このコンテンツを視聴するには、JoVE 購読が必要です。 サインイン又は無料トライアルを申し込む。
Method Article
げっ歯類の下大静脈静脈形成術バルーンモデル
要約
このプロトコルは、非血栓性、血栓性、および血栓性後の深部静脈における静脈形成バルーン (VB) 介入をテストするための生存げっ歯類モデルを提示します。
要約
バルーン静脈形成術は、外傷、先天性解剖学的異常、急性深部静脈血栓症(DVT)、またはステント留置術の結果としての深部静脈狭窄症および閉塞を治療するために一般的に使用される臨床技術です。慢性深部静脈閉塞は、組織病理学的に血栓症、線維症、またはその両方によって特徴付けられます。現在、これらの病理学的プロセスを標的とする直接的な治療法はありません。したがって、新しい介入をテストするための信頼性の高い in vivo 動物モデルが必要です。げっ歯類生存率下大静脈 (IVC) 静脈形成術バルーン モデル (VBM) は、複数の時点にわたる非血栓性および血栓後の状態におけるバルーン静脈形成術の研究を可能にします。コーティングされた静脈形成術およびコーティングされていない静脈形成術バルーンの局所的および全身的な影響は、リアルタイムポリメラーゼ連鎖反応(RT-PCR)、ウェスタンブロット、酵素結合免疫吸着アッセイ(ELISA)、ザイモグラフィー、静脈壁および血栓細胞分析、全血および血漿アッセイ、組織学的分析などの組織、血栓、および血液アッセイによって定量化できます。VBMは再現性があり、外科的なヒト介入を再現し、局所静脈壁血栓タンパク質の変化を特定でき、同じサンプルからの複数の分析を可能にするため、グループごとに必要な動物の数が減少します。
概要
未解決の血栓による深部静脈閉塞は、深部静脈血栓症(DVT)の一般的な結果であり、血栓後症候群(PTS)の原因の1つであり、医療に数十億ドルの費用がかかり、生活の質に大きな影響を与えています1,2,3。PTSは、小動物のDVTモデル5,6,7では完全に捕捉されていない結果として生じる静脈壁線維症4を伴う独特の炎症性病理であり、さらに重要なことに、以前のモデルは治療介入に対する反応を評価できません。血栓性静脈閉塞は、ステント留置術やバルーン静脈形成術によって治療されることがよくあります8,9;このような介入は、再狭窄と閉塞による高い再介入率によって妨げられます 10,11,12。
動脈特異的な狭窄/閉塞に対処するための薬剤コーティングバルーンが利用可能であるにもかかわらず、深部静脈には存在しません。再狭窄の背後にある分子メカニズムは、静脈内ではよく理解されておらず、直接的な治療法はありません4。したがって、私たちの目標は、深部静脈の血栓後閉塞をシミュレートする静脈形成バルーンを介して局所的に送達される治療薬をテストできる動物モデルを提示することです。このモデルは、血栓形成後の状態を再現し、さまざまな種類の静脈形成バルーンが 生体内で静脈壁にどのように影響するかを評価します。
プロトコル
次のプロトコルは、ミシガン大学(UMICH)の施設内動物管理および使用委員会(IACUC)のポリシーとガイドライン、試験、研究、および訓練で使用される脊椎動物の利用とケアに関する米国政府の原則、およびARRIVEガイドライン2.0に従います。UMICH動物福祉保証契約は、OLALおよびUSDAに準拠しており、AAALACインターナショナルによって完全に認定されています。このプロトコルは、ID番号PRO00010841で承認されました。Charles River Laboratoriesの雄の近交系スプレイグ・ドーリーラット(体重450g、生後15週齢)を用いた。
1.ラット麻酔
- 動物をケージから取り出し、5%イソフルランと100%酸素の麻酔術前導入ガスチャンバーに0.8〜1 L / minの流量(気化器制御)で入れ、全身麻酔を誘発します。
- 麻酔導入チャンバーでラットを鎮静させ、チャンバーから取り出し、体重を量り、滅菌眼軟膏で眼を滑らかにしてから、温水循環加熱装置で背側横臥に置きます。ペダル反射(つま先をしっかりとつまむ)を使用して、麻酔の適切な深さを確認します。
- 電動バリカンで腹側腹部を剃ります。全身麻酔を2.5%イソフルランと100%酸素で0.8〜1 L / minで維持し、ノーズコーンを介した非再呼吸回路を使用します。.
2.ラット超音波スキャン
- イメージング中に、呼吸数、心拍数、体温などの生理学的評価をモニタリングします。
- 下大静脈(IVC)の腹部イメージングでは、導電性ゲルを塗布し、トランスデューサーを横方向に向けます。末梢血管をプリセットしながら、2次元イメージングモードまたはBモードを使用し、腹部の中央にあるリニアプローブ(10MHz)を下げ、腹部血管が見えるまで圧力で深さを調整します。
- IVCと腹部大動脈を区別するには、圧縮性を評価し、高周波リニアプローブ(10mHz)によるカラードップラーモードとパルス波ドップラーモードの両方を使用して流量を評価します。
- 超音波プローブで圧縮を適用する場合は、IVCが圧縮性であるのに対し、大動脈はその形状と開存性を維持するため、2つを区別します。
- カラードップラーとパルス波ドップラーの両方を使用して、血流の方向と速度を評価します。カラー ドップラー ウィンドウは、プローブに向かう流れを赤で表し、プローブから離れる流れを青で表します。装置をパルス波ドップラーモード(デュプレックスモード)に切り替えて、血流の方向と速度を経時的に評価します。超音波装置の自動画像キャプチャツールを使用して、必要なフロー画像を取得します(画像取得の正確な仕様は超音波システムによって異なる場合があります)。
- 次の評価方法を使用します:大動脈にはプローブへの血流と三相性波形がありますが、IVCにはプローブから離れる自発的な流れがあり、振幅ははるかに低くなっています。
- 近位IVCが正常な波形を示しているにもかかわらず、遠位に非位相または存在しない(平坦な)波形が検出される場合は、これらの検査ポイント間の静脈閉塞の可能性を探ります。.
- IVCが特定されたら、中央の3分の1の部分にあり、腎静脈より下でIVC分岐部よりも優れている中央部(おそらく腰椎静脈が位置している場所)を特定します。
- IVCの中央部で、断面Bモード画像を使用して横方向の壁間直径を測定し、容器の最も広い直径を記録します。画像取得は、超音波装置によって異なる場合があります。コンパクト ディスク (CD) を使用して DICOM ファイルにデータを保存します。
3.ラットのマイクロサージェリーと回復
- ガーゼに浸したクロルヘキシジンスクラブとクロルヘキシジン溶液3xで腹部を洗浄し、無菌手術状態を確保します。.
- 滅菌したラップをサージカルラップとして置き、ラットの胸部と腹部、顕微鏡のノブ(倍率と焦点)、および接眼レンズを覆います。
- 剣状突起の約2cm下に腹側正中線切開(3cm)を虹彩ハサミで皮膚と腹壁に通し、腹部の内容物を露出させます。滅菌生理食塩水に浸した2インチx2インチのガーゼを使用して、腸を動物の右側に反射させます。
- IVC曝露の場合は、滅菌綿先端のアプリケーターを使用して鈍解剖を行います。.切開部にワイヤー鏡を配置し、IVCの可視化を可能にします。
- 腎臓静脈から腸骨分岐部まで、低温の細い先端の焼灼器を使用してすべてのIVC腰椎枝を焼灼し、7-0非吸収性ポリプロピレン縫合糸で結紮側枝を焼灼します。
- 大動脈から分離され、腎静脈のすぐ下にあるIVCに近位湾曲した血管マイクロクリップを配置します。大動脈から分離され、IVC分岐部よりも優れた遠位IVCにまっすぐなマイクロクリップを配置します。
- 8-0 で配置するナイロン U ステッチ縫合糸は、IVC の前面を中心に左腎静脈に尾側にあります。
- 静脈形成バルーンをバックロードし、逆行性の静脈形成バルーンを逆行させ、静脈内IVCへの血流に逆行させ、湾曲したマイクロクリップに尾側に、0.014mmの鋭利なガイドワイヤーを挿入します。
- セルディンガー法を使用して、バルーンをIVC中部まで進めます。鋭利なガイドワイヤーを取り外し、20mLのインフレーションシリンジを使用して10%から15%のIVCオーバーストレッチで静脈形成バルーンを3分間膨らませ、0-30 Atm.IVCカニューレーションの前に滅菌生理食塩水ですべてのシステムを洗い流し、空気塞栓症を回避します。
注:2.8 mm IVCは、3.22 mm(15%オーバーストレッチ)までの静脈形成術を受けます。.目的のオーバーストレッチに到達するために必要な膨張圧力は、バルーンの特性によって決定され、メーカーのパッケージで入手できます。 - 静脈形成術のバルーンの収縮と取り外し時にUステッチを締めます。マイクロクリップを取り外します。
- 開腹部位を 2 層状に閉じます。5-0ポリグラクチン吸収性合成縫合糸を連続パターンで使用して、腹壁と皮膚の両方を閉じます。
- ブプレノルフィン徐放性注射剤懸濁液 0.65 mg/kg をラットの鎮痛薬として術後に投与します。これは、タンパク質分析のための炎症分子のバイオマーカー パネルに干渉しないためです。
- ラットを個々のケージで回収し、ヒーティングランプ(最小距離-ケージから24インチ離れている)で術後(30分)観察し、元のハウジングユニットに戻ります。
- 各実験群の偽動物については、IVC分岐の結紮、焼灼、およびカニューレ挿入を行わずに、解剖のみを行います。
- 血栓後の状態では、大動脈から分離された、腎静脈のすぐ下にあるIVCに近位のまっすぐな血管マイクロクリップを24時間配置します。.同じ解剖と閉鎖のテクニックを使用してください。
- IVC クリップの視覚化、流れのない遠位 IVC の確認、総腸骨静脈の膨張など、IVC 閉塞の超音波徴候を確認します。フォローアップの時点は、試験の仕様に合わせてカスタマイズできます。
- モデル化されている生理学的変化を観察するには、初期の血栓形成については最初の 72 時間の時点を選択し、後の血栓形成の場合は処置後 3 日から 7 日の時点を選択します。このモデルでテストされた後の時点には、7〜28日が含まれます5,13。
- 血栓後バルーン静脈形成術には、ステップ3.5-3.9の技術を使用しますが、次の注意事項があります:血栓後の状態ではIVCの直径が増加するため、望ましいオーバーストレッチを達成するためには、より高い吸入器圧力が必要になる場合があります。
- げっ歯類の安楽死に関する現在の米国獣医師会ガイドラインに定められた推奨事項に基づいて、動物が復活しないように2つの確認方法(血液除去と生命臓器除去)を実行することにより、安楽死を実行します。
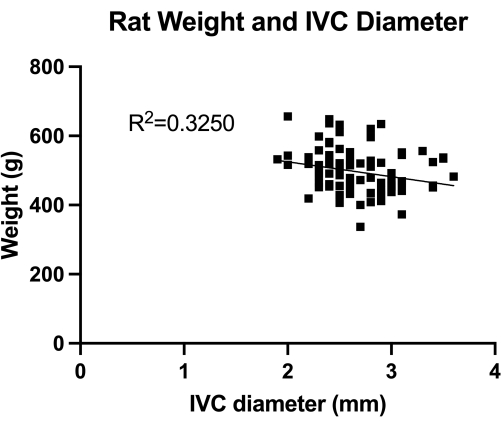
図1:横方向IVC(中部腎下切片)直径の経腹部二重測定の分布。 測定は動物の体重で行われました。重量とIVC直径との間に有意な相関は認められませんでした。 この図の拡大版を表示するには、ここをクリックしてください。
4. サンプル調製
- 高速液体クロマトグラフィー(HPLC)アッセイ技術を使用して、薬剤でコーティングされた静脈形成バルーンの確認組織移植を行います。
- IVCと大動脈を解剖し、ホルマリン固定パラフィン包埋および組織学的分析のために 一括 して除去します。
- タンパク質分析には、希釈濃度60,000 μg/gのサンプルでRIPAバッファーを使用し、4°Cの低温室内で組織ホモジナイザーで処理し、処理後は-80°Cで保存します。
結果
VBMは、完全な止血制御における静脈形成バルーンの効果を評価するモデルです。まず、 図1に示すように、正しいバルーンサイジングのためにIVC(中低腎部)の直径を定量化しました。次に、開発された段階的なマイクロサージュリー技術を 図2に示し、 図3に重要なステップの例も示しています。鋭利なガイドワイヤーを使用?...
ディスカッション
VBMは、2.5〜3.5mmの範囲のバルーンを使用して、250g≥ラットに対して実行できます。750 gを超えるラットの場合、この技術は、IVC直径の変化ではなく、腹腔内の脂肪組織の量と臓器のサイズが多いことによって制限されます。私たちの経験から、静脈壁タンパク質分析または活性アッセイと組織学のためにグループ間の統計的有意性を得るためには、グループごとに10匹以上の動物が必要である...
開示事項
利益相反は宣言されていません。
謝辞
この研究は、American Venous Forum (AVF) 2023 Basic Science research grant と Surmodics, Inc. の支援を受けています。
資料
| Name | Company | Catalog Number | Comments |
| 3-0 DemeCRYL | DemeTech | G183019F4P | 12 per box |
| 5-0 DemeCRYL | DemeTech | G285017B0P | 12 per box |
| 7-0 DemeLENE | DemeTech | PM197011F13M | 12 per box |
| 8-0 DemeLON | DemeTech | NL868007C7P | 12 per box |
| Angled Clip Applier | SCANLAN | 3795-01A | 7-1/4″ (18.5 cm) |
| Baxter 0.9% Sodium Chloride Irrigation Bottle | Baxter | SKU: 195-7124 | 1000mL Bottle |
| Bovie Cautery Low Temperature Fine Tip | Symmetry Surgical Inc | AA00 | 10/bx |
| Buprenorphine | Fidelis Animal Health | SKU- 099114 | 1.3mg/mL, 3mL |
| CD (Sprague Dawley) Rats | Charles River | Strain Code 001 | Male, 400 gm |
| Clip-It Traceable Timer | TRACEABLE PRODUCTS | Cat. No. 5046 | |
| Cotton-Tip Applicator 3" | Dukal Corporation | 922-00037BX100 | 1000/BOX |
| Enzymatic Surgical Instrument Detergent and Presoak | Medline | MDS88000B9 | 1 Gal |
| Eye Lube | Optixcare | SKU-065441 | 20gm |
| Gaymar T/Pump Stryker Localized Warming/Cooling Therapy System | Stryker | TP700 | |
| Heifetz Microclips 12mmx2.25mm | SCANLAN | 3795-20 | Straight |
| Heifetz Microclips 12mmx2.25mm | SCANLAN | 3795-28 | Curved |
| Heifetz Microclips 8mmx1.75mm | SCANLAN | 3795-14 | Straight |
| Heifetz Microclips 8mmx1.75mm | SCANLAN | 3795-18 | Curved |
| Inflation device | Merit Medical | IN4130 | BasixCOMPAK with Analog Display |
| Isoflurane | MFR VETONE | SKU-501017 | 100mL, Liquid for Inhalation |
| Microsurgery kit containing tweezers and forceps | Customized | ||
| Oxygen, E-tank | Medline | MDM1630 | |
| Sharpened Guidewire | Customized | ||
| Sterile Glad Press-n-Seal | Glad | SKU # PSS-140 | ETO Exposed |
| Sterile Nonwoven Gauze Sponges 2s 4ply 4x4 | Medline | SKU PRM21444H | 100Ct |
| Surgical microscope | Zeiss | S100 / OPMI pico | |
| Surgical Scrub & Handwash | Vetoquinol | NDC: 17030-003-16 | 16 oz |
| Ultrasound Gel | Medline | CTR001305 | 8.5 oz, 12/CS |
| Ultrasound system | Siemens | ACUSON Antares | 3-D, Color, Continuous wave (CW), Pulsed wave (PW), Power CW/CW Spectral |
| Venoplasty Balloon | Customized |
参考文献
- Prandoni, P., et al. The long-term clinical course of acute deep venous thrombosis. Ann Inter Med. 125 (1), 1-7 (1996).
- Prandoni, P., Kahn, S. R. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol. 145 (3), 286-295 (2009).
- Gloviczki, P., et al. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II. J Vas Surg Venous Lymph Diso. 12 (1), 101670 (2024).
- Henke, P., Sharma, S., Wakefield, T., Myers, D., Obi, A. Insights from experimental post-thrombotic syndrome and potential for novel therapies. Transl Res. 225, 95-104 (2020).
- Diaz, J. A., et al. Choosing a mouse model of venous thrombosis: A consensus assessment of utility and application. Arterioscl Thrombo Vasc Biol. 39 (3), 311-318 (2019).
- Lee, Y. U., Lee, A. Y., Humphrey, J. D., Rausch, M. K. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology. 52 (3), 235-245 (2015).
- Xie, H., et al. Correspondence of ultrasound elasticity imaging to direct mechanical measurement in aging DVT in rats. Ultrasound Med Biol. 31 (10), 1351-1359 (2005).
- Moini, M., et al. Venoplasty and stenting in post-thrombotic syndrome and non-thrombotic iliac vein lesion. Minim Invasive Ther Allied Technol. 29 (1), 35-41 (2020).
- Chaitidis, N., et al. Management of post-thrombotic syndrome: A comprehensive review. Curr Pharma Des. 28 (7), 550-559 (2022).
- Dangas, G. D., et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 56 (23), 1897-1907 (2010).
- Neglén, P., Berry, M. A., Raju, S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Euro J Vas Endovas Surg. 20 (6), 560-571 (2000).
- Sharifi, M., Mehdipour, M., Bay, C., Smith, G., Sharifi, J. Endovenous therapy for deep venous thrombosis: The TORPEDO trial. Catheter Cardiovasc Interv. 76 (3), 316-325 (2010).
- Nicklas, J. M., Gordon, A. E., Henke, P. K. Resolution of deep venous thrombosis: Proposed immune paradigms. Int J Mol Sci. 21 (6), 2080 (2020).
- Li, J., Kim, S. G., Blenis, J. Rapamycin: One drug, many effects. Cell Metabol. 19 (3), 373-379 (2014).
- Chen, X., et al. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinogen. 49 (6), 603-610 (2010).
- Diaz, J. A., Farris, D. M., Wrobleski, S. K., Myers, D. D., Wakefield, T. W. Inferior vena cava branch variations in C57BL/6 mice have an impact on thrombus size in an IVC ligation (stasis) model. J Thromb Haemo. 13 (4), 660-664 (2015).
- Schönfelder, T., Jäckel, S., Wenzel, P. Mouse models of deep vein thrombosis. Gefässchirurgie. 22 (1), 28-33 (2017).
- de Barros, R. S. M., et al. Morphometric analysis of rat femoral vessels under a video magnification system. J Vasc Bras. 16 (1), 73-76 (2017).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved