Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Production of Transgenic Xenopus laevis by Restriction Enzyme Mediated Integration and Nuclear Transplantation
W tym Artykule
Podsumowanie
This video protocol demonstrates a method for generating transgenic Xenopus laevis by introduction of transgenes into sperm nuclei followed by nuclear transplantation into unfertilized eggs.
Streszczenie
Stable integration of cloned gene products into the Xenopus genome is necessary to control the time and place of expression, to express genes at later stages of embryonic development, and to define how enhancers and promoters regulate gene expression within the embryo. The protocol demonstrated here can be used to efficiently produce transgenic Xenopus laevis embryos. This transgenesis approach involves three parts: 1. Sperm nuclei are isolated from adult X. laevis testis by treatment with lysolecithin, which permeabilizes the sperm plasma membrane. 2. Egg extract is prepared by low speed centrifugation, addition of calcium to cause the extract to progress to interphase of the cell cycle, and a high-speed centrifugation to isolate interphase cytosol. 3. Nuclear transplantation: the nuclei and extract are combined with the linearized plasmid DNA to be introduced as the transgene and a small amount of restriction enzyme. During a short reaction, egg extract partially decondenses the sperm chromatin and the restriction enzyme generates chromosomal breaks that promote recombination of the transgene into the genome. The treated sperm nuclei are then transplanted into unfertilized eggs. Integration of the transgene usually occurs prior to the first embryonic cleavage such that the resulting embryos are not chimeric. These embryos can be analyzed without any need to breed to the next generation, allowing for efficient and rapid generation of transgenic embryos for analyses of promoter and gene function. Adult X. laevis resulting from this procedure also propagate the transgene through the germline and can be used to generate lines of transgenic animals for multiple purposes.
Protokół
Modified versions of this transgenesis approach were initially described in 1 and 2.
A. Sperm nuclei preparation
This nuclei preparation method is adapted from Murray 3, but the protease inhibitors have been omitted as they interfere with subsequent development of eggs transplanted with sperm nuclei. Aliquots are frozen at -80°C and can be used for transplantations for approximately 6 months.
All solutions should be prepared and placed on ice prior to beginning the preparation.
- Anesthetize 1-2 adult male X. laevis by immersion in Tricaine for at least 20 min followed by pithing; remove the testes.
- Roll the testes on a paper towel to remove the blood, blood vessels and fat body, wash them briefly in a 60 mm Petri dish containing 1XMMR, and remove any additional pieces of fat body. Take care not to puncture the testes, as this releases the sperm.
- Transfer testes to a dry 60-mm Petri dish and macerate testes with forceps until there are no visible chunks. Maceration should be done as thoroughly as possible to obtain high yields of nuclei.
- Add 2mLs cold nuclear preparation buffer (NPB;1X from stock) to the macerate and gently pipette up and down.
- Squirt macerate through about 4 thicknesses of cheesecloth on a funnel, collecting into a 15 mL tube (e.g. Falcon 2059); Rinse dish with 8mLs of NPB and put this rinse through the cheesecloth, collecting it in the tube. With gloved hands squeeze the cheesecloth to collect remaining liquid into the tube.
- Pellet the sperm at 3000 rpm for 10 min. at 4°C in a swinging bucket rotor (e.g. 1480g in a Sorvall HB-4 rotor or equivalent) with the appropriate tube adaptors. Decant supernatant; add 8mL NPB to this tube and pipette up and down with a 10 mL pipette to resuspend pellet; Spin down again as above and decant supernatant. Equilibrate 1mL of NPB to room temperature during the spin.
- Resuspend pellet in 1mL room temperature NPB using a 1 mL pipetteman tip, add 50μl of 10mg/mL freshly made lysolecithin, mix gently, and incubate for 5 min. at room temperature.
- Add 10 mL cold 1XNPB+3%BSA to the tube to stop the lysolecithin reaction, mix gently, and spin down for 10 min at 3000 rpm in a swinging bucket rotor. Decant supernatant. The lysolecithin-treated pellet should look slightly more translucent (less opaque white) than it did prior to lysolecithin treatment.
- Decant supernatant and resuspend pellet in 5 mL cold NPB+0.3%BSA, mix gently with a 10 mL pipette and spin down for 10 min at 3000rpm as above.
- Decant supernatant and resuspend pellet in 500μl of sperm storage buffer and transfer to a 1.5 mL tube. This is now your nuclei stock. Store on ice while you check the yield of nuclei.
- To check the yield of nuclei, place 98 μl of sperm dilution buffer (SDB), 1 μl of the nuclear stock and 1μl of 1:100 dilution of the Hoechst stock in a 1.5mL Eppendorf tube. Mix the nuclear stock very well using a razor-clipped (or large-opening) pipette tip just before removing the 1 μl. Mix the diluted SDB/Hoechst/nuclei very well and allow a small amount to flow into the chamber of an improved Neubauer hemacytometer by capillary action. Count nuclei in a square of the hemacytometer under a compound microscope. From one male, you should obtain counts of at least 100-200 (X104 cells/mL) for this 1:100 dilution of the stock for an undiluted stock concentration of 1-2X105 cells/μl. If your stock is less concentrated, let the nuclei settle for several hours, remove some of the supernatant, and recount. Leave the nuclei at 4°C overnight to allow the glycerol to penetrate for better cryopreservation; then mix the nuclear stock well with a large orifice pipette tip, prepare 20μl aliquots, and freeze in liquid nitrogen.
B. Preparation of High Speed Extract
This method is adapted from Murray3 and produces an interphase cytosolic extract that will promote swelling and partial chromatin decondensation of added sperm nuclei. Extract can be frozen in small aliquots at -80°C and thawed before use.
- 3-5 days prior to HCG injection, prime 8-12 adult female X. laevis by injecting 50 U of PMSG into the dorsal lymph sac. The evening before extract preparation, inject each frog with 500 units HCG and place 2 frogs/container into 2 liters 1XMMR. Since one frog with lysing or activating eggs can compromise the extract preparation, it is advisable to separate frogs into pairs for ovulation.
- The next morning, prepare and chill all solutions before beginning the preparation. Gently, manually expel eggs from each frog into large beakers containing 1X MMR. Screen the eggs from each container and omit any batches of eggs with signs of mottling, lysing or activation (visualized by contraction of pigmentation in the animal hemisphere) from the procedure. Collect unbroken eggs with even pigmentation. Good eggs can also be collected from the 1X MMR in the frog buckets. The total volume of eggs should be >100 mL from the 8-12 females.
- De-jelly the eggs. To do this, remove as much MMR as possible, add a small amount of cysteine solution, and swirl the eggs. Replace with fresh cysteine solution several times during de-jellying. De-jelly each batch of eggs separately and discard batches with breakage or egg activation. Combine the rest of the eggs.
- Wash the eggs four times in ~35 mL of extract buffer (XB), and then two times in 25 mL of CSF-XB with protease inhibitors.
- To pack the eggs: transfer the eggs into Beckman ultraclear tubes. Allow the eggs to settle. Remove as much CSF-XB as possible. Centrifuge the eggs using a Beckman SW 40 Ti rotor (or similar rotor) at 1000 rpm (150g) for ~60 sec at 4°C. Remove excess solution from the top of the packed eggs.
- To crush the eggs and generate cytoplasmic extract: centrifuge the eggs at 10,000 rpm (16,000g) for 10 min at 4°C. The eggs should separate into three layers: lipid (top), cytoplasm (center), and yolk (bottom). Collect the cytoplasmic layer from each tube with an 18-gauge needle by inserting the needle through the tube at the base of the cytoplasmic layer. Transfer the cytoplasm to a fresh ultraclear Beckman tube on ice.
- Add protease inhibitors to the isolated cytoplasm to a final concentration of 1X. Recentrifuge the cytoplasm at 16,000g for 10 min at 4°C. Collect the clarified cytoplasm as described above. Expect to obtain 0.75-1 mL cytoplasm/frog.
- Add 1/20 of the extract volume of energy mix to the sample. Transfer the cytoplasm into thick-walled polycarbonate tubes for the Beckman TL-100 ultracentrifuge. Tubes hold about 3 mL each and should be at least half full.
- Add 1 M CaCl2 to each tube to a final concentration of 0.4 mM. Incubate the tubes for 15 min at room temperature. This inactivates CSF and pushes the extract into interphase.
- Balance the tubes and centrifuge them in a Beckman TL-100 ultracentrifuge using a TLA-100.3 rotor at 70,000 rpm (200,000 g) for 1.5 h at 4°C. The cytoplasm will fractionate into four layers, top to bottom: lipid, cytosol, membrane/mitochondria, and glycogen/ribosomes.
- Remove the cytosolic layer from each tube (~1 mL if 2-3 mL was loaded into the tube) by inserting a syringe into the top of the tube through the lipid layer. Transfer the cytosolic fraction to fresh tubes and recentrifuge the samples at 70,000 rpm (200,000g) for 20 min at 4°C.
- Aliquot the supernatant into 25-μl aliquots in 0.5-mL tubes. Quick-freeze the aliquots in liquid nitrogen and store at -80°C until use. To determine whether the extract is effective, sperm nuclei can be incubated in extract and stained with Hoechst to determine whether the nuclei visibly swell (thicken and lengthen) within 10 min of addition at room temperature.
C. Transgenesis reaction and nuclear transfer
Important: Check that solutions, equipment and frogs are all ready before beginning a reaction. Once you begin, you must proceed with the reaction using the approximate timetable described below, since many components do not remain stable for >30 minutes. While the sperm nuclei stock is kept on ice, transgenesis reactions (both diluted and concentrated) should be kept at room temperature.
- Prime female frogs. The evening before transgenesis, inject several (3-5) adult females frog with 800 units HCG in the dorsal lymph sac to obtain freshly laid eggs beginning the next day.
- The day of the transgenesis procedure, prepare or bring to temperature the solutions needed for transgenesis:
- Freshly made cysteine (2.5% in 1XMMR, pH8.0). You will need several hundred milliliters to be used that day.
- 0.2XMMR+6%Ficoll for transplantation dishes and recovery of transgenic embryos and 0.2XMMR+100μg/mL gentamicin (without Ficoll) for raising embryos. Solutions should not be warmer than 18-21°C and transgenic embryos should be raised through early cleavages at temperatures between 16 and 21°C, since higher temperatures adversely affect both frequency of transgenesis and embryonic development.
- Thaw and equilibrate to room temperature frozen aliquots of SDB (sperm dilution buffer) for the day's transplantations, thaw the high speed extract and place on ice, and prepare a 100mM MgCl2 solution.
- Get out the agarose injection dishes and fill with MMR/Ficoll solution, and set up and pre-run the infusion pump to stabilize the flow.
- Check that female frogs are laying and eggs are of high quality. Optimally, eggs should have a firm cortex (hold shape after de-jellying).
- Set up a transgenesis reaction:
- Very gently (using a clipped pipette tip) mix the nuclei stock and combine in a 1.5mL Eppendorf tube:
4μl nuclei (~4-8X105 nuclei)
2μl linearized plasmid (100ng/μl)
Incubate 5 minutes at room temperature. - while incubation is proceeding, dilute 0.5μl of restriction enzyme in 4.5μl H2O and add 1μl of diluted enzyme to 18μl of SDB, 2μl of MgCl2 and 2μl of high speed extract. Add this mixture to the nuclei-plasmid reaction. Incubate 10 more minutes to swell the nuclei.
- During this incubation, squeeze eggs from 2-3 frogs and de-jelly in cysteine solution. Wash them well (5X) with 1XMMR, and use a wide bore pipette to transfer 400-500 dejellied eggs to each agarose well dish containing 0.2X MMR +4% Ficoll. This usually takes about 10 minutes, so once egg dishes are prepared the reaction is usually ready to dilute.
- Approximately 15-20 minutes after starting step a, gently mix the reaction (nuclei/extract/DNA) with a clipped tip and add about 5μl to 150 μl SDB at room temperature. Nuclei in this diluted mixture are stable for about 1hr., whereas they do not retain transplantation capacity as long when left in the concentrated mixture.
- Very gently (using a clipped pipette tip) mix the nuclei stock and combine in a 1.5mL Eppendorf tube:
- Load the needle: Mix the diluted reaction prepared above very gently with a wide orifice pipette tip, then load the needle promptly, as nuclei will settle rapidly in the tube. To backload the needle, place a piece of fine Tygon tubing onto the end of a clipped 200μl pipette tip. Draw the solution into the pipette tip, then attach the tubing to the needle and allow the solution to enter the needle by gravity or gently depressing the pipette plunger.
- Attach needle to pump tubing and begin transplantations of nuclei. Injections should be rapid, shallow and approximately perpendicular to the egg's plasma membrane to avoid doing damage. At the rate of flow suggested (10nl/sec), keep the needle in the egg for ~0.5 seconds. You should complete 1-2 dishes of transplantations within 20-30 minutes.
After injections, leave the dishes at 16-20°C until embryos have reached the 4-8 cell stage. Sort the cleaving embryos away from their non-cleaving neighbors by transferring (with a glass Pasteur pipette with a tip approximately the same diameter as an egg) to a fresh large dish of 0.2XMMR +4% Ficoll. Subdivide the cleaving embryos into smaller groups (10-15 embryos/well of a 6-well plate) and culture in 0.2XMMR (no Ficoll) +100μg/mL gentamicin through early development. Embryos should be checked during gastrulation with any dying embryos removed promptly and the media changed as needed.
Troubleshooting:
- Needle is blocked: solution flow from the needle should be apparent during the transplantations. If the needle becomes blocked by visible particulate matter, change needles or try to remove the debris by clipping the tip with forceps and pushing solution through the needle.
- No cleaving eggs are obtained: check that dilutions of sperm nuclei and injection volume are appropriate and that the needle was not blocked during the transplantation. If injection volume is too high, eggs may also not cleave and instead show discolored pigmentation or damage.
- Many embryos die during gastrulation. Several factors can enhance embryo survival:
- take care with the nuclei during preparation and the enzymatic reaction. Decondensed nuclei are fragile and need to be transplanted using the timetable above. Do not place them on ice.
- The chromosomal damage sustained by the sperm nuclei during the enzyme reaction and nuclear transfer affects both the frequency of transgenesis and the normal development of embryos. Chromosomal damage to the nuclei enhances the efficiency of transgenesis but negatively affects survival/normal development. Decreasing or omitting restriction enzyme during the enzyme incubation can enhance the rate of normal development but may decrease frequency of transgenesis or plasmid copy number introduced into the embryo genome.
- If late blastula through gastrula embryos show signs of discoloration or cell death it is also possible that a reagent used for the transplantations was toxic to the eggs. In addition, if eggs do not have a firm cortex, they may undergo exogastrulation and fail to generate normal post-gastrula embryos.
- The number of embryos expressing the transgene is low. Increase the amount of restriction enzyme.
Representative Transgenic Embryo Results
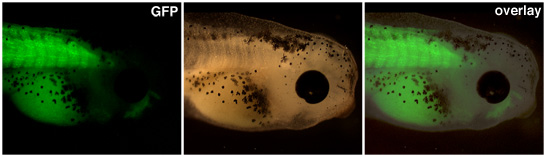
Transgenic embryos resulting from nuclear transfers should be raised until expression from the promoter of interest is visible. In Figure 1 above, a muscle actin promoter drives expression of green fluorescent protein in the somites of this transgenic tadpole.
Dyskusje
For each transgenic construct to be tested, we generally transplant nuclei into 500-1000 eggs; at this scale, we can generate transgenic embryos expressing up to 10 different constructs per day, depending on how many females are induced to lay eggs. Of these transplantations, about one third of the eggs cleave and 60-80% of these cleaving embryos proceed through gastrulation normally. Depending on the reaction conditions used, between 10-50% of these embryos express the transgene of interest. Therefore, once this procedu...
Ujawnienia
Experiments on animals were performed in accordance with the guidelines and regulations set forth by the Animal Care and Use Committee at Washington University School of Medicine.
Podziękowania
Funding for our work is provided by the NIH, the March of Dimes, and the American Cancer Society.
Materiały
| Name | Company | Catalog Number | Comments |
A.Sperm nuclei preparation | |||
Reagents: | |||
| |||
Equipment: | |||
| |||
B. Preparation of High Speed Extract | |||
Reagents: | |||
| |||
Equipment: | |||
| |||
C. Nuclear transplantation. | |||
Reagents: | |||
| |||
Equipment: | |||
Agarose dishes for injection: In a 60 mm plastic petri dish, lay a small 35mmX35mm weigh boat on molten 2.5% agarose in water 0.1XMMR to create a depression with an agarose-coated bottom for filling with eggs. Once agarose has hardened, wrap in parafilm and store at 4 °C until use. Make 2-3 dishes in advance for each transgenic reaction you plan to do. | |||
Infusion pump: We use a single syringe infusion pump from Harvard Apparatus, equipped with a 3 cc syringe/needle filled with mineral oil (Sigma M-8410). Blunt the syringe needle tip (to keep it from perforating the tubing) and attach the fine tygon tubing. Run the pump at ~10nl/sec; this assumes that the time the needle is in each egg will be no greater than 1 sec. Pump should be pre-run for several minutes prior to starting transgenesis for the day to assure that the plunger for the syringe is flush with the piston and that steady positive flow of oil out of the tubing is occurring. | |||
Needles for nuclear transfers. Using a micropipette puller, generate needles with long, sloping tips. Clip these with a forcep under a dissecting microscope equipped with an ocular micrometer to obtain an ~80 micron opening with a beveled shape. | |||
Other equipment: Xenopus laevis females, stereomicroscope, incubator, micromanipulator, microinjection needle puller (e.g. Model P-87, Sutter), syringe needles (26 gauge), glass microinjection needles, ocular micrometer for calibrated clipping of microinjection needle tips to 80μm diameter, petri dishes, weigh boats 35mm, Tygon tubing (ID=1/32 in., OD=3/32 in.) | |||
Odniesienia
- Kroll, K. L., Amaya, E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 122, 3173-3183 (1996).
- Amaya, E., Kroll, K. L. A method for generating transgenic frog embryos. Methods Mol Biol. 97, 393-414 (1999).
- Murray, A. W. Cell cycle extracts. Methods Cell Biol. 36, 581-605 (1991).
- Hartley, K. O., Hardcastle, Z., Friday, R. V., Amaya, E., Papalopulu, N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Developmental Biology. 238, 168-184 (2001).
- Karaulanov, E., Knöchel, W., Niehrs, C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J. 23, 844-856 (2004).
- Ogino, H., Fisher, M., Grainger, R. M. Convergence of a head-field selector Otx2 and Notch signaling: a mechanism for lens specification. Development. 135, 249-2458 (2008).
- Taylor, J. J., Wang, T., Kroll, K. L. Tcf- and Vent-binding sites regulate neural-specific geminin expression in the gastrula embryo. Developmental Biology. 289, 494-506 (2006).
- Marsh-Armstrong, N., Huang, H., Berry, D. L., Brown, D. D. Germ-line transmission of transgenes in Xenopus laevis. Proceedings of the National Academy of Sciences of the United States of America. 96, 14389-14393 (1999).
- Offield, M. F., Hirsch, N., Grainger, R. M. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 127, 1789-1797 (2000).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone