuge Tube | Thermo Scientific | 339651 | |
| 2-Chip Disposable Hemocytometer | Bulldog Bio | DHC-N01 | |
| 75 cm2 Tissue Culture Flask | fisherbrand | FB012937 | |
| A431 Cells | ATCC | CRL-1555 | |
| Calcein AM | Invitrogen | C3099 | |
| Class II Type A2 Biosafety Cabinet | Labgard | NU-543-600 | |
| Custom Components | YangLab | https://github.com/YangLabUNL/PSEP-TEEI | |
| Disposable Centrifuge Tube (50 mL) | fisherbrand | 05-539-6 | |
| DMEM | Gibco | 11965092 | |
| Fetal Bovine Serum | Gibco | A5670401 | |
| Fluid Aspiration System | vacuubrand | 20727403 | |
| HERACELL 240i | Thermo Scientific | 51026331 | |
| Hoechst 33342 | Thermo Scientific | 62249 | |
| Human Plasma Fibronectin | Sigma-Aldrich | FIBRP-RO | |
| Inverted Fluorescent Microscope | Zeiss | 491916-0001-000 | |
| Inverted Microscope | Labomed | TCM 400 | |
| PBS | cytiva | SH30256.02 | |
| PCR Tube 200 µL | Sarstedt | 72.737 | |
| Penicillin / Streptomycin | Gibco | 15140148 | |
| Pipette (0.2-2 µL) | fisherbrand Elite | FBE00002 | |
| Pipette (100-1000 µL) | fisherbrand Elite | FBE01000 | |
| Pipette (20-200 µL) | fisherbrand Elite | FBE00200 | |
| Pipette (2-20 µL) | fisherbrand Elite | FBE00020 | |
| Propidium Iodide | Invitrogen | P1304MP | |
| Reaction Tube 1.5 mL | Sarstedt | 72.690.300 | |
| Sorvall ST 16R Centrifuge | Thermo Scientific | 75004240 | |
| Thincert (24-well) | Greiner Bio-One | 662 641 | 0.4 µm pore diameter, 2x106 cm-2 pore density, transparent PET |
| Tissue Culture Plate (24-well) | fisherbrand | FB012929 | |
| Trypan Blue Solution | Sigma-Aldrich | T8154-20mL | |
| Trypsin | Gibco | 15090046 |
Referencias
- Brooks, J., et al. High throughput and highly controllable methods for in vitro intracellular delivery. Small. 16 (51), e2004917 (2020).
- Stewart, M. P., Langer, R., Jensen, K. F. Intracellular delivery by membrane disruption: Mechanisms, strategies, and concepts. Chem Rev. 118 (16), 7409-7531 (2018).
- Canatella, P. J., Karr, J. F., Petros, J. A., Prausnitz, M. R. Quantitative study of electroporation-mediated molecular uptake and cell viability. Biophys J. 80 (2), 755-764 (2001).
- Pliquett, U., Gift, E. A., Weaver, J. C. Determination of the electric field and anomalous heating caused by exponential pulses with aluminum electrodes in electroporation experiments. Bioelectrochem Bioenerg. 39 (1), 39-53 (1996).
- Pan, J., et al. Cell membrane damage and cargo delivery in nano-electroporation. Nanoscale. 15 (8), 4080-4089 (2023).
- Boukany, P. E., et al. Nanochannel electroporation delivers precise amounts of biomolecules into living cells. Nat Nanotechnol. 6 (11), 747-754 (2011).
- Chang, L., et al. Micro-/nanoscale electroporation. Lab Chip. 16 (21), 4047-4062 (2016).
- Patino, C. A., et al. Multiplexed high-throughput localized electroporation workflow with deep learning-based analysis for cell engineering. Sci Adv. 8 (29), 7637 (2022).
- Sagvolden, G., Giaever, I., Pettersen, E. O., Feder, J. Cell adhesion force microscopy. Proc Natl Acad Sci U S A. 96 (2), 471-476 (1999).
- Ishibashi, T., Takoh, K., Kaji, H., Abe, T., Nishizawa, M. A porous membrane-based culture substrate for localized in situ electroporation of adherent mammalian cells. Sensors Actuators B: Chem. 128 (1), 5-11 (2007).
- Mukherjee, P., Nathamgari, S. S. P., Kessler, J. A., Espinosa, H. D. Combined numerical and experimental investigation of localized electroporation-based cell transfection and sampling. ACS Nano. 12 (12), 12118-12128 (2018).
- Brooks, J. R., et al. An equivalent circuit model for localized electroporation on porous substrates. Biosens Bioelectron. 199, 113862 (2022).
- Brooks, J. R., et al. Transepithelial electrical impedance increase following porous substrate electroporation enables label-free delivery. Small. 20 (25), 2310221 (2023).
- Vindiš, T., et al. Gene electrotransfer into mammalian cells using commercial cell culture inserts with porous substrate. Pharmaceutics. 14 (9), 1959 (2022).
- Ye, Y., et al. Single-cell electroporation with real-time impedance assessment using a constriction microchannel. Micromachines. 11 (9), 856 (2020).
- Bednarek, R. In vitro methods for measuring the permeability of cell monolayers. Methods Protoc. 5 (1), 17 (2022).
- Harhaj, N. S., Antonetti, D. A. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 36 (7), 1206-1237 (2004).
- Hunter, R. J. . Zeta potential in colloid science: Principles and applications. 2, (2013).
- Wong, P. K., Wang, T. -. H., Deval, J. H., Ho, C. -. M. Electrokinetics in microdevices for biotechnology applications. IEEE/ASME Trans Mechatron. 9 (2), 366-376 (2004).
- Qian, K., Wang, Y., Lei, Y., Yang, Q., Yao, C. An experimental and theoretical study on cell swelling for osmotic imbalance induced by electroporation. Bioelectrochemistry. 157, 108637 (2024).
- Fox, M. B., et al. Electroporation of cells in microfluidic devices: A review. Anal Bioanal Chem. 385 (3), 474-485 (2006).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoExplorar más artículos
This article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados