このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
新生児マウス感覚皮質における自発的活動の単一ニューロン分解能でのin vivo可視化
* これらの著者は同等に貢献しました
要約
新皮質の主要な感覚野は、発達中に独特の自発的な活動を示します。この記事では、 in vivoで新生児マウスの領域特異的な同期活動を解析するために、個々のニューロン活動と一次感覚野を可視化する方法について説明します。
要約
哺乳類の脳は、出生前と出生後の期間を通じて、細胞レベルと回路レベルの両方でダイナミックな発達変化を経験します。これらの発生変化に寄与する多数の遺伝子の発見に続いて、現在では、ニューロンの活動もこれらのプロセスを実質的に調節することが知られています。発達中の大脳皮質では、ニューロンは各一次感覚領域に特化した同期した活動パターンを示します。これらのパターンは、成熟した皮質で観察されるパターンとは著しく異なり、領域固有の発達過程を調節する役割を強調しています。発達中のニューロン活動の欠損は、さまざまな脳疾患につながる可能性があります。これらの知見は、ニューロン発生における活動パターンの根底にある調節機構を調べる必要性を浮き彫りにしている。この論文は、新生児マウスの一次感覚野とニューロン活動を視覚化し、 in vivoで2光子顕微鏡を使用して皮質サブフィールド内の個々のニューロンの活動を画像化し、サブフィールド関連の活動相関を分析するための一連のプロトコルをまとめたものです。体性感覚皮質の個々のバレル内でのパッチワークのような同期活動の代表的な結果を示します。また、このプロトコルのさまざまな潜在的なアプリケーションといくつかの制限についても説明します。
概要
大脳皮質には、異なる機能を持ついくつかの感覚領域が含まれています。領域は、対応する感覚器官から入力を受け取り、主に脊髄または脳幹を介して伝達され、視床1,2を介して中継されます。特に、各一次感覚野のニューロンは、発達初期に独自の同期活動を示し、これも感覚器官または下部神経中枢に由来しますが、成熟した皮質で観察される活動とは本質的に異なります3。
例えば、新生児のげっ歯類では、一次視覚野(V1)は波状の活動を示し、これは網膜(網膜波)に由来し、網膜トピーを保存しながら視覚経路全体を通じて伝播する4。一次聴覚野(A1)は、成熟した脳の等周波帯に対応する帯状のサブ領域に編成された同期活動を示します。この活性は、蝸牛の内側の有毛細胞5,6から発せられます。一次体性感覚野(S1)の樽皮質は、個々の樽内の第4層ニューロン、すなわち個々のヒゲに応答するニューロンが同期的に活性化されるパッチワークのような活動パターンを示している7。三叉神経節に由来すると提唱されているが、その活動の源は不明のままである7。その結果、新生児の活動パターンは、各一次感覚領域内と領域内サブフィールドの両方で特殊化されます。ニューロンの活動と一次感覚野の構造を同時に可視化することで、これらの活動パターンが感覚系の発達にどの程度寄与しているのかを調べることができるかもしれません。
この記事では、一連のプロトコルを要約しました:(1)視床皮質軸索に赤色蛍光タンパク質を発現するTCA-RFPマウスを使用して、GCaMPおよび一次感覚野のスパース標識を使用して個々のニューロン活動を視覚化する7、(2)in vivoで2光子顕微鏡を使用して新生児マウスの単一細胞レベルの活動を画像化する、(3)S1バレル皮質内の活動相関を分析する。代表的な結果は、出生後(P)6マウスの個々のバレル内でパッチワークのような同期活性を示しています。いくつかの制限はありますが、この手法は、慢性イメージング、複数の感覚領域にわたる広視野イメージング、およびさまざまな操作実験に使用できます。発達過程における神経活動の多角的な解析は、脳回路形成メカニズムの理解を深めるでしょう。
Access restricted. Please log in or start a trial to view this content.
プロトコル
すべての実験は、熊本大学と国立遺伝学研究所の動物実験ガイドラインに従って行われ、動物実験委員会によって承認されました。
1. 子宮内 エレクトロポレーション(IUE)
- ICRバックグラウンドの雄TCA-RFPマウスと雌の野生型ICRマウスを交配します。膣栓を観察して、翌日の早朝に交尾を確認します。2週間後に腹部を観察して妊娠を確認します。
- 5 ng/μL TRE-nCre、1 μg/μL CAG-loxP-stop-loxP-GCaMP6s-ires-tTA-WPRE、およびddH2O中の0.02 % Trypan Blueを含むプラスミド溶液を調製し、Supernovaシステム8を使用してGCaMPでニューロンをスパースに標識します。
- 0.5 mg / mLのカルプロフェンと0.01 mg / mLのブプレノルフィンを含む鎮痛液を準備します。.光から保護し、室温で保管してください。
- 胚の日(E)14に、次のようにIUEを行います。IUE法は、以前の報告9,10,11と基本的に同じである。
- ラボベンチを70%エタノールまたはグルタルアルデヒド溶液で拭きます。すべての手術器具をオートクレーブ滅菌します。マウスへの感染リスクを減らすために、マスクと白衣を着用してください。
- マイクロピペットプーラーを使用してガラスマイクロピペットを準備します。プラスミド混合物溶液を吸引チューブを使用してガラスマイクロピペットに取り込みます。
- E14で妊娠中のマウスにイソフルラン(空気中2.0%)を使用して麻酔を誘発します。.処置中はマウスをイソフルラン(空気中1.2%)で麻酔しておきます。.つま先をつまんで、麻酔が十分に深いことを確認します。眼の乾燥を防ぐために眼科用軟膏を使用してください。
- ヨウ素ベースのスクラブと70%エタノールを交互に繰り返して、腹部を少なくとも3回消毒します。.手術領域を覆うように滅菌ドレープを配置します。正中線を切開し、子宮をドレープに露出させます。
- 温かい生理食塩水を子宮に繰り返し滴下して、腹部が閉鎖されるまで子宮が乾燥または冷えるのを防ぎます(ステップ1.4.8)。
- プラスミド溶液をマイクロピペットと吸引チューブを使用して、胚の側脳室の片側に1つずつ注入します。
- 鉗子型電極を用いて胚の頭部をつまみ、エレクトロポレーターを用いて1秒間隔で5回、方形電気パルス(40V、50ms)を送達します。
- 子宮を腹腔に戻します。~3 mLの温かい生理食塩水を空洞に塗布します。腹膜と腹部の皮膚を縫合します。鎮痛液を10 μL/gの重量で投与します(カルプロフェンは5 μg/g、ブプレノルフィンは0.1 μg/gの重量)首の後ろの皮下。
- マウスを温熱パッド(37°C)のリカバリーケージに入れて、麻酔から回復させます。マウスを腹の上に置き、舌や唾液が喉を詰まらせないようにします。マウスを他のマウスから離し、正常に動くように回復するまで監視します。マウスをケージに戻します。
- 出生後、ジェノタイピングを実行してRFP対立遺伝子をチェックし、対立遺伝子のない子犬を安楽死させます。このステップは、特にTCA-RFPシグナルが弱く、ステップ2.2.7で確認するのが難しい出生後2週目にイメージングを行う場合に推奨されます。
2.頭蓋窓手術
- 125 mM NaCl、5 mM KCl、10 mM グルコース、10 mM HEPES、2 mM CaCl2、および 2 mM MgSO4 を ddH2O (pH を 1 M NaOH で 7.4 に調整) 12 に含有する皮質緩衝液を手術の日の前に調製します。真空フィルターを使用してバッファーを滅菌します。
注:バッファーは4°Cで最大3ヶ月間保持できます。必要な容量は子犬あたり5〜10mLです。 - P3-12で次の手順を実行します。この手順について説明した以前のレポートも参照してください13,14.
- 50 mgのアガロースを5 mLの皮質緩衝液と混合し、加熱してアガロースを完全に溶解します。溶液の一部を1.5 mLのチューブに入れ、42°Cに保ちます。
- 皮質バッファーを50mLのコニカルチューブに入れ、室温に保ちます。生理食塩水を容器(50mLのコニカルチューブのキャップなど)に入れ、室温に保ちます。
- 0.01 mg / mLブプレノルフィンを含む鎮痛液を調製します。.光から保護し、室温で保管してください。
- すべての手術器具をオートクレーブ滅菌します。蛍光実体顕微鏡を70%エタノールで消毒します。
- イソフルラン気化器を使用して、イソフルラン(空気中で2.0%)で子犬に麻酔を誘発します。子犬に注意を払い、呼吸が少し遅くなったら取り出してください。
注:麻酔が長引くと、子犬の呼吸が数秒間停止することがあります。子犬の呼吸が再開されたとしても、長時間の麻酔により子犬の脳血流が減少し、不可逆的な脳損傷を引き起こす可能性があります。 - 子犬の頭を少なくとも3回、ヨウ素ベースのスクラブと70%エタノールを交互に繰り返して消毒します。子犬を蛍光実体顕微鏡下の加熱パッド(35°C)に置きます。子犬は常にイソフルラン(空気中で1.5%〜2.5%)で麻酔をかけてください。
- TCA-RFPおよびGCaMPを発現する仔犬を、頭蓋骨を通して蛍光を観察して選抜します。両方を表現していない子犬を安楽死させます。
- 出血を引き起こさないように、大脳半球の上の頭皮をできるだけ広く慎重に取り除きます。滅菌生理食塩水に浸した綿棒で頭蓋骨をこすり、結合組織を取り除きます。
- 頭蓋骨が乾いたら、切開した頭皮表面をティッシュ接着剤で頭蓋骨に接着します。
- 子犬を37°Cの加熱パッドに移して、麻酔から回復します。接着剤が固まるまで少なくとも15分待ちます。
注意: 次のステップに進む前に、最大1時間一時停止します。この期間中に必要に応じて、他の子犬も同様の方法で準備します。 - イソフルランで子犬を麻酔します。麻酔をかけた状態で、子犬を蛍光実体顕微鏡(37°C)の加熱パッド(37°C)に置きます(空気中の1.5%-2.5%イソフルラン)。
注:麻酔がエンドポイントの時間(60分)よりも長引く場合は、イソフルランで麻酔をかけている間に斬首して子犬を安楽死させます。 - 頭蓋骨のGCaMP発現位置を滅菌油性マーカーでマークします。その場所に皮質バッファーを適用します。
- カミソリの刃の角を頭蓋骨に挿入します。刃をゆっくりと押して頭蓋骨を削り取り、穴を開けます。ひび割れた頭蓋骨をピンセットでつまんで外します。
- 穴の中の血管を観察して、頭蓋穴が正常に作られていることを確認します。出血が発生した場合は、マイクロピペットを使用して皮質バッファーで穴をすばやくすすいでください。出血が完全に止まるまですすぎを繰り返します。
- 頭蓋骨穴に皮質緩衝液を一滴垂らし、穴の上に直径3mmの滅菌済み丸型カバーグラスを置きます。余分なバッファーを不織布で拭き取ります。ガラスの周りが乾くまで待ちます。
- マイクロピペットを使用して、ガラスの端の周りに温かいアガロース溶液を塗布します。アガロースが多すぎると蛍光シグナルが低下する可能性があるため、ガラスを上から軽く押してガラスの下の余分な溶液を取り除きます。
- ピンセットを使用して、ガラスの上のアガロースを取り除くか、ガラスの端から離します。アガロースはガラスの外側の周囲だけに置いておきます。
- アガロースが固まるまで待ちます。アガロースの収縮によりガラスの下にスペースができる場合は、側面からアガロース溶液を追加してガラスの端全体を覆います。不織布で頭蓋骨の表面から液体を取り除きます。
- アクリル樹脂の粉末と液体をゴム容器に混ぜます。マイクロピペットで混合物を吸引し、ガラスの端を囲むアガロースを樹脂で覆うように注ぎます。
注:粉末と液体を混合するとすぐに樹脂が固化するため、塗布する前に繰り返し混合する必要があります。各混合に必要な量は、液体の場合は~500μL、粉末の場合は~0.15gです。 - 樹脂でチタンバーを反対側の半球に固定します。バーの角度をカバーガラスと平行に保ちます。頭蓋骨全体の表面をレジンで固定します。
- 鎮痛液を10μL / g重量で(ブプレノルフィン重量0.1μg / g重量)首の後ろの皮膚の下に投与します。.子犬を37°Cの加熱パッドに戻して、麻酔を回復させます。樹脂の固化を>60分待ってからイメージングします。
注意: イメージングする前に1〜5時間一時停止します。この間に他の子犬にも同様の方法で手術を行います。
3. 2光子カルシウムイメージング
- 顕微鏡の下にXY位置を置いたステージプレートに、チタンプレートを装着した2軸ゴニオメーターを取り付けます。ステージに加熱パッド(35°C)を設置します。
- 次の条件でスキャンソフトウェアをオンにします: ピクセル、512 x 512;双方向、オン;平均化、なし。イメージングエリア、600 x 600 μm 、 対物レンズ20倍。 スキャンレート が 1Hzより速くなるように設定してください。
- 子犬を加熱パッドに置き、ヘッドマウントチタンバーをネジでチタンプレートに固定します。イソフルラン用のチューブポート(空気中1.5〜2.0%)を配置して、子犬を麻酔し続けます。
- ゴニオメーターでウィンドウの角度を水平に調整します。バックライトをONにし、5倍対物レンズで脳表面を観察し、XYポジショニングで撮像領域を選択します。
- 頭蓋窓に点眼薬を塗ります。対物レンズを20倍水浸レンズに切り替えます。皮質表面を観察して、脳表面に血流が見られることを確認します。
- バックライトをオフにし、1光子モードで脳の表面をスキャンします。レーザー出力を上げて、ガラスと脳の表面の緑色の自家蛍光を見えるようにします。
- イソフルラン濃度を1.0%〜1.5%に下げます。光漏れを防ぐために顕微鏡を覆います。スキャニングソフトウェアを2光子モードに切り替えます。
- GCaMPおよびRFP信号のスキャンに適したレーザー出力と検出器ゲインを調整します。TCA-RFP 信号が見られる深さを見つけます。深さが層 4 で、P6 の脳表面よりも ~300 μm 低い ことを確認してください。GCaMPを発現するニューロンが多く見られるイメージング領域を選択します。
- GCaMPおよびRFP信号のタイムラプスイメージングを開始します。2チャンネル同時スキャンができない場合は、イメージング前にGCaMPおよびTCA-RFP画像をキャプチャします。
- イソフルランを止めて麻酔を弱め、~20分間自発的な活動を観察します。赤外線カメラを使用して、イメージング中の子犬の動きを監視します。苦痛を示す反応が観察された場合は、すぐにイソフルラン麻酔(空気中で2%)を再開します。.
- 子犬の動きが止まったら、ステップ3.9からのイメージングを繰り返します。必要に応じてイメージングエリアを変更します。
- イソフルランの過剰摂取で子犬を安楽死させます。生理食塩水と 4% PFA の経心灌流により脳を固定し、続いて 4% PFA で一晩固定後、接線スライスを調製し、免疫組織化学を行います。それ以外の場合は、麻酔薬の過剰投与で子犬を安楽死させ、その後斬首します。
- 母ネズミに他に子犬がいない場合は、母ネズミを麻酔薬の過剰投与で安楽死させ、その後子宮頸部脱臼を行います。
4. 分析
- MATLAB を起動し、EZcalcium toolbox15 を実行して、グラフィカル ユーザー インターフェイス (GUI) の「初期 GUI」を開きます。
- マウスの動きなどによる画像フレームのずれを補正します。
- 初期 GUI の [Motion Correction ] をクリックして、 モーション補正 GUI を開きます。「Add Files...」をクリックして、イメージングデータのTIFファイルを読み込みます。
- 次のように設定を設定します: 非剛体モーション補正、空白。 アップサンプリング係数、50; 最大シフト、15; 初期バッチサイズ、200; ビン幅、200。「Motion Correctionを実行」をクリックして補正を実行します。モーション補正された画像データは自動的に保存されます。
メモ: 設定は、イメージング データのプロパティに合わせて調整する必要があります。非線形フレームの歪みや皮質の深度方向への移動により、一部のフレームのドリフトが補正されない場合は、ImageJ Fijiで元の画像データを補正せずに開き、フレームを削除してから、手順4.2を再開します。
- ニューロンを検出し、関心領域(ROI)を描画します。
- 初期 GUI で [Automated ROI Detection ] をクリックして、 ROI 検出 GUI を開きます。[ ファイルの追加...] をクリックして、モーション補正された画像データを読み込みます。
- 次のように設定を設定します: 初期化、貪欲。検索方法、楕円。デコンボリューション、制約付きFOOPSI-SPGL1;自己回帰、崩壊;ROIの推定#、60(視覚的に検出された数の2倍以上を推奨)。推定ROI幅、17(~20μm);マージしきい値、0.95;ファッジファクター、0.95;空間ダウンサンプリング、1;温度ダウンサンプリング、1;一時的な反復、5。
メモ: 設定は、イメージング データのプロパティに合わせて調整する必要があります。 - [ ROI 検出の実行 ] をクリックして、検出を実行します。ROIデータは自動的に保存されます。
- 初期 GUI で ROI Refinement をクリックして、 ROI Refinement GUI を開きます。「 Load Data 」をクリックして、ROIデータをロードします。活動頻度が低い (<1 Hz)、頭蓋骨の下に位置している、または他のニューロンの神経突起が含まれていた ROI を選択します。「 ROI を除外 」をクリックして、ROI を分析から除外します。
- 「Data Export Format to XLSX」を選択し、「Export Data」をクリックして、生のdF/F値を含むExcelファイルを取得します。dF/Fは式(1)を使用し、Fは各フレームのピクセルの平均強度、F0はベースライン信号強度、Fbはバックグラウンド蛍光です。
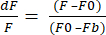 (1)
(1)
- ROI間のdF/Fのピアソンの相関係数を計算し、相関係数行列を作成します。麻酔が弱まり、自発的な活動が起こり始めた後(イソフルランを中止してから~10分後)にのみdF / Fを使用してください。.
- フィジーを使用して、TCA-RFP イメージからバレル エッジを定義します。ROIをそれぞれのバレルまたはセプタムに分類します。同じ銃身内のペアワイズ相関と異なる銃身間のペアワイズ相関を比較します。
- ROIとCa2+ トレース間の対応をランダムにシャッフルすることにより、1,000〜10,000のサロゲートデータを生成します。各サロゲートデータの個々のバレル内の平均相関係数を計算し、実際のデータにおける相関の統計的有意性を評価します。
注: 10,000 個の代理母の 1 つが実際の値より大きい値を持つ場合、統計的有意性は 0.0001 です。ステップ4.5および4.6で説明した分析は、他の場所で実施されているように、複数の動物からのプールされたデータに対して実施することができる7,16。
Access restricted. Please log in or start a trial to view this content.
結果
図1 は、本プロトコルを使用して視覚化されたP6仔のバレル皮質における第4層ニューロン活動の代表的な結果を示しています。緑チャネル(GCaMP)と赤チャネル(TCA-RFP)の2光子画像を時間平均化し、 図1Aに示します。TCA-RFP蛍光はGCaMP蛍光よりもはるかに弱かったため、GCaMP信号は赤チャネルに漏れました(図1A1
Access restricted. Please log in or start a trial to view this content.
ディスカッション
自発的な活動が感覚器官または下部神経系から発生し、成熟した神経系3と同等の経路を通じて一次感覚領域に移動することを考えると、一次感覚領域とその領域内の画像化されたニューロンの位置を定義することが重要です。このプロトコルでは、視床皮質軸索を可視化するトランスジェニックマウスと、GCaMPをまばらに発現するSupernovaシステム8?...
Access restricted. Please log in or start a trial to view this content.
開示事項
著者は、宣言する競合する金銭的利益を持っていません。
謝辞
本研究は、日本学術振興会学術振興会学術変革領域研究(B)(22H05092, 22H05094)、学術研究助成20K06876、AMED(課題番号21wm0525015)、武田科学振興財団、内藤財団、加藤記念生命科学振興財団、公益財団法人興和生命科学振興財団、国立遺伝学研究所JOINT (24A2021) (to H.M.);日本学術振興会科学研究費助成事業基盤研究費19K06887・22K06446、児玉記念医学研究基金、上原記念財団、加藤記念バイオサイエンス財団、武田科学振興財団(N.N-T.へ)TCA-RFPマウスを提供してくださった岩里卓司博士に感謝いたします。
Access restricted. Please log in or start a trial to view this content.
資料
| Name | Company | Catalog Number | Comments |
| 20× objective lens (water immersion) | |||
| 250 mL Vacuum Filter/Storage Bottle System | Corning | 431096 | |
| 4%-paraformaldehyde phosphate buffer solution (4% PFA) | Nacalai | 09154-85 | |
| Acrylic resin (UNIFAST II) | GC | N/A | |
| Agarose | Sigma | A9793 | |
| Aspirator tube assembly | Drummond | 2-040-000 | |
| CaCl2•2H2O | Nacalai | 06731-05 | |
| Electroporator | BEX | GEB14 | |
| Eye drop (Scopisol) | Senju Pharmaceutical | N/A | |
| Fluorescence stereo microscope | Leica | M165FC | |
| Glucose | Nacalai | 16806-25 | |
| Heating pad | Muromachi Kikai | FHC-HPS | |
| HEPES | Gibco | 15630-080 | |
| Isoflurane | Pfizer | N/A | |
| KCl | Nacalai | 28514-75 | |
| MgSO4•7H2O | Wako | 131-00405 | |
| Micropipette puller | Narishige | PC-100 | |
| Multiphoton laser | Spectra-Physics | Mai Tai eHP DeepSee | |
| Multiphoton microscope | Zeiss | LSM 7MP | |
| NaCl | Nacalai | 31320-05 | |
| Non-woven fabric (Kimwipe) | Kimberly Clark | S-200 | |
| Phosphate buffered saline (PBS) | Nacalai | 27575-31 | |
| Plasmid: CAG-loxP-STOP-loxP-GCaMP6s-ires-tTA-WPRE | Addgene | pK175 | |
| Plasmid: TRE-nCre | Addgene | pK031 | |
| Precision calibrated micropipets | Drummond | 2-000-050 | |
| Razor blade | Feather | FA-10 | |
| Rimadyl (50 mg/mL Carprofen) | Zoetis JP | N/A | |
| Round cover glass, 3-mm-diameter | Matsunami | CS01078 | |
| Saline | Otsuka | 035175315 | |
| Sodium pentobarbital | Nacalai | 26427-72 | |
| Stage for imaging living pup (two single-axis translation stage for XY positioning, two-axis goniometer, base plate, adjustable pillar for z positioning) | ThorLabs | LT1/M, GN2/M, BM2060/M, MLP01/M | |
| TCA-RFP mouse | N/A | N/A | Mizuno et al., 2018a |
| Tissue adhesive (Vetbond) | 3M | 1469SB | |
| Titanium bar | Endo Scientific Instrument | N/A | Custom made (Mizuno et al., 2018b) |
| Titanium bar fixing plate | N/A | Custom made (Mizuno et al., 2018b) | |
| Trypan blue | Sigma | T8154 | |
| Tweezers with platinum plate electrode, 5 mm diameter | BEX | CUY650P5 | |
| Wild-type ICR mouse | Nihon SLC | Slc:ICR |
参考文献
- Rao, M. S., Mizuno, H. Elucidating mechanisms of neuronal circuit formation in layer 4 of the somatosensory cortex via intravital imaging. Neuroscience Research. 167, 47-53 (2021).
- Iwasato, T., Erzurumlu, R. S. Development of tactile sensory circuits in the CNS. Current Opinion in Neurobiology. 53, 66-75 (2018).
- Martini, F. J., Guillamón-Vivancos, T., Moreno-Juan, V., Valdeolmillos, M., López-Bendito, G. Spontaneous activity in developing thalamic and cortical sensory networks. Neuron. 109 (16), 2519-2534 (2021).
- Ackman, J. B., Burbridge, T. J., Crair, M. C. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 490 (7419), 219-225 (2012).
- Tritsch, N. X., Yi, E., Gale, J. E., Glowatzki, E., Bergles, D. E. The origin of spontaneous activity in the developing auditory system. Nature. 450 (7166), 50-55 (2007).
- Babola, T. A., et al. Homeostatic control of spontaneous activity in the developing auditory system. Neuron. 99 (3), 511-524.e5 (2018).
- Mizuno, H., et al. Patchwork-type spontaneous activity in neonatal barrel cortex layer 4 transmitted via thalamocortical projections. Cell Reports. 22 (1), 123-135 (2018).
- Mizuno, H., et al. NMDAR-regulated dynamics of layer 4 neuronal dendrites during thalamocortical reorganization in neonates. Neuron. 82 (2), 365-379 (2014).
- Tabata, H., Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 103 (4), 865-872 (2001).
- Fukuchi-Shimogori, T., Grove, E. A. Neocortex patterning by the secreted signaling molecule FGF8. Science. 294 (5544), 1071-1074 (2001).
- Saito, T., Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Developmental Biology. 240 (1), 237-246 (2001).
- Holtmaat, A., et al. Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nature Protocols. 4 (8), 1128-1144 (2009).
- Mizuno, H., Nakazawa, S., Iwasato, T. In vivo two-photon imaging of cortical neurons in neonatal mice. Journal of Visualized Experiments. 140, e58340(2018).
- Egashira, T., et al. In vivo two-photon calcium imaging of cortical neurons in neonatal mice. STAR Protocols. 4 (2), 102245(2023).
- Cantu, D. A., et al. EZcalcium: Open-source toolbox for analysis of calcium imaging data. Frontiers in Neural Circuits. 14, 25(2020).
- Maruoka, H., et al. Lattice system of functionally distinct cell types in the neocortex. Science. 358 (6363), 610-615 (2017).
- Antón-Bolaños, N., et al. Prenatal activity from thalamic neurons governs the emergence of functional cortical maps in mice. Science. 364 (6444), 987-990 (2019).
- Guillamón-Vivancos, T., et al. Input-dependent segregation of visual and somatosensory circuits in the mouse superior colliculus. Science. 377 (6608), 845-850 (2022).
- Cardin, J. A., Crair, M. C., Higley, M. J. Mesoscopic imaging: Shining a wide light on large-scale neural dynamics. Neuron. 108 (1), 33-43 (2020).
- Chen, J. L., Voigt, F. F., Javadzadeh, M., Krueppel, R., Helmchen, F. Long-range population dynamics of anatomically defined neocortical networks. eLife. 5, e14679(2016).
- Ota, K., et al. cell-resolution, contiguous-wide two-photon imaging to reveal functional network architectures across multi-modal cortical areas. Neuron. 109 (11), 1810-1824 (2021).
- Zariwala, H. A., et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. The Journal of Neuroscience. 32 (9), 3131-3141 (2012).
- Murakami, T., Matsui, T., Uemura, M., Ohki, K. Modular strategy for development of the hierarchical visual network in mice. Nature. 608 (7923), 578-585 (2022).
- Pnevmatikakis, E. A., et al. Simultaneous denoising, deconvolution, and demixing of Calcium imaging data. Neuron. 89 (2), 285-299 (2016).
- Shemesh, O. A., et al. Precision calcium imaging of dense neural populations via a cell-body-targeted calcium indicator. Neuron. 107 (3), 470-486 (2020).
- Giovannucci, A., et al. CaImAn an open source tool for scalable calcium imaging data analysis. Elife. 8, e38173(2019).
- Pachitariu, M., et al. Suite2p: beyond 10,000 neurons with standard two-photon microscopy. BioRxiv. , (2017).
- Sitdikova, G., et al. Isoflurane suppresses early cortical activity. Annals of Clinical and Translational Neurology. 1 (1), 15-26 (2014).
- Marques-Smith, A., et al. A Transient translaminar GABAergic interneuron circuit connects thalamocortical recipient layers in neonatal somatosensory cortex. Neuron. 89 (3), 536-549 (2016).
- Tuncdemir, S. N., et al. Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron. 89 (3), 521-535 (2016).
- Nakazawa, S., Yoshimura, Y., Takagi, M., Mizuno, H., Iwasato, T. Developmental phase transitions in spatial organization of spontaneous activity in postnatal barrel cortex layer 4. The Journal of Neuroscience. 40 (40), 7637-7650 (2020).
- Yu, Y. -C., et al. Preferential electrical coupling regulates neocortical lineage-dependent microcircuit assembly. Nature. 486 (7401), 113-117 (2012).
- Siegel, F., Heimel, J. A., Peters, J., Lohmann, C. Peripheral and central inputs shape network dynamics in the developing visual cortex in vivo. Current Biology. 22 (3), 253-258 (2012).
- Nakagawa, N., Hosoya, T. Slow dynamics in microcolumnar gap junction network of developing neocortical pyramidal neurons. Neuroscience. 406, 554-554 (2019).
- Valiullina, F., et al. Developmental changes in electrophysiological properties and a transition from electrical to chemical coupling between excitatory layer 4 neurons in the rat barrel cortex. Frontiers in Neural Circuits. 10, 1(2016).
- Avitan, L., et al. Spontaneous and evoked activity patterns diverge over development. Elife. 10, e61942(2021).
- Mölter, J., Avitan, L., Goodhill, G. J. Detecting neural assemblies in calcium imaging data. BMC Biology. 16 (1), 143(2018).
- Nakazawa, S., Mizuno, H., Iwasato, T. Differential dynamics of cortical neuron dendritic trees revealed by long-term in vivo imaging in neonates. Nature Communications. 9 (1), 3106(2018).
Access restricted. Please log in or start a trial to view this content.
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved