Method Article
GC-MS Analysis of Deuterium Enrichment of Retinol in Serum and Estimation of Vitamin A Total Body Stores
In This Article
Summary
This method involves extracting retinol from serum, separating it using HPLC, and determining labeled and non-labeled retinol isotopes using GC-MS. The ratio of labeled to non-labeled retinol is used to estimate total body stores of vitamin A.
Abstract
This method describes the determination of deuterium enrichment of retinol in serum and the estimation of vitamin A stores in the body. The process involves extracting retinol from 0.4 mL of serum using 0.5 mL of 0.85% saline solution, 100 µL of internal standard solution, and 5 mL of chloroform-methanol (2:1 v/v) solution. After centrifugation and removal of the lower chloroform layer, the mixture is dried under nitrogen and resuspended in 0.1 mL of ethanol, and the retinol fraction is separated from other constituents using an HPLC system equipped with a PE C18 column. The retinol fraction can be collected manually or with a fraction collector. Subsequently, the retinol fraction is dried under nitrogen and derivatized with O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) containing 10% trimethylchlorosilane. Finally, labeled and non-labeled retinol isotopes are quantified using a GC-MS system equipped with a 19091z-431 HP-1 methyl siloxane capillary column, employing electron capture negative chemical ionization with helium as the carrier gas and methane as the ionization agent. The ratio of labeled to non-labeled retinol is then used in the Olson, Green, or mass balance equations to estimate vitamin A stores.
Introduction
Vitamin A is an essential nutrient needed for the visual system and maintenance of cell function for growth, epithelial integrity, red blood cell production, immunity, and reproduction1. Vitamin A deficiency is a serious public health problem worldwide, affecting more than 100 countries. It disproportionately impacts young children and pregnant women in low-income countries. Approximately 190 million children globally suffer from vitamin A deficiency, making it a critical issue for public health and child development2.
In response to this situation, several programs, including vitamin A supplementation with biannual distribution of high doses of vitamin A to children under 5 years of age and vitamin A fortification of certain food commodities, have been implemented for decades in many low-income countries. However, these interventions often overlap, exposing some populations to inadvertent chronic excessive vitamin A intake3,4. This dual risk of deficiency and excess highlights the need for a biomarker that can accurately assess vitamin A status across the full spectrum, from deficiency to toxicity, to guide program evaluation.
Vitamin A biomarkers are crucial for assessing nutritional status. The most commonly used biomarkers are serum concentrations of retinol and retinol-binding protein (RBP). However, it's important to note that these biomarkers can be temporarily suppressed by infections and inflammation, which may lower the specificity of vitamin A assessments in certain populations5,6,7.
While liver biopsy or autopsy samples are considered the gold standard for assessing vitamin A status, the most sensitive indirect indicator of total liver vitamin A reserves is the retinol isotope dilution (RID) method8. RID provides a quantitative estimate of vitamin A status across the full spectrum, ranging from deficient to excessive stores9. In most research applications, the RID method involves administering an oral dose of deuterium (2H) or 13C-labeled retinyl acetate, which then mixes with body stores over a period of 14 to 21 days. After this period, a blood sample is collected, and the serum is stored at -80 °C. The ratio of labeled to total retinol is then analyzed using mass spectrometry to estimate vitamin A stores10, employing the Olson equation11, the mass-balance equation12, or the Green equation13. The protocol presented in this paper is valid for the administration of deuterium (2H)- or 13C-labeled retinyl acetate and is based on the work of Tang et al.14. The overall goal of this method is to accurately assess and monitor vitamin A status in the body11. It is a powerful method that provides a quantitative estimate of vitamin A concentrations across a broad spectrum of statuses, from deficiency to excess15. It is more accurate and precise than other methods, which often rely on indirect measures9.
Protocol
The protocol was approved by the Ethics Committee of the Ministry of Public Health (No. 2015/02/550/CE/CNERSH/SP), and informed consent was obtained from parents/guardians.
NOTE: Because vitamin A is light-sensitive, it is crucial that all procedures be conducted in dim light or under gold fluorescent lighting16. The materials used are detailed in Table of Materials.
1. Preparation of reagents
- Sodium chloride (0.85% w/v): Dissolve 0.85 g NaCl in distilled water in a 100 mL volumetric flask. Fill till the graduation mark with distilled water and mix.
- Chloroform-methanol (2:1 v/v): Transfer 300 mL of chloroform into a 500 mL volumetric flask with a measuring cylinder. Add 150 mL of methanol and mix.
- Mobile phase A for HPLC: Acetonitrile/Tetrahydrofuran/ultrapure water (50/20/30, v/v/v): Transfer 500 mL of acetonitrile into a 1000 mL volumetric flask. Add 200 mL of tetrahydrofuran and 300 mL of water. Mix the solution, filter it using a membrane filter with a pore size of 0.45 µm, and sonicate (amplitude 100%, frequency 40 kHz and duration 15 min).
- Mobile phase B for HPLC: Acetonitrile/Tetrahydrofuran/ ultrapure water (50/44/6, v/v/v): Transfer 500 mL of acetonitrile into a 1000 mL volumetric flask. Add 440 mL of Tetrahydrofuran and 60 mL of water. Mix the solution, filter it using a membrane filter with a pore size of 0.45 µm, and sonicate.
2. Preparation of standard solutions
- Prepare the stock solution by dissolving 40 mg of the standard in ethanol in a 100 mL volumetric flask, as illustrated in Table 1. Fill till the graduation mark with the solvent and mix thoroughly.
- Diluted stock solutions: Prepare the diluted stock solution by transferring 1 mL of the stock solution into a 50 mL volumetric flask, as shown in Table 2. Fill till the graduation mark with the appropriate solvent and mix thoroughly.
- Determination of the concentration of diluted stock solutions: Place an aliquot (1 mL) of the diluted solution in a quartz tube and measure its absorbance in the spectrophotometer at the specified wavelength (Table 3) using ethanol as the blank. Calculate the using the Lambert-Beer Law17. Ensure the spectrophotometer is calibrated before analysis by warming up the instrument for 15-30 min, selecting the desired wavelength, and zeroing the instrument by placing the blank in the sample holder. Calculate the concentration using the following formula:
Concentration = (absorbance/absorption coefficient) x 106 (µg/dL)
NOTE: To convert values from conventional units (µg/dL) to S.I. units (µmol/L), multiply the conventional value by the following conversion factors: 0.0304 for Retinyl acetate and 0.0349 for Retinol18. - Preparation of working standards: Using a volumetric pipette, transfer 2 mL of the diluted stock solutions into a 100 mL conical flask. Fill the flask to the graduation mark with ethanol, mix thoroughly, and close with a stopper.
- To determine the exact concentration of each solution, assess the purity of each component using HPLC equipped with a C18 column and a diode-array detector set to 340 nm. Program the pump to use Mobile Phase A and Mobile Phase B according to the schedule shown in Table 4. Ensure that the HPLC system is calibrated before analysis by validating various components such as the pump, detector, autosampler, and the entire system's performance.
- Transfer 1 mL of the diluted stock solutions into crimp vials and inject the samples into the HPLC system according to the normal procedure. Determine the purity using the following equation:
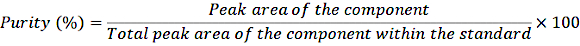
EQUATION 1
With this correction for purity, calculate the exact concentration of the different components in the standard solution.
- Preparation of internal standard (retinyl acetate OD ~ 0.2): Using a volumetric pipette, transfer 30 mL of the Retinyl acetate working standard into a 100 mL conical flask. Fill the flask to the graduation mark with ethanol, mix thoroughly, and close with a stopper.
- Preparation of working solutions: Add 20 µg of Non-Labelled-Retinol to 10.00, 3.33, 1.00, 0.33, and 0.00 µg of Labelled-Retinol to provide Labelled- and Non-labelled-retinol ratios of 0.500, 0.167, 0.050, 0.0167 and 0.00.
3. Sample analysis
NOTE: The serum samples used in this study were collected on the 14th day from children who received an oral dose (2 mg retinol equivalents) of D8-retinol as part of a study designed to monitor and assess the vitamin A status of children in Cameroon.
- Extraction of retinoids in serum: This extraction is based on the work of Folch et al.19 as modified by Tang et al.14. Follow the steps described below.
- Allow frozen serum samples to thaw gently at room temperature (20-25 °C) prior to analysis. Aliquot 400 µL of serum into a 16 x 100 mm disposable culture tube. Add 500 µL of 0.85% saline plus 100 µL of Internal Standard and 5 mL of chloroform-methanol (2:1 v/v) solution.
- Vortex for 30 s and centrifuge at 1157 x g for 10 min at 4 °C. Using a glass Pasteur pipette, carefully remove the chloroform lower layer to a 13 x 100 mm disposable culture tube. Dry under nitrogen gas in a water bath (40 °C) and resuspend the residue in 100 µL of ethanol. Vortex and sonicate for 30 s. Transfer the sample into a crimp vial with an insert, close the well, and label. The sample is ready for HPLC retinol collection.
- Perform HPLC retinol collection as described below.
- Inject 70 µL of each serum sample into an HPLC system equipped with a C18 column and a diode-array detector set to 340 nm. Program the pump to deliver Mobile Phases A and B at a constant flow rate of 1 mL/min, following the schedule shown in Table 4.
- Before injecting the serum samples, inject 70 µL of the retinol working solution into the HPLC system and record the retention time of the retinol peak. Then, set up the fraction collector to collect the eluted retinol fraction within a specified range in a 5 mL tube with a ground-glass stopper. In this case, the retinol fraction was collected over a 3 min interval, from 7.5-10.5 min.
NOTE: The concentration of retinol can also be calculated in this step by using a calibration curve of retinol standards and the recovery rate obtained from the internal standard.
- Perform derivatization of retinol as described below.
- Dry the retinol fraction collected from HPLC under nitrogen gas in a water bath at 40 °C for at least 3 h. Add ethanol to facilitate moisture evaporation during drying (samples should be completely dry, as the derivatization is moisture-sensitive). Add 20 µL of BSTFA with 10% TMCS to the tube.
- Place the tube in a dry block heater set to 70 °C and incubate for 30 min. Using a glass Pasteur pipette, transfer the reaction mixture into a crimp vial with an insert, seal tightly with crimp cap using a cap crimper, and label. The sample is now ready for GC/MS analysis, and it can be stored in a desiccator at 4 °C until analysis.
- Additionally, dry a 100 µL set of working standard solutions and derivatize as described above to calibrate the GC/MS.
NOTE: The derivatization of retinol using BSTFA involves a chemical reaction where the hydroxyl group (-OH) of retinol is replaced by trimethylsilyl group (-Si(CH3)3), forming retinyl trimethylsilyl ether. This process enhances the volatility and stability of retinol, making it more suitable for GC/MS analysis20.
- GC/MS analysis: GC/MS analysis is performed using the procedure described by Tang et al.14. Follow the steps described below.
- Inject 3 µL of the derivatized retinol sample using an autosampler into the GC with a cool on-column injector connected through a zero dead volume connector to a 15 m x 0.25 mm inner diameter fused silica capillary column coated with a DB-1 stationary phase of 0.25 µm film thickness.
- Program the column oven and on-column injector temperatures to increase from 50 °C to 285 °C at a rate of 15 °C/min, and set the GC/MS interface temperature to 285 °C. Use helium as the carrier gas to elute the trimethylsilyl derivative of retinol over approximately 12 min.
- Detect the GC eluate with a quadrupole mass spectrometer using 0.5 torr methane negative ion chemical ionization, with the ion source temperature set to 150 °C. Set the mass spectrometer to scan between 260 and 280 daltons.
4. Data analysis
- Use the GC/MS data analysis software.
- Extract all desired ions based on their mass-to-charge (m/z) ratios, integrate the peak's area (use manual integration to adjust the start and end points of the peak) and transfer the results to a spreadsheet file.
NOTE: Depending on the isotope used, the desired ions will be as follows: 268-270 m/z for native retinol; 271-274 m/z for [2H4]-retinol; 278-280 m/z for [13C]-retinol; and 276-280 m/z for [2H8]-retinol. - Calculate the sum of peaks for labeled retinol (ΣD) by adding peak area at m/z 274, 275, 276, 277 and 278. Calculate the sum of peaks for unlabeled retinol (ΣH) by adding peak area at m/z 268, 269 and 270.
- Calculate the enrichment of labeled retinol (D) using the following equation: D = ΣD/(ΣH + ΣD). Calculate the amount of unlabeled retinol (H) using the following equation: H = 1 - D.
NOTE: Gas Chromatography Electron Capture Negative Chemical Ionisation Mass Spectrometry of retinyl trimethylsilyl ether gives no molecular ion but a major fragment ion at m/z 268 to 271 for unlabeled retinol, at m/z 272 to 275 for D4-retinol, and at m/z 276 to 280 for D8-retinol14. - To control the system, calculate a linear regression equation between the weight ratios of the calibration standards and the integrated areas for labeled retinol and non-labeled retinol.
5. Estimation of vitamin A stores
NOTE: This step allows for the assessment of an individual's vitamin A status.
- Calculate vitamin A total liver stores using the Olson equation11
Vitamin A Total Liver Stores = F x Dose x [S x a x (H/D - 1)]
where F is a factor for the efficiency of absorption and storage of the orally administered dose (F = 0.50), Dose is the amount of labeled vitamin A administered orally (µmol), S is a factor that corrects for the inequality of the plasma to the liver ratio of labeled to nonlabeled retinol (S = 0.65), a is a factor that corrects for irreversible loss of labeled vitamin A during the mixing period; specifically, a = e-kt, where k is the estimated system fractional catabolic rate (k = ln 2/32 days for children), and t is time, expressed as days since dose; D/H is the serum isotopic ratio of labeled to non-labeled retinol, -1 corrects for the contribution of the dose of labeled vitamin A to the total body vitamin A pool. - For calculating vitamin A total body stores (TBS) use the Green equation13
TBS = Fa × S × (1/SAp)
where Fa is the fraction of the orally labeled dose of VA absorbed and found in the body's exchangeable storage pools at time t, and S is the ratio of retinol-specific activity in serum to that in stores at time t (for children, Fa × S = 0.642 at 14 days)21. SAp is the fraction of dose in serum per µmol dose (i.e., [labeled retinol] / ([unlabeled + labeled retinol] in serum) / oral dose of labeled retinol (µmol)).
Results
The injection of 3 µL of a derivatized sample of a dried solution containing approximately 50 pM/µL of calibrants (retinol and D8-retinol) into the GC/MS showed no molecular ion but exhibited a major fragment ion at m/z 268 for retinol (Figure 1) and m/z 278 for D8-retinol (Figure 2). This indicates that the molecular ions of retinyl trimethylsilyl ether, formed during the derivatization of retinol and D8-retinol, are not stable under the ionization conditions used in GC/MS. They break down into smaller fragments, primarily through alpha cleavage. This common fragmentation pattern involves the breaking of the bond adjacent to the trimethylsilyl ether group. The alpha cleavage results in the loss of the trimethylsilyl group (TMS, which has a mass of 73 Da) and a hydrogen atom, leading to a fragment ion with a mass of 268 Da for retinol and 278 Da for D8-retinol22. This mechanism helps in identifying and confirming the presence of retinol and its derivatives in the sample by analyzing the specific fragment ions produced during mass spectrometry. Injection of mixtures of retinol and D8-retinol showed two major fragment ions at m/z 268 for retinol and m/z 276 for D8-retinol (Figure 3), indicating the presence of these compounds in the sample. The calibration curve exhibited excellent linearity, as indicated by a high correlation coefficient (Figure 4), showing that the relationship between peak ratio and weight ratio has a very straight and predictable pattern.
The GC/MS response obtained from the injections of serum samples (serum from children who received an oral dose of 2 mg retinol equivalents of D8-retinol; Figure 5) shows the presence of retinol and D8-retinol, with the two major fragment ions observed at m/z 268 and m/z 274. Results obtained after extraction of ions and integration of peak areas at m/z 274, 275, 276, 277, and 278 for D8-retinol and at m/z 268, 269, and 270 for unlabeled retinol are presented in Table 5. These results are to be integrated into the Olson equation11 or the Green equation13 to calculate vitamin A stores in the body.
The stable isotope dilution technique provides measurements of vitamin A levels that cannot be obtained by other methods, enabling accurate assessments of vitamin A status. This method is valuable for nutritional studies, clinical diagnostics, and epidemiological research.
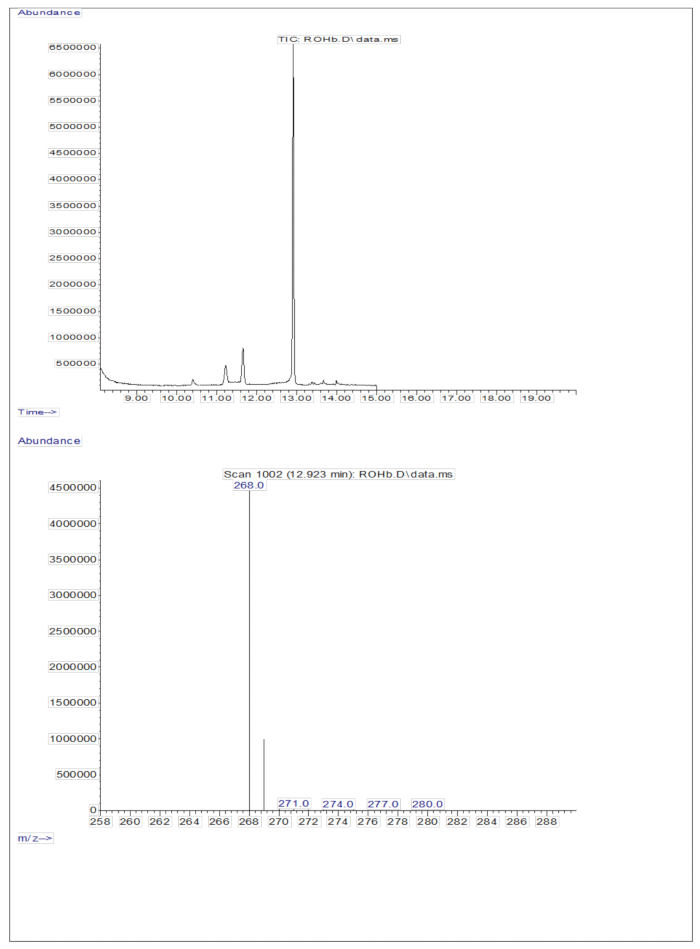
Figure 1: Chromatogram and mass spectrum of retinol. The figure shows the gas chromatography/methane electron capture negative chemical ionization-mass spectrometry chromatogram (top panel) from the analysis of the derivatized retinol standard. The bottom panel is a mass spectrum showing m/z 268 for retinol. Please click here to view a larger version of this figure.

Figure 2: Chromatogram and mass spectrum of D8-retinol. The figure shows the gas chromatography/methane electron capture negative chemical ionization-mass spectrometry chromatogram (top panel) from the analysis of the derivatized D8-retinol standard. The bottom panel is a mass spectrum showing m/z 278 for D8-retinol. Please click here to view a larger version of this figure.

Figure 3: Chromatogram and mass spectrum of a retinol and D8-retinol mixture. The figure shows the gas chromatography/methane electron capture negative chemical ionization-mass spectrometry chromatogram (top panel) from the analysis of a derivatized mixture of retinol and D8-retinol standards. The bottom panel is a mass spectrum showing m/z 268 for retinol and m/z 276 for D8-retinol. Please click here to view a larger version of this figure.
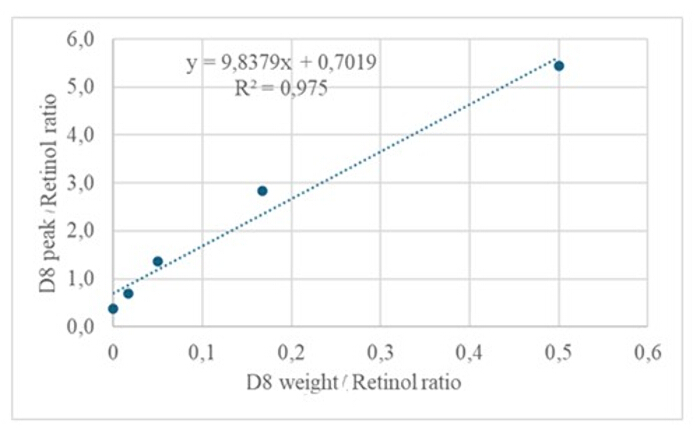
Figure 4: Calibration curve. This figure illustrates the relationship between the GC/MS response and the concentration of unlabeled and labeled retinol. It is described by the equation y = 9.8379x + 0.7019, where y (the area ratios) represents the instrument response, 9.8379 is the sensitivity, x (the weight ratios) represents the analyte concentration, and 0.7019 is the background signal. Please click here to view a larger version of this figure.
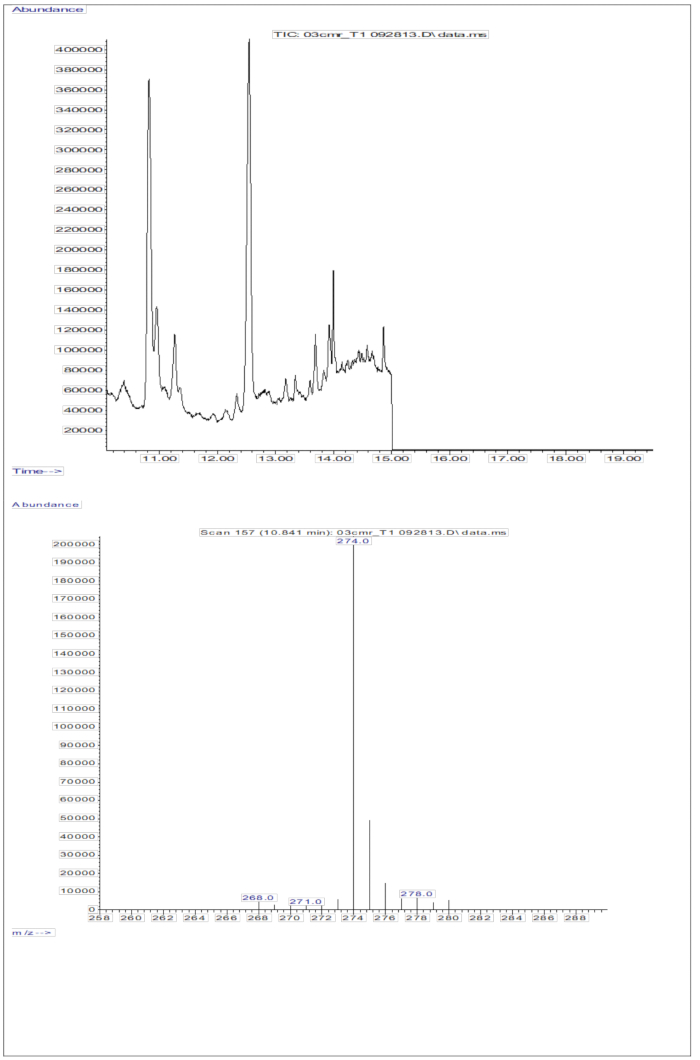
Figure 5: Chromatogram and mass spectrum of serum sample. This figure illustrates the gas chromatography/methane electron capture negative chemical ionization-mass spectrometry chromatogram (top panel) from the analysis of the derivatized retinol fraction of serum. The bottom panel is a mass spectrum showing m/z 274 for D8-retinol. Please click here to view a larger version of this figure.
| Standard | Weight (mg) | Volumetric flask (mL) | Solvent |
| Retinol | 40 | 100 | Ethanol |
| Retinyl acetate | 40 | 100 | Ethanol |
| Deuterium-labeled retinyl acetate | 40 | 100 | Ethanol |
Table 1: Preparation of stock solution. This table demonstrates how to prepare concentrated solutions of retinol and deuterium-labeled retinyl acetate, which can then be diluted to lower concentrations for future experiments.
| Standard | Stock solution (mL) | Volumetric flask (mL) | Solvent |
| Retinol | 1 | 50 | Ethanol |
| Retinyl acetate | 1 | 50 | Ethanol |
| Deuterium-labeled retinyl acetate | 1 | 50 | Ethanol |
Table 2: Preparation of diluted stock solutions. This table demonstrates how to prepare ready-to-use solutions of retinol and deuterium-labeled retinyl acetate.
| Standard | Wavelength (nm) | E1%1 cm |
| Retinol | 325 | 1850 |
| Retinyl acetate | 326 | 1550 |
Table 3: Wavelength and E1% 1 cm (Absorption coefficient).
| Time (min) | Flow (mL/min) | Mobile Phase A (%) | Mobile Phase B (%) |
| 0 – 6 | 1 | 100 | 0 |
| 6 – 13 | 1 | 100 → 50 | 0 → 50 |
| 13 - 18 | 1 | 50 | 50 |
| 18 - 20 | 1 | 50 → 0 | 50 → 100 |
| 20 - 28 | 1 | 0 | 100 |
| 28 - 29 | 1 | 0 → 100 | 100 → 0 |
Table 4: Time schedule for HPLC mobile phases. This table illustrates the planned sequence and duration of different phases during the HPLC chromatographic run.
| SH | SD | D | H | |
| Subject 1 | 64030809 | 566089.7 | 0.008763 | 0.991237 |
| Subject 2 | 194354 | 43861.39 | 0.184125 | 0.815875 |
| Subject 3 | 793490 | 80179.28 | 0.091773 | 0.908227 |
| Subject 4 | 2002063 | 45286.7 | 0.02212 | 0.97788 |
| Subject 5 | 80999193 | 355980.7 | 0.004376 | 0.995624 |
| Subject 6 | 32196717.7 | 216152.7 | 0.006669 | 0.993331 |
| Subject 7 | 40905724.5 | 334818.1 | 0.008119 | 0.991881 |
| Subject 8 | 28336711.5 | 218924.1 | 0.007667 | 0.992333 |
| Subject 9 | 8695135.5 | 542077 | 0.058684 | 0.941316 |
| Subject 10 | 103260212 | 1717728 | 0.016363 | 0.983637 |
| SH: sum of peak area at m/z 268, 269 and 270 | ||||
| SD: sum of peak area at m/z 274, 275, 276, 277 and 278 | ||||
| D: enrichment of labeled retinol | ||||
| H: level of unlabeled retinol |
Table 5: Results of GC/MS of derivatized retinol fraction of serum. This table reports GC/MS outputs needed for the calculation of vitamin A total body store. H is the sum of the peak area at m/z 268, 269, and 270; D is the sum of the peak area at m/z 274, 275, 276, 277, and 278; D is the enrichment of labeled retinol; H is the level of unlabeled retinol; TBS is vitamin A Total Body Store.
Discussion
The successful implementation of this protocol relies on the effective execution of each step. Proper preparation of solutions and standards is crucial to ensure that the data collected is accurate and reliable. The procedures described in the protocol were tested in various settings and are suitable for obtaining solutions and standards that meet the objectives of sample analysis.
The analysis of samples starts with the extraction and separation of retinol in the serum. Storing serum samples at -80 °C until analysis is essential to prevent the degradation of vitamin A. Additionally, working in dim light is necessary16. The HPLC procedure used to collect the retinol fraction is designed to separate retinol from other fat-soluble components to avoid interference in the derivatization process. It also allows running hundreds of samples without flushing the column.
The retinol fraction collected from HPLC is derivatized with BSTFA at 70 °C for 30 min. The derivatization step is critical for improving the volatility of retinol and its detectability in GC-MS. Since it is sensitive to water, it is crucial to completely dry the sample before derivatization and to allow sufficient time for the derivatization reaction to occur before GC-MS analysis. It was noticed that the derivatization process with BSTFA is very mild and efficient compared to the one using N-methyl-N-(tert-butyldimethylsilyl) trifluoroacetamide (MTBSTFA)23,24 and provides a very sharp peak for derivatized retinol, with no tailing or background peaks in the scanned mass range14. Derivatized retinol in a sealed vial can be kept in a desiccator at 4 °C for 1 month without degradation14.
For the GC-MS analysis, it is critical to ensure proper calibration and maintenance of the column, and to optimize the injection volume, temperature, and flow rates. The conditions used here with on-column injection showed good and reliable results14. During GC-MS analysis of deuterated and unlabeled retinol, Tang et al.14 observed that the peaks of deuterated retinol appeared both in the administered dose and in the serum of subjects who received the dose. This pattern was not seen with unlabeled retinol. Therefore, they concluded that the peaks of deuterated retinol are pre-formed in the dose and not a result of fragmentation in the mass spectrometer, suggesting that the deuterated retinol maintains its structure through the metabolic process, providing a reliable marker for tracking retinol in biological studies. The enrichment of deuterated retinol in serum after the administration of 2 mg of D8-retinol in preschool-age children began to increase at 7 h and reached its peak at 14 days, which is the shorter optimal sampling time25. In 200 µL of human serum, the minimum detectable percent enrichment of retinol is 0.01%, demonstrating that the method is sensitive enough to analyze serum samples collected from subjects with a wide range of vitamin A statuses14.
While the stable isotope dilution technique presented here offers significant advantages for assessing vitamin A status, it should be noted that this method requires sophisticated equipment and technical expertise, making it expensive and less accessible for routine use in many settings26. Therefore, it is essential to consider this limitation when planning to use this technique.
Disclosures
This manuscript is part of a series entitled Utilizing the Retinol Isotope Dilution Method for Assessing Vitamin A Body Stores and Liver Vitamin A Concentration supported by IAEA.
Acknowledgements
We learned this protocol during a fellowship at the Carotenoids and Health Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, Boston, USA, under the supervision of Guangwen Tang and with the financial support of the International Atomic Energy Agency (IAEA).
Materials
| Name | Company | Catalog Number | Comments |
| 13x100 mm disposable culture tubes | 99445-13 | PYREX Disposable Rimless Culture Tubes | |
| 16x100 mm disposable culture tubes | 99445-16 | PYREX Disposable Rimless Culture Tubes | |
| 24 Position N-EVAP Nitrogen Evaporator | Organomation Associates, Inc | 11250 | N-EVAP 112, Nitrogen Evaporator, with OA-SYS heating system |
| Acetonitrile | Sigma-Aldrich | 00687 | Acetonitrile, suitable for HPLC, gradient grade, ≥99.9% |
| Amber colored Crimp vials, 2 mL, | SU860033 | Short thread autosampler vial, amber vial 11.6 x 32 mm | |
| Analytical Balance | Mettler Toledo | 30133525 | Precision Balance MS303TS/00 |
| C18 column | Perkin-Elmer Inc | 2580195 | Brownlee Pecosphere RA C18 Cartridge Column - 33 mm x 4.6 mm I.D., Pkg. 5 |
| cap crimper | MilliporeSigma | Z114243 | Hand-operated aluminum cap crimper O.D. 20 mm |
| Capillary column | J & W Scientific | 122-1011 | 15 m × 0.25 mm i.d. fused silica capillary column coated with a DB-1 stationary phase of 0.25 mm film thickness |
| Centrifuge | Sigma 3-18KS | ||
| Chloroform | Sigma-Aldrich | 528730 | Chloroforme, HPLC grade, ≥ 99.9% |
| Conical Flasks: 100 mL, | 4980016 | Borosil Erlenmeyer Flasks Graduated Conical NM Borosilicate | |
| Crimp caps with PTFE seal | Supelco | 27455-U | Crimp seals with PTFE/silicone septa |
| D8-Retinyl acetate | Cambridge Isotope Laboratories Inc. | DLM-2244-PK | Vitamin A acetate 3-4% cis (10, 14, 19, 19, 19, 20, 20, 20-D8, 90%) |
| Dispenser for 1-10 mL | Gilson | F110103 | DISPENSMAN Bottle-top Dispenser |
| Dry Block Heater | Grant | Grant QBH2 High Performance Digital Dry Block Heater | |
| Ethanol | Sigma-Aldrich | 459844 | Ethyl alcohol, Pure, ≥ 99.5%, ACS reagent, 200 proof |
| GC-MS | Agilent | Agilent 7890 A Series Gas Chromatography with 5975C Mass Spectrometer System equipped with a 5975C inert XL EI/CI MSD/DS Turbo CI System, a 7693A Auto‐injector Includes transfer turret and a 7693 sample Tray | |
| Glass stoppered volumetric Flasks: 2000 mL | 956854 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Glass stoppered volumetric Flasks: 100 mL | 956849 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Glass stoppered volumetric Flasks: 1000 mL | 956853 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Glass stoppered volumetric Flasks: 25 mL | 956841 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Glass stoppered volumetric Flasks: 50 mL | 956847 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Glass stoppered volumetric Flasks: 500 mL | 956852 | BRAND BLAUBRAND volumetric flask, glass stopper, clear glass | |
| Helium (highest purity) | Air Liquide | UN 1046 Helium compressed, Class 2.2 | |
| HPLC | Varian | Varian 940LC HPLC with fraction collector | |
| Inserts for crimp vials, 5 mm, 175 μL, | AR0-4521-12 | Verex insert, 5 mm Dia, 175 µl, clear, conical bottom, w/bottom spring | |
| Measuring Cylinders: 100 mL | 213902402 | DURAN Measuring Cylinder, with Hexagonal Base, Class A | |
| Measuring Cylinders: 250 mL | 213903604 | DURAN Measuring Cylinder, with Hexagonal Base, Class A | |
| Methane (highest purity) | Air Liquide | UN1971 Methane compressed, Class 2.1 | |
| Methanol | Sigma-Aldrich | 34860 | Methanol, suitable for HPLC, ≥ 99.9% |
| N, O-bis(trmethylsilyi)trifluoroacetamide (BSTFA) with 10% Trimethylchlorosilane (TMCS) | Thermo Scientific | 043939.22 | |
| Nitrogen | Produced by Parker Balston NitroVap Generator | ||
| Pasteur Pipettes, glass, | 13-678-20A | Fisherbrand Disposable Borosilicate Glass Pasteur Pipets | |
| Quartz glass Cuvettes | EW-83301-12 | Cole-Parmer Standard Single Quartz Cuvettes | |
| Retinol | Sigma-Aldrich | 17772 | ≥95.0% (HPLC), ~2700 U/mg |
| Retinyl acetate | Sigma-Aldrich | R0635 | analytical standard grade |
| Sodium chloride | Sigma-Aldrich | S9888 | Chlorure de sodium, ACS reagent, ≥ 99.0% |
| Spectrophotometer | Shimadzu | Uvmini-1240 UV-Vis Spectrophotometer | |
| Tetrahydrofuran | Sigma-Aldrich | 439215 | Tetrahydrofurane, HPLC grade, ≥ 99.9%, inhibitor-free |
| Ultrasonic cleaner | Bransonic | CPX-952-339R | Branson CPX Bransonic Ultrasonic Bath |
| Volumetric Pipettes: 100-1000 µL | 3123000063 | Eppendorf 1-canal micropipette with T.I.P.S. Box 2.1 | |
| Volumetric Pipettes: 20-200 µL | 3123000055 | Eppendorf 1-canal micropipette with T.I.P.S. Box 2.0 | |
| Vortex mixer | Ika | Vortx Genius 3 |
References
- D'Ambrosio, D., Clugston, R., Blaner, W. Vitamin A metabolism: an update. Nutrients. 3 (1), 63-103 (2011).
- WHO. Global prevalence of vitamin A deficiency in populations at risk 1995-2005. WHO Global Database on Vitamin A Deficiency. , (2009).
- Kraemer, K., et al. Are low tolerable upper intake levels vitamin A undermining effective food fortification efforts. Nutr Rev. 66 (9), 517-525 (2008).
- Allen, L. H., Haskell, M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr. 132 (9 Suppl), 2907S-2919S (2002).
- Filteau, S. M., et al. Influence of morbidity on serum retinol of children in a community-based study in northern Ghana. Am J Clin Nutr. 58 (2), 192-197 (1993).
- Rubin, L. P., Ross, A. C., Stephensen, C. B., Bohn, T., Tanumihardjo, S. A. Metabolic effects of inflammation on vitamin A and carotenoids in humans and animal models. Adv Nutr. 8 (2), 197-212 (2017).
- Suri, D. J., et al. Inflammation adjustments to serum retinol and retinol-binding protein improve specificity but reduce sensitivity when estimating vitamin A deficiency compared with the modified relative dose-response test in Ghanaian children. Curr Dev Nutr. 5 (8), nzab098 (2021).
- Tanumihardjo, S. A. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr. 94 (2), 658S-665S (2011).
- Furr, H. C., et al. Stable isotope dilution techniques for assessing vitamin A status and bioefficacy of provitamin A carotenoids in humans. Public Health Nutr. 8 (6), 596-607 (2005).
- Haskell, M. J., Ribaya-Mercado, J. D. the Vitamin A Tracer Task Force. Handbook on Vitamin A Tracer Dilution Methods to Assess Status and Evaluate Intervention Programs. Technical Monograph 5. , (2005).
- Furr, H. C., Amedee-Manesme, O., Bergen, H. R., Anderson, D. P., Olson, J. A. Vitamin A concentrations in liver determined by isotope dilution assay with tetradeuterated vitamin A and by biopsy in generally healthy adult humans. Am J Clin Nutr. 49 (4), 713-716 (1989).
- Gannon, B. M., Tanumihardjo, S. A. Comparisons among equations used for retinol isotope dilution in the assessment of total body stores and total liver reserves. J Nutr. 145 (5), 847-854 (2015).
- Green, M. H. Evaluation of the "Olson equation", an isotope dilution method for estimating vitamin A stores. Int J Vitam Nutr Res. 84 (Suppl 1), 9-15 (2014).
- Tang, G., Qin, J., Gregory, G., Dolnikowski, G. G. Deuterium enrichment of retinol in humans determined by gas chromatography electron capture negative chemical ionization mass spectrometry. J Nutr Biochem. 9 (7), 408-414 (1998).
- Tanumihardjo, S., et al. . Appropriate uses of vitamin A tracer (stable isotope) methodology. Vitamin A tracer task force. , (2004).
- Barua, A. B., Furr, H. C. Properties of retinoids: structure, handling, and preparation. Mol Biotechnol. 2 (2), 167-182 (1998).
- Swinehart, D. F. The Beer-Lambert Law. J Chem Edu. 39, 333-335 (1962).
- Barua, A. B., Furr, H. C. Properties of retinoids. Structure, handling, and preparation. Methods Mol Biol. 89, 3-28 (1998).
- Folch, J., Lees, M., Stanley, S. G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226 (1), 497-509 (1957).
- Knapp, D. R. . Handbook of analytical derivatization reactions. , (1979).
- Green, M. H., Green, J. B. Use of model-based compartmental analysis and theoretical data to further explore choice of sampling time for assessing vitamin A status in groups and individual human subjects by the retinol isotope dilution method. J Nutr. 157 (7), 2068-2074 (2021).
- Todd, J. F. J. Recommendations for nomenclature and symbolism for mass spectroscopy (including an appendix of terms used in vacuum technology). (Recommendations 1991). Pure and Appl Chem. 63 (10), 1541-1566 (1991).
- Handelman, G. J., Haskell, M. J., Jones, A. D., Clifford, A. J. An improved protocol for determining ratios of retinol-d4 to retinol isolated from human plasma. Anal Chem. 65 (15), 2024-2028 (1993).
- Tang, G., Andrien, B. A., Dolnikowski, G., Russell, R. M. . Methods in Enzymology, Vitamins and Coenzymes. Part L. 282, 140-154 (1997).
- Haskell, M. J., et al. Population-based plasma kinetics of an oral dose of [2H4]retinyl acetate among preschool-age Peruvian children. Am J Clin Nutr. 77 (3), 681-686 (2003).
- Lopez-Teros, V., et al. International experiences in assessing vitamin A status and applying the vitamin A-labeled isotope dilution method. Int J Vitam Nutr Res. 84 (Suppl 1), 40-51 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved