A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Effects of Mechanical Methods Used in Peri-implantitis Treatment on Implant Surface Decontamination and Roughness
In This Article
Summary
The present protocol describes an experimental model based on ink-staining which can be used for in vitro implant surface decontamination and roughness research to contribute to clinical decision-making.
Abstract
Various mechanical methods have been proposed for decontaminating dental implant surfaces with varying success. This in vitro study evaluated the decontamination efficiency of an air abrasion (AA) system with erythritol powder, a polyether-ether-ketone (PEEK) ultrasonic tip, and titanium curettes (TIT) and their effects on implant surface topography using scanning electron microscopy (SEM). A total of 60 implants were stained with permanent red ink and placed in 3D-printed Class 1A and Class 1B peri-implantitis defects, forming six groups (n=10 per group) based on defect type and treatment protocol. Additionally, one positive and one negative control implant was used. Erythritol powder, PEEK ultrasonic tips, and titanium curettes were applied for 2 min in Class 1A defects and 3 minutes in Class 1B defects. Residual red ink areas were quantified with digital software, and implant surface changes were analyzed using SEM and EDS. None of the methods achieved complete decontamination. However, erythritol powder was significantly the most effective, leaving a residual ink rate of 24% ± 6% (p < 0.001). PEEK ultrasonic tips resulted in 41% ± 4% residual ink, while titanium curettes left 55% ± 3%. Significant differences were observed among all methods. No significant difference in decontamination efficacy was found between Class 1A and Class 1B defects. SEM analysis showed minimal surface damage with erythritol powder and PEEK tips, whereas titanium curettes caused moderate to severe damage. Based on both decontamination efficiency and surface preservation, erythritol powder and PEEK tips are safe and effective options for peri-implantitis treatment, while titanium curettes are less effective and cause considerable surface damage. These findings may assist clinicians in peri-implantitis treatment planning.
Introduction
Dental implant treatment is the most common and preferred protocol for replacing missing teeth worldwide. Long-term follow-up studies have shown that the use of implant-supported restorations in the treatment of complete or partial edentulism provides predictable results and high success rates in terms of survival. However, various complications affecting the hard and soft tissues may arise following the surgical placement and restoration of implants1. In 2017, the World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions introduced definitions and differential diagnoses for diseases affecting peri-implant tissues2. According to this definition, peri-implantitis is an irreversible pathological condition characterized by clinical signs of inflammation, including bleeding on probing and/or suppuration, increased probing depths, and/or recession of the mucosal margin in the peri-implant mucosa, and radiographic loss of supporting bone2. The etiology of peri-implant diseases is multifactorial, and some individuals are more susceptible to this condition than others. Specific predispositions of individuals may increase the risk of peri-implant disease development, which may lead to implant loss. Other factors that play a role in the etiology of peri-implant diseases are patient-related factors (smoking, systemic diseases, periodontal disease history, oral hygiene); the condition of the keratinized mucosa, quantity and quality of bone and soft tissues at the implant site; forces on the implant and surrounding tissues; complications encountered during implant placement; and the experience and skill of the physician performing surgical and prosthetic treatments2. In addition, a new risk assessment and treatment concept has recently been introduced, the Implant Disease Risk Assessment Tool (IDRA)3. This tool was developed as a functional diagram consisting of eight parameters, each with a documented association with peri-implantitis. The vectors of the octagon are the history of periodontitis, percentage of implant and tooth sites with bleeding on probing (BoP), number of teeth/implants with probing pocket depths ≥ 5 mm, rate of periodontal bone loss (radiographs in relation to a patient's age), susceptibility to periodontitis, frequency of supportive periodontal therapy (SPT), and design of the prosthesis.
Recent systematic reviews have shown that the prevalence of peri-implantitis is 19.53% at the patient level and 12.53% at the implant level3. Regarding approximately more than 5 million implants being placed each year worldwide, with a market size of more than 4 billion USD, peri-implantitis represents a major health problem for the population. If left untreated, peri-implantitis results in the loss of the affected implant and the implant-supported prosthesis, causing a big distress for both the dentist and the patient.
The treatment of peri-implant diseases can be divided into non-surgical and surgical approaches. Although there is a reasonable expectation for the success of endpoints in the treatment of periodontitis4, comparable evidence for the treatment of peri-implantitis is still scarce. Therefore, the rationale for a staged approach and non-surgical therapy of peri-implantitis is to attempt biofilm and inflammation control with relatively simple approaches before increasing treatment invasiveness and to perform the surgical step when better biofilm and risk factor control is achieved. This includes OH instructions and motivation, risk factor control, control of biofilm-retaining factors, and prosthesis cleaning/removal/modification, including assessment of prosthesis components, supramarginal and sub-marginal instrumentation, and concomitant periodontal treatment when needed. Thus, non-surgical therapy should always be the first step5. For early peri-implantitis, reducing risk factors and non-surgical treatment may suffice, but complete biofilm removal in deep pockets after bone loss is often challenging. During the reevaluation phase after non-surgical treatment, persistent pocket depths (≥ 6 mm) and bleeding on probing (BoP) indicate potential progression of peri-implantitis. If these signs are present, surgical interventions are recommended6. The surgical therapy of peri-implantitis includes (i) open flap debridement, (ii) resective flap surgery, (iii) the management of peri-implant osseous defects using reconstructive approaches, (iv) additional methods for implant surface decontamination and (v) adjunctive use of local/systemic antibiotics7.
The major etiological factor of peri-implantitis is the pathogenic biofilm colonized on the implant surface6. Removing this biofilm is the main principle and goal of all treatment protocols, which involve mechanical, chemical, and laser decontamination methods7.
Mechanical debridement employs plastic, carbon, and titanium curettes, ultrasonic devices with plastic and metal tips, titanium brushes, and air-abrasive (AA) systems with various powders. Although complete elimination of the biofilm is difficult to achieve, these therapies provide clinical benefits. Various clinical interventions, including mechanical debridement protocols with or without antiseptics8, antibiotics9, as well as resective and regenerative surgery10, have been used with varying degrees of clinical success. However, they also induce changes in the chemical and physical properties of the implant surface, possibly complicating new bone formation and re-osseointegration.
Among mechanical methods, AA procedures using different powder compositions have shown the best cleaning efficacy11,12,13. However, the presence of residual particles can alter surface topography and reduce biocompatibility14. Glycine, followed by sodium bicarbonate, is the most used powder in AA systems8. Recently, smaller air-abrasive particles like erythritol (14 µm) have gained interest for effective decontamination with reduced surface damage9. Titanium and plastic curettes, which cause less surface damage than steel tips, are effective in biofilm decontamination15. Ultrasonic scaler tips made from poly-ether-ether-ketone (PEEK) also reduce bacterial load with minimal surface damage10. Decontamination methods must consider the high roughness of implant surfaces and aim to remove bacterial biofilm without causing significant surface damage. Although extensive in vitro, in vivo, and clinical research has been performed, there is still no consensus and a gold standard protocol for peri-implantitis treatment to date. The increasing prevalence of peri-implant diseases due to numerous dental implants necessitates an evidence-based, predictable approach to treating contaminated surfaces. This study aims to evaluate the effectiveness of different decontamination methods -air abrasive (AA) systems, PEEK ultrasonic tips, and titanium curettes-on implant surface decontamination and to assess their impact on implant surface roughness by SEM analysis.
Protocol
The study protocol was approved by the ethical committee (TBAEK-363) of Akdeniz University, Antalya, Turkey. This study was supported by the Akdeniz University Research Fund (Project number: TDH-2024-6676). The study utilized a screw-shaped dental implant (PrimeTaper EV Implant) with dimensions of 4.2 mm x 11 mm, featuring a micro-thread design measuring 1.7 mm on the collar. Surface preparation with sandblasting and acid-etching with diluted hydrofluoric acid to achieve the well-defined OsseoSpeed surface.
1. Preparation of experimental peri-implantitis models
NOTE: Three decontamination mechanical treatment methods (air abrasive (AA), polyetheretherketone (PEEK) ultrasonic, and titanium curettes; Table of Materials) in two different peri-implantitis defect types11 (Class 1A and Class 1B) were analyzed. Thus, there were six experimental groups (Figure 1). A total of 62 implants were used, including one positive and one negative control implant. This in vitro study design, initially developed by Sharhmann et al.16, has been modified by various researchers12,13,14,15,16,17,18 in the literature (Figure 2). Assuming a 10% difference in biofilm removal efficacy between groups, the sample size was determined as 60 (10 for each group) for six groups with G*power, an effect size of 0.50, a type I error of 5%, and 80% power.
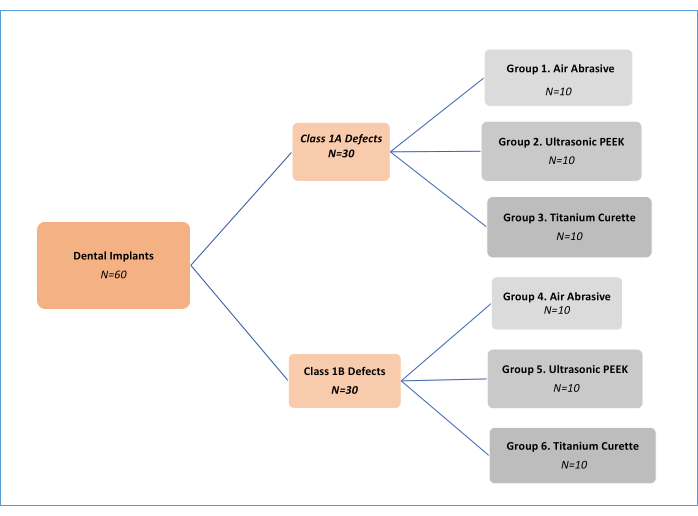
Figure 1: Flowchart of experimental groups. Please click here to view a larger version of this figure.
- Remove one first molar from the educational mandibular phantom model. The plastic teeth are fixed to this phantom model with screws. Unscrew the first molar tooth and remove the thread from the socket. Fill the socket by molding the soft silicone material to cover the socket to create a flat alveolar ridge.
NOTE: A simulation of an implant placed in an edentulous area has been created. - Scan the prepared model in a laboratory scanner to create a digital design.
- Create 3D Class 1A and Class 1B11 peri-implantitis bone defects digitally. Open the Exocad software program. Upload the scanned model file. Then click Design and select Expert Mode. Remove irregular areas with the Edit Mesh option. Then right-click and select Save Scan as a File on the related folder on the computer.
- Select Tools > Add/Remove Mesh option, select Wax Scanning> Upload File, and select the file just saved in the folder. After that, click Wizard Mode on the right.
- In the resulting new STL model, create a defect simulation in the tooth socket. To do this, click Add/Remove on the left side. Select Oval Shape Brush Size. Afterward, create the defect on the model by using shift and left click at the same time. Adjust the length of the defect as 5 mm and the width as 4.2 mm (corresponding to the diameter of the implant) on the buccal surface of the implant for the Class 1A defects and 5-5-5 mm for the Class 1B defects.
- To narrow the horizontal width of the defect, select the Cusps by clicking on Anatomy on the left side and narrowing the vestibular width of the defect inwards. Then, right-click and save the scene as a file.
- To create a final model, restart the program and reload the saved folder. Then select the Wizard Mode and Model Alignment option on the right. Select the model type as Digital Waxup Model on the left. Click Next several times until you reach the model design section on the left. Select the Full Model option and click Next two times. Once the model file is complete, open it with Explorer, and it is ready to print.
- Forward the STL files of the designed model to the 3D printer. Print the created digital models using a model resin.
- Rinse the experimental models in 96% ethanol for 5-10 min. After the cleaning process, place the models in the light-emitting curing device and cure with light for 5 min at the dose setting in accordance with the manufacturer's instructions.
NOTE: Use a resin model with high tensile, flexural, and compressive strength that is suitable for implant drilling. Clean the printed model with alcohol-based solutions according to the manufacturer's instructions and ensure it is adequately light-cured.
2. Staining of implants
- Submerge the test implants in viscous water-resistant red ink. Ensure all parts of the implant surface are completely and homogeneously covered with ink for 15 s. Remove the implants from the sterile container using driver handpieces or impression posts without hand contact.
NOTE: This staining will simulate an optically visible biofilm surrogate for the photographic analysis. - Air-dry the stained implants with a dental unit air syringe in order to have an even dispersion of the ink. Dry the stained implants further for 24 h at room temperature. Dry the implants in isolation with the handpieces, without hand contact.
3. Placement of stained implants
- Adjust the settings of a dental physio dispenser as follows: 800 rpm, 40 N torque with no saline irrigation.
- Create the implant socket with the surgical implant drills on the experimental models to place the implants of 11 mm in length and 4.2 mm in width. Prepare the same implant socket for models with both types of defects (Class 1A and 1B defect models).
- Use the implant drills sequentially according to the manufacturer's instructions to achieve primary stability. Clean the remaining debris after drilling with an air-water syringe, then place the implants. Stabilize the experimental peri-implantitis models on the working platform with a clamp to avoid micromovement of the implants and to prevent microcracks on models.
- Insert the implants with a carrier handpiece into the sockets. Leave 5 mm of exposed area on the buccal surface. Ensure the implants are submerged at the same level on the lingual bone crest of the model. Avoid touching the stained implant surface.
4. Decontamination of implants
- Start decontaminating the implants in groups without removing them from the experimental 1A and 1B defect models.
- Air abrasive system: Set the device at full power with water irrigation with 14 µm erythritol powder. Hold the tip of the device at 2-3 mm from the implant surface and apply the powder evenly to the exposed peri-implantitis defect. Limit the working time to 2 min for 1A defects and 3 min for 1B defects.
- Polyetheretherketone (PEEK) ultrasonic tip: Set the device at 8 power (80%) with maximum water irrigation. Hold the PEEK handpiece in a manner suitable for ultrasonic use. Perform decontamination on the implant surface with linear and parallel movements. Apply the PEEK tip between the threads as much as its design allows. Limit the working time to 2 min for 1A defects and 3 min for 1B defects.
- Titanium curettes: Apply consecutive contacts with constant pressure at 60°-90° to the implant surface with an approximate 0.75 N force on the 5 mm exposed implant surface for 2 min for 1A defects and 3 min for 1B defects.
- After decontamination, remove the implant using the driver piece without hand contact. If the models become deformed after decontamination, proceed to the backup models. Stabilize the experimental peri-implantitis models on the working platform with a clamp.
NOTE: All methods should be calibrated and applied by a single researcher.
5. Photographic imaging
- Remove the implants from the model with a compatible implant driver piece. Air-dry the implants for 20 s to remove any loosened particles/remnants on the surface.
- Place the implants on custom-designed acrylic photographic models to shoot flat views, 30° apical views, and 30° coronal views to evaluate the apical and coronal parts of the threads on the implant surface.
- Place the camera on a tripod and standardize the camera settings (distance 15 cm, ISO 160, aperture f/16, exposure time 1/250 s). Ensure that the room is adequately lit. It is necessary to stabilize the camera with a tripod.
- Take the digital photos in RAW format with a flash. Obtain a total of 90 buccal photos (one flat, one 30° apical, and one 30°coronal for each implant surface) for Class1A defects and 270 photos (flat, 30° apical, and 30°coronal from each buccal, mesial, and distal surface for each implant) for Class 1B defects. Deposit all digital photo files on a hard disk for further image analysis.
6. Image analysis
- Perform all analysis on digital image software (ImageJ). Before the analysis, make the background of the photographs black using a Photoshop program (Photoroom) to ensure that only the implant is visible in the image. Open the application then add each image from the gallery. Remove the background from the image and select Black Background from the options.
- Drag and drop the image on ImageJ. Draw a square in the image to cover 5 mm coronal to the implant. Then click Image > Crop for standardization. Repeat the same process for each image.
- Convert the images to 8-bit format by clicking Image ˃ Type ˃ 8-bit and adjust the thresholds by clicking Image ˃ Adjust ˃ Threshold for area calculations.
- Calculate the whole implant surface area and the red color residue area by clicking Analyze ˃ Measure ˃ Area.
- Record the obtained pixel area in a spreadsheet file. Create a separate spreadsheet file to record the raw image data.
- In order to obtain the percentage of red color remnants, multiply the red-displayed area by 100 and divide by the total implant surface area.
7. SEM analysis
- Store all implants in their sterile boxes until the day of analysis.
- Before the SEM analysis, randomly select one representative sample from each treatment group. In addition to the samples selected from each group add one sterile implant and only the implant completely covered with ink. Thus, prepare a total of eight implant samples for SEM analysis.
- Spray nitrogen gas using a gas gun for 20 s in order to remove any micro-powder on the implant surface before SEM analysis.
NOTE: No additional gold coating was applied due to the advanced technology of the device. - Mount each implant on SEM stubs with conductive carbon adhesive discs in a way that allows analysis of the buccal flat surface without hand decontamination. Arrange them in order by number to avoid confusion of the groups.
- Select an area from the implants placed in the instrument and capture images at different magnifications. Repeat the same procedure for different regions of the implant surfaces (mesial or distal). Use 100x, 1000x, and 5000x magnification for images using an SEM device operating at 10-30 kV with an average working distance of 12 mm.
NOTE: The second microstrand from the collar area and the second macrostrand from the body were selected for each implant to ensure standardization during SEM analysis. For some images, it is recommended to perform elemental analysis (EDS) during imaging for comparison.
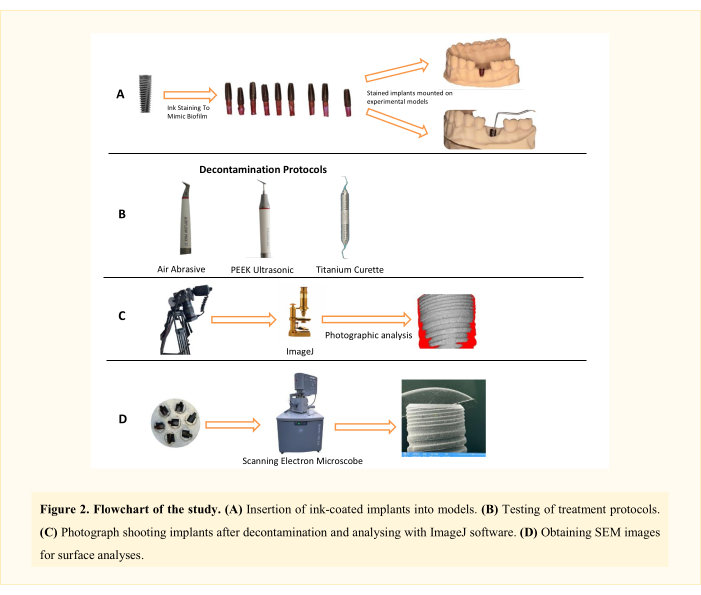
Figure 2: Flowchart of the study. Please click here to view a larger version of this figure.
8. Statistical analysis
- Express categorical variables as numbers and percentages and continuous variables as mean and standard deviation. Confirm the normality of distribution for continuous variables with the Shapiro-Wilk test.
- For comparison of continuous variables between defect groups, use the Student's t-test. For comparison of more than two groups, use one-way ANOVA or Kruskal Wallis test depending on whether the statistical hypotheses were fulfilled or not.
- For normally distributed data, regarding the homogeneity of variances, use Tukey tests for multiple comparisons of groups. For nonnormally distributed data, use the Bonferroni-adjusted Mann-Whitney U test for multiple comparisons of groups. All statistical analyses were carried out using IBM SPSS 20. The statistical level of significance for all tests was considered to be 0.05.
Results
The experimental protocol described here for analyzing the decontamination of implant surfaces revealed significant differences among various treatment procedures. In addition, the post-treatment SEM protocol also showed significant changes on the implant surfaces with varying degrees among study groups.
Implant-level comparisons (Total implant means) after decontamination
Implant-level comparisons were carried out by comparing the general means of each implant (the meas...
Discussion
The methodology of in vitro surface analysis of dental implants affected by peri-implant disease has always been challenging due to the inflammatory and bacterial nature of the pathogenic mechanisms occurring on the rough surfaces of the implant. Several concerns include the sample material choice, mimicking biofilm on the surface, choosing the peri-implantitis defect type, representing clinical conditions during the in vitro procedures, variations of the decontamination procedures, and the methods of determinin...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The implants used in the study were supported by Dentsply Sirona.
Materials
| Name | Company | Catalog Number | Comments |
| 3D Printer | DentaFab, Istanbul, Turkey | To produce experimental periimplantitis defects | |
| 3D Printing Resin | Alias, Istanbul,Turkey | To produce experimental periimplantitis models | |
| 3D Scanner | DOF Inc. EDGE, Seoul ,Republic of Korea | Used to scan the dental phantom model | |
| Air Abrasive system | AIRFLOW Plus PowderE.M.S., Electro Medical Systems S.A., Nyon, Switzerland | Used to decontaminate implant surface | |
| CAD/CAM Software | Exocad 3.2 Elefsina | To produce experimental periimplantitis defects | |
| Camera | Canon EOS 70D, Japan | In order to obtain photographic records of implants | |
| Dental implant | DS PrimeTaper, Dentsply Sirona, Hanau, Germany | ||
| Light-Curing Unit | Solidilite V, Japan | Used to curing experimental models in laboratory | |
| Permanent ink | Edding, Germany | Used to stain the implant surface for mimicking biofilm | |
| Physiodispenser | Dentsply Sirona, Hanau, Germany | To place the implants in the experimental models | |
| SEM Device | FEI QUANTA FEG 250 FEI Technologies Inc. (Oregon, United States | Used to analyze topograhic changes on the implant surface | |
| Surgical implant set | Dentsply Sirona, Hanau, Germany | To place the implants in the experimental models | |
| Titanium Currette | Langer ½ Titanium Currette, Hu-Friedy, Chicago, IL, USA | Used to decontaminate implant surface | |
| Ultrasonic PEEK Tip | PI-MAX Implant Scaler, E.M.S., Electro Medical Systems S.A., Nyon, Switzerland | Used to decontaminate implant surface |
References
- Buser, D., et al. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. 14 (6), 839-851 (2012).
- Berglundh, T., et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World workshop on the classification of periodontal and peri-implant diseases and conditions. J Clin Periodontol. 45, S286-S291 (2018).
- Diaz, P., Gonzalo, E., Villagra, L. J. G., Miegimolle, B., Suarez, M. J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 22 (1), 1-13 (2022).
- Herrera, D., et al. Prevention and treatment of peri-implant diseases—The EFP S3 level clinical practice guideline. J Clin Periodontol. 50 (S26), 4-76 (2023).
- Heitz-Mayfield, L., Mombelli, A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 29 Suppl, 325-345 (2014).
- Heitz-Mayfield, L. J. A., Heitz, F., Lang, N. P. Implant disease risk assessment IDRA–a tool for preventing peri-implant disease. Clin Oral Implants Res. 31 (4), 397-403 (2020).
- Monje, A., Cha, J. K. Strategies for implant surface decontamination in peri-implantitis therapy. Int J Oral Implantol. 15 (3), 213-248 (2022).
- Francis, S., Iaculli, F., Perrotti, V., Piattelli, A., Quaranta, A. Titanium surface decontamination: A systematic review of in vitro comparative studies. Int J Oral Maxillofac Implants. 37 (1), 76-84 (2022).
- Pujarern, P., et al. Efficacy of biofilm removal on the dental implant surface by sodium bicarbonate and erythritol powder airflow system. Eur J Dent. 18 (4), 1022-1029 (2024).
- Polizzi, E., D’orto, B., Tomasi, S., Tetè, G. A micromorphological/microbiological pilot study assessing three methods for the maintenance of the implant patient. Clin Exp Dent Res. 7 (2), 156-162 (2021).
- Monje, A., et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 21 (4), 635-643 (2019).
- Khan, S. N., Koldsland, O. C., Tiainen, H., Hjortsjö, C. Anatomical three-dimensional model with peri-implant defect for in vitro assessment of dental implant decontamination. Clin Exp Dent Res. 10 (1), e841-e848 (2024).
- Matsubara, V. H., et al. Cleaning potential of different air abrasive powders and their impact on implant surface roughness. Clin Implant Dent Relat Res. 22 (1), 96-104 (2020).
- Ronay, V., Merlini, A., Attin, T., Schmidlin, P. R., Sahrmann, P. In vitro cleaning potential of three implant debridement methods. Simulation of the non-surgical approach. Clin Oral Implants Res. 28 (2), 151-155 (2017).
- Sahrmann, P., et al. In vitro cleaning potential of three different implant debridement methods. Clin Oral Implants Res. 26 (3), 314-319 (2015).
- Hart, I., Wells, C., Tsigarida, A., Bezerra, B. Effectiveness of mechanical and chemical decontamination methods for the treatment of dental implant surfaces affected by peri-implantitis: A systematic review and meta-analysis. Clin Exp Dent Res. 10 (1), e839-e844 (2024).
- Korello, K., Eickholz, P., Zuhr, O., Ratka, C., Petsos, H. In vitro efficacy of non-surgical and surgical implant surface decontamination methods in three different defect configurations in the presence or absence of a suprastructure. Clin Implant Dent Relat Res. 25 (3), 549-563 (2023).
- Luengo, F., et al. In vitro effect of different implant decontamination methods in three intraosseous defect configurations. Clin Oral Implants Res. 33 (11), 1087-1097 (2022).
- Keim, D., et al. In vitro efficacy of three different implant surface decontamination methods in three different defect configurations. Clin Oral Implants Res. 30 (6), 550-558 (2019).
- Al-Hashedi, A. A., Laurenti, M., Benhamou, V., Tamimi, F. Decontamination of titanium implants using physical methods. Clin Oral Implants Res. 28 (8), 1013-1021 (2017).
- Sanz-Martín, I., et al. Significance of implant design on the efficacy of different peri-implantitis decontamination protocols. Clin Oral Investig. 25 (6), 3589-3597 (2021).
- Mensi, M. Comparison between four different implant surface debridement methods: an in vitro experimental study. Minerva Stomatol. 69 (5), 286-294 (2020).
- Sirinirund, B., Garaicoa-Pazmino, C., Wang, H. L. Effects of mechanical instrumentation with commercially available instruments used in supportive peri-implant therapy: An in vitro study. Int J Oral Maxillofac Implants. 34 (6), 1370-1378 (2019).
- Wiessner, A., et al. In vivo biofilm formation on novel PEEK, titanium, and zirconia implant abutment materials. Int J Mol Sci. 24 (2), 1779 (2023).
- Cai, Z., et al. Disinfect Porphyromonas gingivalis biofilm on titanium surface with combined application of chlorhexidine and antimicrobial photodynamic therapy. Photochem Photobiol. 95 (3), 839-845 (2019).
- Azizi, B., et al. Antimicrobial efficacy of photodynamic therapy and light-activated disinfection on contaminated zirconia implants: An in vitro study. Photodiagnosis Photodyn Ther. 21, 328-333 (2018).
- Sahrmann, V., et al. In vitro cleaning potential of three different implant debridement methods. Clin Oral Impl Res. 26 (3), 314-319 (2015).
- Tuchscheerer, V., et al. In vitro surgical and non-surgical air-polishing efficacy for implant surface decontamination in three different defect configurations. Clin Oral Investig. 25 (4), 1743-1754 (2021).
- Iatrou, P., et al. In vitro efficacy of three different nonsurgical implant surface decontamination methods in three different defect configurations. Int J Oral Maxillofac Implants. 36 (2), 271-280 (2021).
- Petersilka, G. J. Subgingival air-polishing in the treatment of periodontal biofilm infections. Periodontol 2000. 55 (1), 124-142 (2011).
- Giffi, R., et al. The efficacy of different implant surface decontamination methods using spectrophotometric analysis: an in vitro study. J Periodontal Implant Sci. 53 (4), 295 (2023).
- Laleman, I., et al. Subgingival debridement: end point, methods and how often. Periodontol 2000. 75 (1), 189-204 (2017).
- Regidor, E., Derks, J., Ortiz-Vigón, A. The use of air abrasive devices for implant surface decontamination. Perio Clinica. 27 (2), 23-38 (2023).
- Khan, S. N., et al. The decontamination effect of an oscillating chitosan brush compared with an ultrasonic PEEK-tip: An in study using a dynamic biofilm model. Clin Oral Implants Res. 36 (1), 73-81 (2025).
- Louropoulou, A., Slot, D. E., van der Weijden, F. The effects of mechanical instruments on contaminated titanium dental implant surfaces: a systematic review. Clin Oral Implants Res. 25 (10), 1149-1160 (2014).
- Lang, M. S., Cerutis, R., Miyamoto, T., Nunn, E. Cell attachment following instrumentation with titanium and plastic instruments, diode laser, and titanium brush on titanium, titanium-zirconium, and zirconia surfaces. Int J Oral M axillofac Implants. 31, 799-806 (2016).
- Harrel, S. K., Wilson, T. G., Pandya, M., Diekwisch, T. G. H. Titanium particles generated during ultrasonic scaling of implants. J Periodontol. 90 (3), 241-246 (2019).
- Schwarz, F., Nuesry, E., Bieling, K., Herten, M., Becker, J. Influence of an Erbium, Chromium-Doped Yttrium, Scandium, Gallium, and Garnet (Er,Cr:YSGG) laser on the reestablishment of the biocompatibility of contaminated Titanium implant surfaces. J Periodontol. 77 (11), 1820-1827 (2006).
- Hakki, S. S., Tatar, G., Dundar, N., Demiralp, B. The effect of different cleaning methods on the surface and temperature of failed titanium implants: an in vitro study. Lasers Med Sci. 32 (3), 563-571 (2017).
- Chegeni, E., Espanã-Tost, A., Figueiredo, R., Valmaseda-Castellón, E., Arnabat-Domínguez, J. Effect of an Er,Cr:YSGG laser on the surface of implants: A descriptive comparative study of 3 different tips and pulse energies. Dent J. 8 (4), 109-118 (2020).
- Mei, L., Guan, G. Profilometry and atomic force microscopy for surface characterization. Nano TransMed. 2 (1), e9130017-e9130024 (2023).
- Martelo, J. B., Andersson, M., Liguori, C., Lundgren, J. Three-dimensional scanning electron microscopy used as a profilometer for the surface characterization of polyethylene-coated paperboard. Nord Pulp Paper Res J. 36 (2), 276-283 (2021).
- Kimoto, K., et al. Unsupervised machine learning combined with 4D scanning transmission electron microscopy for bimodal nanostructural analysis. Sci Rep. 14 (1), 2901-2909 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved