Method Article
Three-Dimensional Imaging of Tumor-Bearing Tissue Using the Iterative Bleaching Extends Multiplexity Approach
In This Article
Summary
This article describes a protocol for multiplex immunofluorescence optimized to characterize the three-dimensional architecture of peritoneal metastases.
Abstract
The spatial heterogeneity of the tumor microenvironment (TME) is a critical determinant of therapeutic response, particularly for immune-oncology agents, where success depends on the distribution of specific immune cell subpopulations. Over the past decade, multiple sophisticated technologies have been introduced to achieve detailed resolution of the TME using two-dimensional sections from either formalin-fixed, paraffin-embedded (FFPE) or fixed-frozen tissues. While these thin sections are easier to procure and analyze, they lack the three-dimensional architecture needed to reliably and comprehensively characterize a tumor. To address this limitation, a tissue mounting and imaging technique was developed to enable the three-dimensional analysis of tumor lesions in their native in vivo state. This protocol outlines the procurement of human tumor tissue, the mounting of samples on custom-printed platforms, and staining procedures for post-fixation samples. The multiplexed immunofluorescence technique, IBEX (Iterative Bleaching Extends Multiplexity), was adapted to characterize the three-dimensional TME with up to 15 markers for tumor, immune, and stromal cells using commercially available antibodies. Imaging depths of up to 100 µm were achieved using an inverted white-light laser confocal microscope with a custom-printed imaging adaptor and commercial glass-bottom dishes to ensure optimal tissue orientation. This protocol highlights the potential of the IBEX method to expand multiplexed immunofluorescence studies, providing a more comprehensive understanding of TME composition.
Introduction
Solid tumors are highly variable, with an intricate composition of cell types (malignant and non-malignant), extracellular matrix proteins, and soluble factors1,2. Ultimately, this highly complex, heterogeneous tumor microenvironment (TME) determines susceptibility to treatments like immunotherapy3,4. As such, technologies as highly multiplexed immunofluorescence5,6 and spatial transcriptomics7 have been applied to characterize the complexity of the TME in cell layer-thick (~4 µM) sections.
As opposed to cell dispersion approaches such as single-cell sequencing and flow cytometry8,9,10, these techniques preserve spatial relationships to contribute critical information about cellular crosstalk and can be employed to identify potential biomarkers for prediction of treatment response11,12,13. However, tumor lesions are inherently three-dimensional (3D) structures, and 2D approaches like those mentioned above do not adequately capture the complex cellular landscape. The importance of 3D biology has been appreciated in the field, as evidenced by the wide utilization of patient-derived tumor organoid cultures14. However, organoids do not retain the cellular complexity of the original in vivo TME, which limits applicability for use with immunomodulatory drugs or for characterization of the diverse TME cell populations.
Although it may be possible to perform analyses on serial 2D sections and computationally "stitch" the images together for a 3D rendering, this technique is cost-prohibitive and challenging to accomplish with high fidelity15. To cost-effectively characterize 3D tissues (e.g., tumors) in a reproducible manner, a unique method for the preparation and multiplex immunofluorescence staining/analysis was developed and demonstrated using peritoneal tumor lesions from a patient with primary peritoneal cancer.
Based on an adaptation of the well-established IBEX technique (Iterative Bleaching Extends Multiplexity)16,17, this protocol details 3D-printing of a tissue holder and imaging adapter, peritoneal tissue procurement, and mounting, multiple cycles of staining with various tumor and cell-type markers, and high-resolution confocal 3D-imaging.
Protocol
The study was conducted with approval from the National Institutes of Health Institutional Review Board under protocol NCT01915225. Human tissue was obtained with written informed consent at the time of diagnostic laparoscopy. All tissue was tumor-bearing, as determined by visible inspection and confirmed through final histopathologic examination. Details of all reagents and equipment used are listed in the Table of Materials. A schematic illustration of the entire workflow is provided in Figure 1.
1. Printing platforms, imaging adaptor, and incubation plate
- Slicing/ 3D printing/ post-processing procedures for the platform and 9-well plate parts (outlined in Figure 2A-C)
- Slicing: Open the slicing software and import the design files in .STL format (Supplementary File 1 and Supplementary File 2). Orient the models for optimal printing and add supports. Select the medical grade printer, select the clear resin, and 50 µm layer height. Check printability and send the job to the printer.
- 3D printing: Check that the printer is ready (correct resin cartridge, tank, and platform in place). On the printer's touchscreen, select the uploaded job from the slicer software to start the print. Once the print is complete, use the removal tool to detach the printed part from the platform.
- Post-processing: Rinse the part in the wash unit filled with 99% isopropyl alcohol (IPA) for 15 min. Remove parts from the wash unit and soak in fresh IPA for 5 min.
- Post-cure the part for 60 min at 60 °C. Remove supports using flush cutters. Sand down any support marks using fine-grit sandpaper if necessary.
- Slicing/ 3D printing/post-processing procedures for height adjuster and outer lid (outlined in Figure 2A-C)
- Slicing: Open the print software and import the design files in .STL format (Supplementary File 3 and Supplementary File 4). Orient the models and assign materials. Select the desired print mode (High mix, high speed), and set the model settings to finish to Matte. Check printability and send the job to the printer.
- 3D printing: Ensure the printer has the correct materials loaded and the build tray is clean. On the printer's monitor interface, select the uploaded job to start the print. Once the print is complete, use the scraper tool to gently remove the printed part from the build tray.
- Post-processing: Place the printed pieces into a water jet cleaner or an agitation cleaning system to remove the support material. Perform any manual support removal, if necessary, using tools like tweezers or pliers.
- Laser cutting of platform slider (shown in Figure 2A,C)
- Preparing the design: Import the *.DWG design file in the vector software (Supplementary File 5). Set the line colors to red (RGB) for vector cutting. Send the job to the laser cutter.
- Setting up the laser cutter: Open the laser cutter software and import the design file. Select the material type and adjust settings for power, speed, and resolution for 1/32" thick acrylic. Place the material on the laser bed, ensure it is properly aligned, and focus the laser.
- Laser cutting: Start the laser cutting process and monitor to ensure the material is being cut correctly.
2. Tissue mounting on printed platforms
- Preparation of harvest medium and prep table
- Supplement 100 mL of DMEM (low glucose) with Anti-Anti and place in a 37 °C incubator until needed (harvest medium).
- Set up a "prep table" in the operating room with the following items: autoclaved 3D printed platforms, pack of 2-0 silk sutures ties, heating plate, sample cup with 50 mL prewarmed harvest medium, 15 cm culture plate containing 30 mL of prewarmed harvest medium and a 24 well plate with 4 wells each containing 1.5 mL of harvest medium (place all on heating plate), 1 pair of forceps and 1 pair of surgical shears.
- Tissue resection and mounting
NOTE: Tissue is obtained from the operating room. Areas of peritoneum with foci of tumor of less than 0.5 mm in thickness and diameter are identified on visual inspection (Figure 2D). Of note, ~ 2 cm2 of tissue (peritoneum) is needed to prepare one platform.- Carefully separate the tumor-bearing peritoneum from the underlying abdominal wall musculature and fascia (performed by the surgeon). Transfer the resulting tissue sheets to a sample cup containing warm harvest medium and place on a heating plate (prep table).
- Mount the tissue on platforms in the 15 cm plate containing the harvest medium. Carefully drape the tumor-bearing tissue (mesothelial side facing up) over the small orifice side of the platform and secure it in place with a 2-0 silk suture. Place the prepared tissue platform inversely (submerged) in a 24-well plate containing harvest medium (Figure 2E-G).
NOTE: Mount the tissue taught enough to prevent folds on the platform surface, but do not generate tension as this will induce unwanted biomechanical and physiologic changes.
3. Fixation/staining/bleaching procedure
NOTE: Changes from the original protocol tailored to 4 µm thin FFPE or fixed frozen sections are outlined in Figure 3. Table 1 provides a list of antibodies (with dilutions) used for IBEX rounds 1-6.
- Fixation
- Prepare 40 mL of fixation buffer by adding 10 mL of Fixative stock to a 50 mL conical tube containing 30 mL of cold 1x PBS.
- Using a pair of forceps, transfer the tissue mounted on platforms to the fixation buffer and incubate for 24 h at 4 °C.
- Decant the fixative and replace it with 40 mL of cold 1x PBS and incubate for 10 min at 4 °C.
- Staining
- Add 1 mL of blocking buffer (1% BSA, 0.3% Triton-X-100, 1% Fc Block in 1x PBS filtered with 0.22 µm syringe filter) to each well and insert tissue platforms. Block for at least 2 h at room temperature on a rocker set to low speed (30 rpm).
NOTE: Ensure the notch on the platform and the grove in the well align. This step is not time-sensitive and can be done overnight at 4 °C. - Prepare 0.8 mL of antibody/dye solution in a microcentrifuge tube and centrifuge at 10,000 x g for 5 min at room temperature. Transfer 0.75 mL to a clean well of the 9-well incubation plate and insert a washed platform.
- Wrap the plate in aluminum foil and incubate for at least 2 h at room temperature on a rocker set to low speed (30 rpm).
- Wash the platform in a 50 mL conical tube with 40 mL of 1x PBS for 10 min at 4 °C. The platform is now ready to be inserted into the imaging adaptor.
- Add 1 mL of blocking buffer (1% BSA, 0.3% Triton-X-100, 1% Fc Block in 1x PBS filtered with 0.22 µm syringe filter) to each well and insert tissue platforms. Block for at least 2 h at room temperature on a rocker set to low speed (30 rpm).
- Bleaching
- Following each round of staining and imaging, bleach the fluorophores using the ester-reducing agent lithium borohydride (LiBH4). This treatment will eliminate fluorescence signals from Alexa Fluor (AF)488, AF647, and AF750 but not from AF594 and the nuclear dye Hoechst.
CAUTION: Lithium borohydride is extremely reactive with water. Flammable hydrogen gases are released upon contact with water; spent lithium borohydride and disposables contaminated with lithium borohydride need to be disposed of as hazardous waste. - Prepare 5 mL of 1.5 mg/mL LiBH4 solution in distilled water (in a chemical hood) and incubate for 30 min at room temperature.
NOTE: LiBH4 solution will lose its reducing/bleaching effectiveness when prepared >4 h before use. - Transfer 1 mL of LiBH4 solution to a clean well of the 9-well incubation plate and insert the washed platform for 60 min at room temperature.
- Wash the platform briefly in 20 mL of 1x PBS in a conical tube and check for the remaining fluorescence signal using the highest laser setting for Z compensation in the preceding imaging round before proceeding to subsequent rounds of staining.
- Repeat blocking of the sample for 2 h at room temperature before proceeding to the next antibody incubation step.
- Following each round of staining and imaging, bleach the fluorophores using the ester-reducing agent lithium borohydride (LiBH4). This treatment will eliminate fluorescence signals from Alexa Fluor (AF)488, AF647, and AF750 but not from AF594 and the nuclear dye Hoechst.
4. Imaging procedure
- Assembling of imaging adaptors/platforms
- Add 0.1 mL of 1x PBS to the center of the glass bottom of a 3.5 cm imaging dish and set aside. Screw the height adjustment ring loosely clockwise into the outer lid and mount the platform in the inner plastic holder (notch-grove alignment). Secure the platform with the slider.
- Place the assembled imaging adaptor on the glass bottom dish and carefully lower the height adjustment ring until the tissue touches the buffer. Avoid pivoting the platform on the glass bottom.
- Once the optimal distance from the glass is reached, add 0.2 mL of 1x PBS onto the tissue (now the bottom side up) to avoid tissue dehydration during imaging.
- Confocal imaging
- Turn on all components of the inverted confocal microscope, including the computer, microscope, laser, and LED light source for eyepiece usage. Start the image acquisition software.
- Select the appropriate objective (e.g., 20x or 40x) and add the appropriate immersion medium. For example, a 20x water objective is used in this study; ~200 µL of water is added to the objective to avoid drying during long image acquisitions.
- Place the assembled imaging dish into the sample holder on the microscope stage and ensure the specimen is secure (does not move).
NOTE: When placing the assembled sample on the microscope stage, take note of the notch position and use the same position for iterative cycles to facilitate image alignment. It is important to keep the imaging adaptor at the optimum height setting for consecutive rounds. - Center the specimen using the X-Y control and bring the objective up to the cover glass. Looking through the eyepiece and using the Hoechst signal as a reference, move the objective to obtain fine focus on the tissue.
- Select appropriate laser lines or adjust WLL to the excitation and emission spectra of the fluorophores used to stain the tissue sample. Eliminate overlap between channels by adjusting the settings and add sequences if fluorophores have similar excitation spectra.
- Choose the following image acquisition parameters: scan speed 600 Hz, XY resolution 1024 x 1024, 3-line averages, and bidirectional scan.
- Choose the following Z-stack parameters: (1) use Z-step size optimized to the objective being used, (2) define the beginning and end of the stack, (3) adjust the Z-compensation parameters to achieve equal brightness throughout the stack, and (4) acquire a 1-tile test stack with one average.
- Define the region to be imaged and acquire a high-resolution tile-Z stack.
Results
The peritoneum is a membranous structure less than 1 mm thick that lines the surface of the abdominal wall and is contiguous with the surfaces of abdominal organs. It is composed of surface mesothelium and underlying connective tissue that is interspersed with adipose cells, lymphocytes, macrophages, and fibroblasts, as well as blood and lymphatic vessels. Peritoneal malignancies most often stem from abdominal tumor metastases (e.g., ovarian cancer or gastric cancer)18 or, less frequently, can be primary tumors of the peritoneum (e.g., mesothelioma)19. These often small (<1 cm) tumor lesions are highly complex but are amenable to 3D characterization using the technique outlined. A maximum projection of five confocal sections is used to view the entire sample mounted on a platform (Figure 4, inset in upper left corner). Three primary conjugated antibodies (AF488-CD44, AF594-CD45, and AF647-Podoplanin) together with the nuclear dye Hoechst were used for the first round of IBEX (IBEX1, Figure 4). Tumor lesions become evident by the rosette-like structures that are double-positive for CD44 and Podoplanin and show an arrangement of nuclei characteristic of papillary mesothelioma.
Tumor cells were identified using cell morphology and two antibodies since both anti-Podoplanin and anti-CD44 recognize non-tumor cells, as evidenced by the detection of lymphatic vessels via anti-Podoplanin and the binding of the CD44 antibody to subsets of immune cells. Smaller regions of interest (ROIs) are then captured using a tile scan format in higher resolution at optimized Z-step sizes for rendering 3D projections (Figure 5A). Platforms were taken through the iterative staining procedures, and the same ROIs were imaged in IBEX cycles 1-6. Representative images for tumor (yellow box) and tumor-tertiary lymphoid structure (TLS) interfaces (blue box) are assembled in Figure 5B-M.
The presence of TLS and density of CD3-positive cells indicate significant immune cell infiltration20 and possibly responsiveness to immunomodulatory drugs21. Of note, TLS and the bulk of the tumor lesions are present in different Z-depths of the tissue, as demonstrated in comparing stack projections of different optical sections in Figure 6, highlighting the benefits of 3D imaging and the potential missed biology when 2D imaging is applied to 3D structures. The 3D character of these lesions becomes more evident in the movie files (Movie 1-12), which depict volume renderings of the individual lesions in Figure 5.
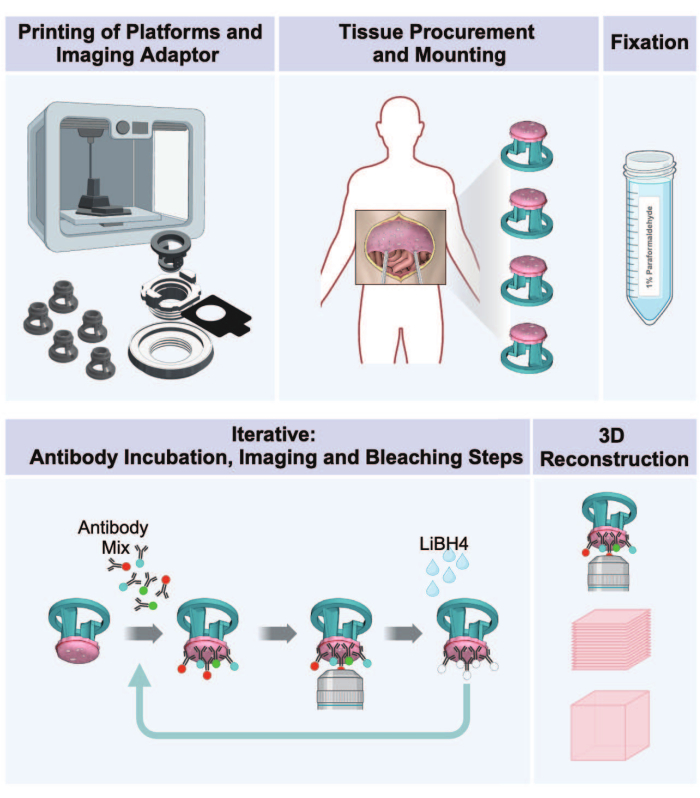
Figure 1: Workflow from 3D printing to 3D imaging of peritoneal tumors. The protocol consists of a sequence of five steps to prepare 3D multiplexed images from tumor-bearing peritoneal tissue. (1) Printing of tissue-receiving platforms, imaging adaptor parts, and incubation plate. (2) Tissue procurement and mounting procedures. (3) Fixation with paraformaldehyde. (4) IBEX staining and imaging cycles. (5) 3D reconstruction using imaging software. Please click here to view a larger version of this figure.

Figure 2: Hardware components with tissue interface for 3D imaging. (A) Schematic diagram of platform assembly within imaging adaptor. (B) A 9-well incubation plate. (C) Photographs of image adaptor components with assembly. (D) Mesothelioma-bearing peritoneum as observed during diagnostic laparoscopy. (E) Mounting of tissue on the platform. (F,G) Tumor-bearing peritoneum mounted on platforms. Please click here to view a larger version of this figure.
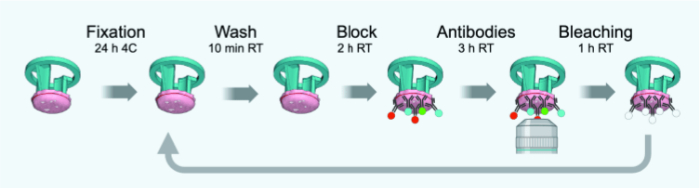
Figure 3: Timeline schematic of IBEX cycles. Please click here to view a larger version of this figure.
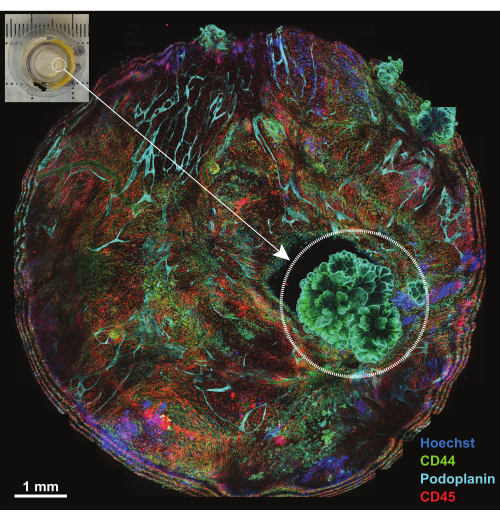
Figure 4: Overview of platform following incubation with IBEX1 panel. Maximum projection of the entire platform following incubation with IBEX1 panel (Hoechst, AF488-CD44, AF594-CD45, and AF647-Podoplanin). The dashed circle highlights the tumor. Inset: Photograph of tumor-bearing peritoneum mounted on the imaging platform. Scale bar: 1 mm. Please click here to view a larger version of this figure.
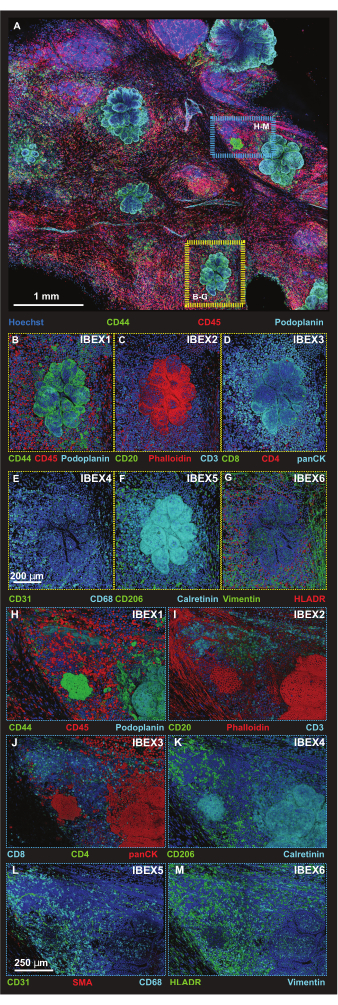
Figure 5: High-resolution immunofluorescence images of select platform regions. (A) Maximum projection of merged 56-tile Z-stack image acquired following incubation with IBEX1 panel (Hoechst, AF488-CD44, AF594-CD45 and AF647-Podoplanin). Yellow rectangle: tumor lesion shown in panels (B-G). Blue rectangle: tumor-tertiary lymphoid structure interface shown in (H-M). (B-G) One individual tumor lesion with IBEX panel 1-6 in volume rendering. (B) IBEX1, green: CD44, red: CD45, cyan: Podoplanin. (C) IBEX2, green: CD20, red: F-actin, cyan: CD3. (D) IBEX3, green: CD8, red: CD4, cyan: panCK. (E) IBEX4, green: CD31, cyan: CD68. (F) IBEX5, green: CD206, cyan: Calretinin. (G) IBEX6, green: Vimentin, red: HLADR. (H-M) One individual tumor-tertiary lymphoid structure interface with IBEX panel 1-6 in volume rendering. (H) IBEX1, green: CD44, red: CD45, cyan: Podoplanin. (I) IBEX2, green: CD20, red: F-actin, cyan: CD3. (J) IBEX3, green: CD4, red: panCK, cyan: CD8. (K) IBEX4, green: CD31, red: SMA, cyan: CD68. (L) IBEX5, green: CD206, cyan: Calretinin. (M) IBEX6, green: HLADR, cyan: Vimentin. All panels, blue: Hoechst. Scale bars: (A, 1 mm); (B-M, 250 µm). Please click here to view a larger version of this figure.
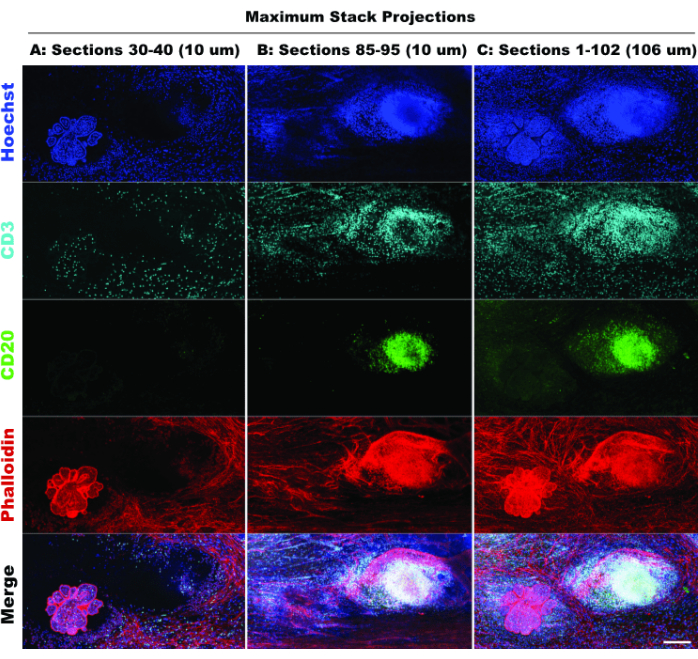
Figure 6: Simulated 2D imaging. Comparison of limited (10 optical sections) and full stack (102 optical sections) projections confirms loss of tissue context in 2D simulation. (A) Maximum projection of sections 30-40 (10 µm). (B) Maximum projection of sections 85-95 (10 µm). (C) Maximum projection of sections 1-102 (~100 µm). All panels, IBEX2 (Hoechst, AF488-CD20, AF647-CD3, AF790-phalloidin). Scale bar: 200 µm. Please click here to view a larger version of this figure.
Table 1: Antibodies used for the individual IBEX panels. Please click here to download this Table.
Movie 1: Animated volume rendering of the individual lesion with IBEX panel 1 (blue: Hoechst, green: CD44, red: CD45, cyan: Podoplanin). Please click here to download this Movie.
Movie 2: Animated volume rendering of the individual lesion with IBEX panel 2 (blue: Hoechst, green: CD20, red: F-actin, cyan: CD3). Please click here to download this Movie.
Movie 3: Animated volume rendering of the individual lesion with IBEX panel 3 (blue: Hoechst, green: CD8, red: CD4, cyan: panCK). Please click here to download this Movie.
Movie 4: Animated volume rendering of the individual lesion with IBEX panel 4 (blue: Hoechst, green: CD31, cyan: CD68). Please click here to download this Movie.
Movie 5: Animated volume rendering of the individual lesion with IBEX panel 5 (blue: Hoechst, green: CD206, cyan: Calretinin). Please click here to download this Movie.
Movie 6: Animated volume rendering of the individual lesion with IBEX panel 6 (blue: Hoechst, green: Vimentin, red: HLADR). Please click here to download this Movie.
Movie 7: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 1 (blue: Hoechst, green: CD44, red: CD45, cyan: Podoplanin). Please click here to download this Movie.
Movie 8: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 2 (blue: Hoechst, green: CD20, red: F-actin, cyan: CD3). Please click here to download this Movie.
Movie 9: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 3 (blue: Hoechst, green: CD8, red: CD4, cyan: panCK). Please click here to download this Movie.
Movie 10: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 4 (blue: Hoechst, green: CD31, red: SMA, cyan: CD68). Please click here to download this Movie.
Movie 11: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 5 (blue: Hoechst, green: CD206, cyan: Calretinin). Please click here to download this Movie.
Movie 12: Animated volume rendering of tertiary lymphoid structure-lesion interface with IBEX panel 6 (blue: Hoechst, green: Vimentin, red: HLADR). Please click here to download this Movie.
Supplementary File 1: Print file for the incubation plate. Please click here to download this File.
Supplementary File 2: Print file for the 36 individual platforms. Please click here to download this File.
Supplementary File 3: Print file for the height adjustment ring. Please click here to download this File.
Supplementary File 4: Print file for the outer lid. Please click here to download this File.
Supplementary File 5: Laser cut file for the slider. Please click here to download this File.
Discussion
Current multiplex techniques for fluorescence imaging are restricted to thin sections and do not provide 3D context. The present study describes a protocol to apply the IBEX imaging method16,17 to intact tumors mounted on custom-designed platforms. Peritoneal tumor lesions were chosen to highlight the technique as patients commonly present with multiple, ideally sized tumor deposits that are taken along with the surrounding normal peritoneum as part of standard cytoreduction surgery undertaken in most tertiary referral centers across the world22,23. The protocol is nonetheless applicable to parenchymal metastases and mouse model tumors alike using tumor slices as have been previously described24,25. Moreover, the diameter of the mounting ring on the platforms can be adjusted to experimental/tissue needs, although larger areas are prone to tissue sagging and extended imaging times. Irrespective of the tissue source and size, the time between procurement and preservation must be kept minimal to ensure optimal quality26. The setup of a prep table in the operating room guarantees prompt processing when patients are involved. The time from procurement to fixation of the tissue should be noted to ensure comparability between experiments.
Tissue samples are taken through the IBEX protocol as whole mounts, i.e., fixation and imaging as well as bleaching steps, are performed with the intact tissue affixed to the platform. This guarantees sample preservation through the iterative staining/imaging/bleaching cycles, since there is no semi-permanent section-glass interface and no need to repeatedly place/remove a cover glass from the tissue, both of which can lead to tissue loss. To save on reagents, the custom-designed 9-well plate guarantees an optimum fit of the platform with minimal "dead volume". All antibodies used for staining are primary conjugated antibodies, and all fluorophore conjugates used (except for the Hoechst dye) are Alexa Fluor dyes, which were chosen for their excellent brightness27. Other fluorophores Antibody panels were designed according to data deposited from the IBEX imaging community, an open, global repository collecting datasets, protocols, and feedback from an international group of scientists using IBEX (https://ibeximagingcommunity.github.io/ibex_imaging_knowledge_base/). The sequence of antibody panels was carefully chosen. In general, targets with low abundance were placed in earlier cycles. IBEX cycle 1 (CD45, CD44, and Podoplanin) is an exception since these three markers were used to identify tumor lesions in the initial staining round.
Antibody panels are comparably small to reduce channel crosstalk at high laser intensity settings, which is necessary to image the tissue deeper using a confocal microscope. AF750 conjugates were included wherever possible to expand the panel from 2 to 3 antibodies. Of note, there are not many AF750 conjugates commercially available, and the fact that the target for this channel should be highly abundant limits the use of this fluorophore. An example is the AF750-conjugated anti-SMA antibody that was used in this protocol. Although this is a clone that works reliably conjugated to AF488 or AF594, the signal intensity for this antibody conjugated with AF750 is significantly lower, which makes it difficult to generate high-quality Z-stacks.
Antibody incubations of 3 h at room temperature are short compared to the original protocol and reflect most likely the lower cell density in peritoneal tissue samples. In contrast, the bleaching step with 1.5 mg/mL lithium borohydride for 60 min was more stringent. These parameters were determined empirically and can vary with tissue and antibodies being used. Of note is that due to incubation in the reactive bleaching solution, small air bubbles can be trapped in the tissue. Whenever this became evident during the imaging step of the procedure, samples were degassed by exposing the platform in the 9-well incubation plate to vacuum using a suction canister. Simple ITK sample registrations, often applied in IBEX studies, could not be performed due to overwhelming data input to the image analysis software. Nonetheless, identical cell populations are easily identified by comparing individual datasets. Overall, the iterative cycles were timed such that one cycle could be completed in one day, with imaging taking up most of an entire cycle (~ 8 h).
The technique does have limitations that deserve mention. The time requirement of the protocol makes this method unlikely to be adopted broadly but rather in select circumstances. We envision this protocol best utilized to characterize changes in the tumor immune microenvironment pre- and post-treatment (e.g., bispecific antibodies for cancer immunotherapy) or to predict general treatment outcomes. Although this protocol offers 3D characterization, it is not known how much area must be interrogated to effectively describe a tumor, including its complex cell populations28. Access to fresh human tissue may also be difficult in certain centers, but we envision this protocol is applicable to animal models as well29.
The applications for this protocol are diverse, from basic research analyzing the TME composition across multiple solid tumors to pre/ post-tissue biopsies for patients enrolled in clinical trials. Although the data obtainable from a single cell-layer thick tissue section is immense, 3D biology can be easily overlooked. We see 3D imaging as an adjunct to other spatial biology techniques, likely aiding in the selection of 2D sections for subsequent analyses. Additionally, a protocol to incorporate a live imaging component over multiple hours using the described tissue mounting and imaging setup is actively ongoing.
Disclosures
None.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (NCI). This research was also partially supported by the CAT-I, a research collaboration between NIAID and NCI, led by Ronald Germain. We would like to extend our gratitude to Andrea Radtke for her enthusiastic, collaborative efforts. Her expertise greatly benefited this study.
Materials
| Name | Company | Catalog Number | Comments |
| Alexa Fluor Plus 750 Phalloidin | Invitrogen | A30105 | F-actin dye |
| Alexa Fluor 488 anti-human CD206 (MMR) Antibody | BioLegend | 321114 | antibody |
| Alexa Fluor 488 anti-human CD31 Antibody | BioLegend | 303110 | antibody |
| Alexa Fluor 488 anti-human CD4 Antibody | BioLegend | 300519 | antibody |
| Alexa Fluor 488 anti-human HLA-DR Antibody | BioLegend | 307656 | antibody |
| Alexa Fluor 488 anti-mouse/human CD44 Antibody | BioLegend | 103016 | antibody |
| Alexa Fluor 594 anti-human CD45 Antibody | BioLegend | 304060 | antibody |
| Alexa Fluor 647 Anti-Calretinin antibody [EP1798] | Abcam | ab214244 | antibody |
| Alexa Fluor 647 anti-human CD3 Antibody | BioLegend | 300416 | antibody |
| Alexa Fluor 647 anti-human CD8 Antibody | BioLegend | 344726 | antibody |
| Alexa Fluor 647 anti-human Podoplanin Antibody | BioLegend | 337007 | antibody |
| Alexa Fluor 647 anti-Vimentin Antibody | BioLegend | 677807 | antibody |
| Alexa Fluor 647 CD68 Antibody (KP1) | Santa Cruz | sc-20060AF647 | antibody |
| Alexa Fluor 750 Cytokeratin, pan Antibody (AE-1/AE-3) | Novus | NBP2-33200AF750 | antibody |
| Alexa Fluor 750 Human alpha-Smooth Muscle Actin Antibody | R&D | IC1420S | antibody |
| Alexa Fluor 488 CD20 Monoclonal Antibody (L26) eBioscience | Thermo Fisher | 53-0202-82 | antibody |
| Antibiotic-Antimycotic | Gibco | 15240096 | supplement harvest medium |
| BioMed Clear Resin (Form 3) | FormLabs | RS-F2-BMCL-01 | resin used for platform + incubation plate |
| BSA | Sigma | A7906-500g | blocking solution component |
| Cast acrylic 1/32" thick | material used for cutting slider | ||
| CleanStation DT3 | Stratasys | DT3 | post processing for height adjuster & outer lid |
| Container, Specimen | McKesson | 870203 | transfer of tissue from perating table to prep table |
| CorelDraw | CorelDRAW | Software to prepare the vector-based design file for laser cutting | |
| Cytofix | BD Bioscience | 554655 | fixative |
| Dish 15 cm | Falcon | 353025 | dish used during mounting |
| Dish 35 mm No. 1.5 Coverslip 14 mm Glass Diameter Uncoated | Matek | P35G-1.5-14-C | imaging dish |
| DMEM (no glucose) | Gibco | 11966025 | harvest medium |
| Fc Block | BD Bioscience | 564220 | blocking solution component |
| Form 3B+ | Formlabs | Form 3B+ | printer used for platform + incubation plate |
| Form Cure | Formlabs | FH-CU-01 | post processing for platform + incubation plate |
| Form Wash | Formlabs | FH-WA-01 | post processing for platform + incubation plate |
| GrabCAD Print | GrabCAD | GrabCAD Print | Software to prepare models for Stratasys printers |
| Hoechst 33342 10 mg/mL | Biotium | 40046 | nuclear dye |
| J826 Prime 3D Printer | Stratasys | J826 | printer used for height adjuster & outer lid |
| LAS X | Leica | LAS X | Confocal software |
| Laser cutting system | Universal Laser Systems | ULS PLS6.150D | CO2 Laser cutting used for slider |
| Lithium Borohydride | STREM Chemicals | 93-0397 | bleaching chemical |
| PBS, pH 7.4 | Gibco | 10010023 | base Buffer for washing, blocking, staining |
| PreForm | FormLabs | PreForm | Software to prepare models for Formlab printers |
| Silk sutures 2-0 | Ethicon | A305.O35 | affix tissue to platform |
| Stellaris 8 WLL confocal microscope | Leica | STELLARIS 8 | Confocal Imaging |
| Syringe filter | filter ab solution | ||
| Triton X-100 | American Bio | AB02025-00100 | permeabelizing reagent, blocking solution component |
| Vero ContactClear | Stratasys | CTT610, 4 KG | resin used for height adjuster & outer lid |
| Warming Tray | Spring USA | ST-1220 | keep media and tissue warm |
| Water, distilled | Gibco | 15230-170 | diluent for LiBH4 |
References
- Anderson, N. M., Simon, M. C. The tumor microenvironment. Curr Biol. 30 (16), R921-R925 (2020).
- Li, Z., Li, J., Bai, X., Huang, X., Wang, Q. Tumor microenvironment as a complex milieu driving cancer progression: A mini-review. Clin Transl Oncol. , (2024).
- Fridman, W. H., Zitvogel, L., Sautes-Fridman, C., Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 14 (12), 717-734 (2017).
- Roma-Rodrigues, C., Mendes, R., Baptista, P. V., Fernandes, A. R. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci. 20 (4), 840 (2019).
- Sheng, W., et al. Multiplex immunofluorescence: A powerful tool in cancer immunotherapy. Int J Mol Sci. 24 (4), 3086 (2023).
- Bollhagen, A., Bodenmiller, B. Highly multiplexed tissue imaging in precision oncology and translational cancer research. Cancer Discov. 14 (11), 2071-2088 (2024).
- Jin, Y., et al. Advances in spatial transcriptomics and its applications in cancer research. Mol Cancer. 23 (1), 129 (2024).
- Kashyap, A., et al. Quantification of tumor heterogeneity: From data acquisition to metric generation. Trends Biotechnol. 40 (6), 647-676 (2022).
- Chang, Q., Hedley, D. Emerging applications of flow cytometry in solid tumor biology. Methods. 57 (3), 359-367 (2012).
- Zhang, L., et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell. 181 (2), 442-459.e29 (2020).
- Wang, X. Q., et al. Spatial predictors of immunotherapy response in triple-negative breast cancer. Nature. 621 (7980), 868-876 (2023).
- Maestri, E., et al. Spatial proximity of tumor-immune interactions predicts patient outcome in hepatocellular carcinoma. Hepatology. 79 (4), 768-779 (2024).
- Wang, Q., et al. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 12 (10), 11149-11165 (2023).
- Veninga, V., Voest, E. E. Tumor organoids: Opportunities and challenges to guide precision medicine. Cancer Cell. 39 (9), 1190-1201 (2021).
- Lin, J. R., et al. Multiplexed 3d atlas of state transitions and immune interaction in colorectal cancer. Cell. 186 (2), 363-381.e19 (2023).
- Radtke, A. J., et al. Ibex: An iterative immunolabeling and chemical bleaching method for high-content imaging of diverse tissues. Nat Protoc. 17 (2), 378-401 (2022).
- Radtke, A. J., et al. Ibex: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proc Natl Acad Sci U S A. 117 (52), 33455-33465 (2020).
- Van Baal, J. O., et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 49 (1), 95-105 (2017).
- Bridda, A., Padoan, I., Mencarelli, R., Frego, M. Peritoneal mesothelioma: A review. MedGenMed. 9 (2), 32 (2007).
- Demuytere, J., Ernst, S., Van Ovost, J., Cosyns, S., Ceelen, W. The tumor immune microenvironment in peritoneal carcinomatosis. Int Rev Cell Mol Biol. 371, 63-95 (2022).
- Wang, Q., et al. Heterogeneity of tertiary lymphoid structures predicts the response to neoadjuvant therapy and immune microenvironment characteristics in triple-negative breast cancer. Br J Cancer. , (2024).
- Kepenekian, V., et al. Peritoneal mesothelioma: Systematic review of hyperthermic intraperitoneal chemotherapy (HIPEC) protocol outcomes. Indian J Surg Oncol. 14 (Suppl 1), 39-59 (2023).
- Mcquade, C., Renton, M., Chouhan, A., Macdermott, R., O'brien, C. Review of imaging peritoneal disease and treatment. Can Assoc Radiol J. , (2024).
- Kenerson, H. L., et al. Tumor slice culture as a biologic surrogate of human cancer. Ann Transl Med. 8 (4), 114 (2020).
- Arrizabalaga, L., et al. Tumor slice culture system for ex vivo immunotherapy studies. Methods Cell Biol. 189, 55-69 (2024).
- Neumeister, V. M., et al. Quantitative assessment of effect of preanalytic cold ischemic time on protein expression in breast cancer tissues. J Natl Cancer Inst. 104 (23), 1815-1824 (2012).
- Panchuk-Voloshina, N., et al. Alexa dyes: A series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 47 (9), 1179-1188 (1999).
- Rajaram, S., et al. Sampling strategies to capture single-cell heterogeneity. Nat Methods. 14 (10), 967-970 (2017).
- Bella, A., et al. Mouse models of peritoneal carcinomatosis to develop clinical applications. Cancers (Basel). 13 (5), 963 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved