A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Rodent Inferior Vena Cava Venoplasty Balloon Model
In This Article
Summary
The protocol presents a survival rodent model to test venoplasty balloon (VB) interventions in non-thrombotic, thrombotic, and post-thrombotic deep veins.
Abstract
Balloon venoplasty is a commonly used clinical technique to treat deep vein stenosis and occlusion as a consequence of trauma, congenital anatomic abnormalities, acute deep vein thrombosis (DVT), or stenting. Chronic deep venous obstruction is histopathologically characterized by thrombosis, fibrosis, or both. Currently, no direct treatment is available to target these pathological processes. Therefore, a reliable in vivo animal model to test novel interventions is necessary. The rodent survival inferior vena cava (IVC) venoplasty balloon model (VBM) allows the study of balloon venoplasty in non-thrombotic and post-thrombotic conditions across multiple time points. The local and systemic effect of coated and uncoated venoplasty balloons can be quantified via tissue, thrombus, and blood assays such as real-time polymerase chain reaction (RT-PCR), western blot, enzyme-linked immunosorbent assay (ELISA), zymography, vein wall and thrombus cellular analysis, whole blood and plasma assays, and histological analysis. The VBM is reproducible, replicates surgical human interventions, can identify local vein wall-thrombi protein changes, and allows multiple analyses from the same sample, decreasing the number of animals required per group.
Introduction
Deep vein obstructions from unresolved thrombi are a common consequence of deep vein thrombosis (DVT) and are one of the causes of post-thrombotic syndrome (PTS), costing billions of dollars in healthcare and significantly impacting the quality of life1,2,3. PTS is a unique inflammatory pathology with consequent vein wall fibrosis4 not fully captured in small animal DVT models5,6,7, and more importantly, previous models cannot evaluate the response to therapeutic interventions. Thrombotic venous obstructions are often treated by stenting and balloon venoplasty8,9; such interventions are hampered by a high reintervention rate due to restenosis and occlusion10,11,12.
Despite the availability of drug-coated balloons to address arterial-specific stenosis/occlusions, none exist for deep veins. The molecular mechanism behind restenosis is poorly understood in veins and has no direct therapy4. Therefore, our goal is to present an animal model that can test locally delivered therapeutics via venoplasty balloons simulating deep vein post-thrombotic obstructions. This model reproduces the post-thrombotic condition and evaluates how different types of venoplasty balloons affect the vein wall in vivo.
Protocol
The following protocol follows the University of Michigan (UMICH) Institutional Animal Care and Use Committee (IACUC) policies and guidelines, the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training, and the ARRIVE guidelines 2.0. The UMICH Animal Welfare Assurance agreement is compliant with OLAW and USDA and is fully accredited by AAALAC International. This protocol was approved with the ID number PRO00010841. Male outbred Sprague Dawley rats from Charles River Laboratories, weighing 450 g and 15 weeks of age, were used.
1. Rat anesthesia
- Remove animals from their cage and place them in an anesthetic pre-operative induction gas chamber at 5% isoflurane and 100% oxygen at a flow rate of 0.8 to 1 L/min (vaporizer-controlled) to induce general anesthesia.
- Sedate rats in the anesthetic induction chamber, remove them from the chamber, weigh, lubricate their eyes with sterile ophthalmic ointment, and then place them in dorsal recumbency on a warm water circulating heating device. Confirm adequate depth of anesthesia using pedal reflex (firm toe pinch).
- Shave the ventral abdomen with electric clippers. Maintain general anesthesia at 2.5% isoflurane and 100% oxygen at 0.8 to 1 L/min with a non-rebreathing circuit through a nosecone.
2. Rat ultrasound scanning
- Monitor physiological assessments, including respiratory rate, heart rate, and temperature, during imaging.
- For abdominal imaging of the Inferior Vena Cava (IVC), apply conducting gel and orient the transducer in a transverse position. While presetting the peripheral vascular, use two-dimensional imaging mode or B-mode, lower the linear probe (10 MHz) on the center of the abdomen, adjusting depth with pressure until the abdominal vessels come into view.
- Differentiate IVC from the abdominal aorta by assessing compressibility and by assessing flow using both color and pulsed-wave Doppler mode with a high-frequency linear probe (10 mHz).
- When applying compression with the ultrasound probe, differentiate the two as the IVC is compressible, whereas the aorta maintains its shape and patency.
- Assess the direction and velocity of blood flow by using both color and pulsed-wave Doppler. The color Doppler window represents the flow toward the probe in red and flows away from the probe in blue. Switch the instrument to pulse-wave Doppler mode (Duplex mode) to assess blood flow direction and velocity over time. Acquire necessary flow images by using the ultrasound machine's automated image capture tool (The exact specifications for image acquisition may vary between ultrasound systems).
- Use the following assessment method: the aorta has blood flow toward the probe and a triphasic waveform, whereas the IVC has spontaneous flow away from the probe with a much lower amplitude.
- If the proximal IVC displays a normal waveform, yet a nonphasic or absent (flat) waveform is detected distally, explore the possibility of a venous obstruction between these points of examination.
- Once the IVC is located, identify the mid-section, which is in the middle third portion, inferior to the renal veins and superior to the IVC bifurcation, most likely where the lumbar veins are located.
- Measure at the mid-section IVC the wall-to-wall diameter in the transverse view using the cross-sectional B-mode image, recording the widest diameter of the vessel. Imaging acquisition may vary in different ultrasound machines. Store data using compact disks (CDs) in DICOM files.
3. Rat micro-surgery and recovery
- Clean the abdomen with gauze-soaked chlorhexidine scrub and chlorhexidine solution 3x to ensure an aseptic surgical condition. Administer buprenorphine extended-release injectable suspension, 0.65 mg/kg subcutaneous (SC), as an analgesic for rats since it does not interfere with the biomarker panels of inflammatory molecules for protein analysis.
- Place sterilized cling film as a surgical wrap to cover the rat thorax and abdominal area, microscope knobs (magnification and focus), and eyepiece.
- Make a ventral midline incision (3 cm) approximately 2 cm below the xiphoid process with iris scissors through the skin and abdominal wall, exposing the abdominal contents. Use sterile saline-soaked 2 inch x 2 inch gauze to reflect the intestines to the animal's right side.
- For IVC exposure, perform a blunt dissection using a sterile cotton-tipped applicator. Place a wire speculum in the incision, allowing IVC visualization.
- Cauterize all IVC lumbar branches using a low-temperature fine tip cautery, from the renal veins to the iliac bifurcation, and ligate side branches with 7-0 non-absorbable polypropylene sutures.
- Place a proximal curved vascular micro-clip on the IVC, which is separated from the aorta and just inferior to the renal veins. Place a straight microclip on the distal IVC, which is separated from the aorta and superior to the IVC bifurcation.
- Place an 8-0 nylon U stitch suture caudal to the left renal vein centered on the anterior surface of the IVC.
- Insert a 0.014 mm sharpened guidewire with the venoplasty balloon backloaded, retrograde to blood flow into the infrarenal IVC, caudal to the curved micro-clip.
- Advance the balloon into the mid-IVC using the Seldinger technique. Remove the sharpened guidewire and inflate the venoplasty balloon for 3 min with a 10% to 15% IVC overstretch using a 20 mL inflation syringe to generate positive pressure over a range of 0-30 Atm. Flush all systems with sterile saline before IVC canulation to avoid air embolism.
NOTE: A 2.8 mm IVC would undergo venoplasty to 3.22 mm (15% overstretch). The required inflation pressure to reach the desired overstretch is determined by the characteristics of the balloon and is available on the manufacturer's packaging. - Tighten the U stitch upon venoplasty balloon deflation and removal. Remove the micro-clips.
- Close the laparotomy site in a two-layer fashion. Use a 5-0 polyglactin absorbable synthetic suture in a continuous pattern to close both the abdominal wall and skin.
- Recover rats in an individual cage, observe post-operatively (30 min) under a heating lamp (minimum distance - 24 inch away from the cage), and then return to their original housing units.
- For sham animals from each experimental group, perform only the dissection, without the IVC branches ligation, cauterization, and cannulation.
- For post-thrombotic conditions, place a proximal straight vascular micro-clip on the IVC, which is separated from the aorta, just inferior to the renal veins, for 24 h. Use the same dissection and closing techniques.
- Check for the ultrasound signs of IVC occlusion, which include the visualization of the IVC clip, confirmation of distal IVC without flow, and common iliac veins distended. Follow-up timepoints can be customized to fit the study's specifications.
- To observe the physiologic changes that are being modeled, select timepoints in the first 72 h for early thrombi formation and from 3 to 7 days postprocedural for later thrombi resolution. Later timepoints tested in this model include 7 to 28 days5,13.
- Use the techniques in steps 3.5-3.9 for post-thrombotic balloon venoplasty with the following caveats: A higher insufflator pressure might be required to achieve the desired overstretch since the IVC diameter increases in post-thrombotic conditions.
- Perform euthanasia under the recommendations set forth by the current American Veterinary Medical Association Guidelines on Euthanasia for rodents by performing two confirmatory methods (blood removal and vital organ removal) to ensure the animal will not revive.
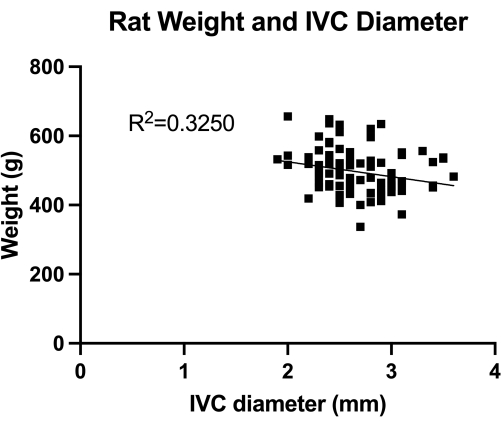
Figure 1: Distribution of transabdominal duplex measures of transverse IVC (mid-infrarenal section) diameter. The measurement was done by animal weight. No significant correlation was found between the weight and the IVC diameter. Please click here to view a larger version of this figure.
4. Sample preparation
- Perform confirmatory tissue transfer of drug-coated venoplasty balloons using high-performance liquid chromatography (HPLC) assay technique.
- Dissect the IVC and aorta and remove en bloc for formalin-fixed paraffin embedding and histological analysis.
- For protein analysis, use RIPA buffer in a diluting concentration of 60,000 µg/g of sample, process with a tissue homogenizer in low-temperature room settings at 4 °C, and store at -80 °C after processing.
Results
The VBM is a model that evaluates the effect of venoplasty balloons in complete hemostasis control. First, we quantified the IVC (mid-infrarenal section) diameter, as shown in Figure 1, for correct balloon sizing. Second, the developed step-by-step microsurgical technique is illustrated in Figure 2, and we also show examples of the critical steps in Figure 3. Notice the retrograde approach used for the IVC canulation with the sharpe...
Discussion
The VBM can be performed on rats ≥ 250 g using balloons in the 2.5-3.5 mm range. For rats exceeding 750 g, the technique is limited by a higher amount of adipose tissue in the abdominal cavity and organ size rather than changes in IVC diameter. From our experience, we have found that 10 or more animals per group are needed to obtain statistical significance between the groups for vein wall protein analysis or activity assays and histology. Periodic temperature control and respiration rate surveillance during surgic...
Disclosures
No conflicts of interest were declared.
Acknowledgements
This study is supported by the American Venous Forum (AVF) 2023 Basic Science research grant and Surmodics, Inc.
Materials
| Name | Company | Catalog Number | Comments |
| 3-0 DemeCRYL | DemeTech | G183019F4P | 12 per box |
| 5-0 DemeCRYL | DemeTech | G285017B0P | 12 per box |
| 7-0 DemeLENE | DemeTech | PM197011F13M | 12 per box |
| 8-0 DemeLON | DemeTech | NL868007C7P | 12 per box |
| Angled Clip Applier | SCANLAN | 3795-01A | 7-1/4″ (18.5 cm) |
| Baxter 0.9% Sodium Chloride Irrigation Bottle | Baxter | SKU: 195-7124 | 1000mL Bottle |
| Bovie Cautery Low Temperature Fine Tip | Symmetry Surgical Inc | AA00 | 10/bx |
| Buprenorphine | Fidelis Animal Health | SKU- 099114 | 1.3mg/mL, 3mL |
| CD (Sprague Dawley) Rats | Charles River | Strain Code 001 | Male, 400 gm |
| Clip-It Traceable Timer | TRACEABLE PRODUCTS | Cat. No. 5046 | |
| Cotton-Tip Applicator 3" | Dukal Corporation | 922-00037BX100 | 1000/BOX |
| Enzymatic Surgical Instrument Detergent and Presoak | Medline | MDS88000B9 | 1 Gal |
| Eye Lube | Optixcare | SKU-065441 | 20gm |
| Gaymar T/Pump Stryker Localized Warming/Cooling Therapy System | Stryker | TP700 | |
| Heifetz Microclips 12mmx2.25mm | SCANLAN | 3795-20 | Straight |
| Heifetz Microclips 12mmx2.25mm | SCANLAN | 3795-28 | Curved |
| Heifetz Microclips 8mmx1.75mm | SCANLAN | 3795-14 | Straight |
| Heifetz Microclips 8mmx1.75mm | SCANLAN | 3795-18 | Curved |
| Inflation device | Merit Medical | IN4130 | BasixCOMPAK with Analog Display |
| Isoflurane | MFR VETONE | SKU-501017 | 100mL, Liquid for Inhalation |
| Microsurgery kit containing tweezers and forceps | Customized | ||
| Oxygen, E-tank | Medline | MDM1630 | |
| Sharpened Guidewire | Customized | ||
| Sterile Glad Press-n-Seal | Glad | SKU # PSS-140 | ETO Exposed |
| Sterile Nonwoven Gauze Sponges 2s 4ply 4x4 | Medline | SKU PRM21444H | 100Ct |
| Surgical microscope | Zeiss | S100 / OPMI pico | |
| Surgical Scrub & Handwash | Vetoquinol | NDC: 17030-003-16 | 16 oz |
| Ultrasound Gel | Medline | CTR001305 | 8.5 oz, 12/CS |
| Ultrasound system | Siemens | ACUSON Antares | 3-D, Color, Continuous wave (CW), Pulsed wave (PW), Power CW/CW Spectral |
| Venoplasty Balloon | Customized |
References
- Prandoni, P., et al. The long-term clinical course of acute deep venous thrombosis. Ann Inter Med. 125 (1), 1-7 (1996).
- Prandoni, P., Kahn, S. R. Post-thrombotic syndrome: prevalence, prognostication and need for progress. Br J Haematol. 145 (3), 286-295 (2009).
- Gloviczki, P., et al. The 2023 Society for Vascular Surgery, American Venous Forum, and American Vein and Lymphatic Society clinical practice guidelines for the management of varicose veins of the lower extremities. Part II. J Vas Surg Venous Lymph Diso. 12 (1), 101670 (2024).
- Henke, P., Sharma, S., Wakefield, T., Myers, D., Obi, A. Insights from experimental post-thrombotic syndrome and potential for novel therapies. Transl Res. 225, 95-104 (2020).
- Diaz, J. A., et al. Choosing a mouse model of venous thrombosis: A consensus assessment of utility and application. Arterioscl Thrombo Vasc Biol. 39 (3), 311-318 (2019).
- Lee, Y. U., Lee, A. Y., Humphrey, J. D., Rausch, M. K. Histological and biomechanical changes in a mouse model of venous thrombus remodeling. Biorheology. 52 (3), 235-245 (2015).
- Xie, H., et al. Correspondence of ultrasound elasticity imaging to direct mechanical measurement in aging DVT in rats. Ultrasound Med Biol. 31 (10), 1351-1359 (2005).
- Moini, M., et al. Venoplasty and stenting in post-thrombotic syndrome and non-thrombotic iliac vein lesion. Minim Invasive Ther Allied Technol. 29 (1), 35-41 (2020).
- Chaitidis, N., et al. Management of post-thrombotic syndrome: A comprehensive review. Curr Pharma Des. 28 (7), 550-559 (2022).
- Dangas, G. D., et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 56 (23), 1897-1907 (2010).
- Neglén, P., Berry, M. A., Raju, S. Endovascular surgery in the treatment of chronic primary and post-thrombotic iliac vein obstruction. Euro J Vas Endovas Surg. 20 (6), 560-571 (2000).
- Sharifi, M., Mehdipour, M., Bay, C., Smith, G., Sharifi, J. Endovenous therapy for deep venous thrombosis: The TORPEDO trial. Catheter Cardiovasc Interv. 76 (3), 316-325 (2010).
- Nicklas, J. M., Gordon, A. E., Henke, P. K. Resolution of deep venous thrombosis: Proposed immune paradigms. Int J Mol Sci. 21 (6), 2080 (2020).
- Li, J., Kim, S. G., Blenis, J. Rapamycin: One drug, many effects. Cell Metabol. 19 (3), 373-379 (2014).
- Chen, X., et al. Rapamycin regulates Akt and ERK phosphorylation through mTORC1 and mTORC2 signaling pathways. Mol Carcinogen. 49 (6), 603-610 (2010).
- Diaz, J. A., Farris, D. M., Wrobleski, S. K., Myers, D. D., Wakefield, T. W. Inferior vena cava branch variations in C57BL/6 mice have an impact on thrombus size in an IVC ligation (stasis) model. J Thromb Haemo. 13 (4), 660-664 (2015).
- Schönfelder, T., Jäckel, S., Wenzel, P. Mouse models of deep vein thrombosis. Gefässchirurgie. 22 (1), 28-33 (2017).
- de Barros, R. S. M., et al. Morphometric analysis of rat femoral vessels under a video magnification system. J Vasc Bras. 16 (1), 73-76 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved