Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Step-by-step Method for the Reconstitution of an ABC Transporter into Nanodisc Lipid Particles
W tym Artykule
Podsumowanie
Nanodiscs are small discoid particles that incorporate membrane proteins into a small patch of phospholipid bilayer. We provide a visual protocol that shows the step-by-step incorporation of the MalFGK2 transporter into a disc.
Streszczenie
The nanodisc is a discoidal particle (~ 10-12 nm large) that trap membrane proteins into a small patch of phospholipid bilayer. The nanodisc is a particularly attractive option for studying membrane proteins, especially in the context of ligand-receptor interactions. The method pioneered by Sligar and colleagues is based on the amphipathic properties of an engineered highly a-helical scaffold protein derived from the apolipoprotein A1. The hydrophobic faces of the scaffold protein interact with the fatty acyl side-chains of the lipid bilayer whereas the polar regions face the aqueous environment. Analyses of membrane proteins in nanodiscs have significant advantages over liposome because the particles are small, homogeneous and water-soluble. In addition, biochemical and biophysical methods normally reserved to soluble proteins can be applied, and from either side of the membrane. In this visual protocol, we present a step-by-step reconstitution of a well characterized bacterial ABC transporter, the MalE-MalFGK2 complex. The formation of the disc is a self-assembly process that depends on hydrophobic interactions taking place during the progressive removal of the detergent. We describe the essential steps and we highlight the importance of choosing a correct protein-to-lipid ratio in order to limit the formation of aggregates and larger polydisperse liposome-like particles. Simple quality controls such as gel filtration chromatography, native gel electrophoresis and dynamic light scattering spectroscopy ensure that the discs have been properly reconstituted.
Protokół
Overall Reconstitution Process
The reconstitution process starts by mixing the membrane scaffold protein (MSP) with the purified MalFGK2 complex in the presence of detergent-solubilized phospholipids. The step is followed by the slow removal of the detergent by an adsorbent polystyrene material called Bio-Beads or Amberlite (Figure 1). The auto-assembly process occurs most likely because of the apolar interactions between the hydrophobic phospholipids, the MalFGK2 complex and the surface of the MSP amphipathic protein. The final product is a discoid particle made of two molecules of MSP wrapping around the MalFGK2 complex. The particles are separated from the adducts and aggregates by ultra-centrifugation and analytical size-exclusion chromatography. The particles are characterized by native-gel electrophoresis and dynamic light scattering spectroscopy.
1. Preparation of the Membrane Scaffold Protein, MSP
- The his-tagged MSP (version MSP1D11) is produced from plasmid pMSP1D1 in E. coli BL21 (DE3) induced at OD600~0.5 with 0.5 mM isopropyl β-D-1-thiogalactopyranoside for 3 hr at 37 °C.
- The cells are harvested by centrifugation at 5,000 x g for 10 min at 4 °C and resuspended in TSG10 buffer containing 100 μM phenylmethanesulfonyl fluoride.
- The cells are lysed with a French press (3x) at 8,000 psi and the insoluble material is removed by centrifugation at 5,000 x g for 10 min at 4 °C. The soluble fraction containing the MSP is isolated by ultra-centrifugation at 125,000 x g for 45 min.
- The MSP is purified by nickel-chelating chromatography using ~1.5 ml Ni Sepharose HP in TSG10 buffer. Contaminants are washed away with TSG10 buffer containing 50 mM imidazole. The MSP is eluted with TSG10 buffer containing 600 mM imidazole. The purified protein is dialyzed in TSG10 buffer and stored in -70 °C at a protein concentration of ~10-15 mg/ml.
2. Preparation of the MalFGK2 Complex
- The MalFGK2 complex, his-tagged at the C-terminus of MalK, is expressed from plasmid pBAD22-FGK in E. coli BL21 (DE3) and induced at OD600~0.5 with 0.2% L-arabinose for 3 hr at 37 °C.
- Following cell lysis and centrifugation as in step 1.3, the membrane pellet is resuspended in TSG20 buffer at a final concentration of 5 mg/ml. The material is solubilized with 1% w/v n-dodecyl-β-maltopyranoside (DDM) for 3 hr at 4 °C with gentle shaking.
- The insoluble material is removed by ultra-centrifugation as in step 1.3 and the supernatant containing the solubilized MalFGK2 complex is collected and purified as in step 1.4, but without dialysis.
- Further purification is achieved by gel filtration chromatography in TSGD buffer on a Superdex 200 HR 10/300 column at a flow rate of 0.5 ml/min.
3. Preparation of Phospholipids
- An E. coli total lipid extract dissolved in chloroform is separated into 1,000 nmol aliquots in screw cap microfuge tubes. The solvent is evaporated under a gentle stream of nitrogen and dried further overnight in a vacuum desiccator.
- The lipid film is dissolved in TS buffer at 5 nM final concentration and vortexed vigorously and sonicated. The dissolved lipids will appear slightly opaque due to their general insolubility in aqueous TS buffer, but they should remain in suspension. DDM is added to a final concentration of 0.5% (~10 mM), at which the solution becomes clear.
- The lipid mixture is placed in a water bath and pulse sonicated for 5 times for ~5 sec. The lipid mixture is stored at 4 °C for a maximum of 1 week.
4. Preparation of Bio-Beads
- Approximately 10-15 ml (dry volume) Bio-Beads is placed into a 50 ml tube.
- The beads are successively washed with 50 ml of 100% methanol, 95% ethanol, milliQ H2O, and finally TS buffer (twice each).
- The washed beads are stored at 4 °C in ~10 ml TS buffer.
5. Nanodisc Reconstitution
- The MSP is diluted in TSGD buffer at the final concentration of ~7 mg/ml (~0.3 mM).
- ~2 nmol of purified MalFGK2 complex are mixed at a protein:MSP:lipid ratio of 1:3:60 or 1:3:400 in TSGD buffer. The final concentration is 6 μM MalFGK2, 18 μM MSP and 360 μM lipids (1:3:60) or 2.4 mM lipids (1:3:400). The final volume is 300 μl. The final DDM concentration is 0.08% (~1.6 mM). The final concentration of glycerol depends on the amount of lipid added but remains around 5-10% v/v.
- ~50 μl of Bio-Bead suspension is added to the tube and the mixture is incubated overnight on a rocking table at 4 °C.
- The beads are sedimented by gravity and the solution is pipetted through a narrow tip to avoid as much Bio-Beads as possible.
- Large precipitates are removed by ultra-centrifugation at 100,000 x g for 20 min. The discs are purified by gel filtration chromatography as in step 2.4 in TSG10 buffer. Fractions containing the discs are pooled and an aliquot should be re-injected on the same gel filtration column to test the stability of the preparation.
- The purified discs can stored at -70 °C for an extended period of time. Following thawing on ice, the discs should be subjected to ultra-centrifugation as in step 5.5 to remove potential precipitates. An aliquot should be analyzed by gel filtration chromatography to ensure that significant aggregation or precipitation did not occur during the thawing process.
6. Native Gel Electrophoresis
- 1 μM of nanodiscs (~0.2 mg/ml) are analyzed by native PAGE6, 7 (Tris-HCl pH 8.8, 4-12%) to assess the quality of the reconstitution (Figure 3A).
- The binding of MalE (1 μM) to the MalFGK2-complex reconstituted in nanodiscs is also assessed by native-PAGE (Figure 3A).
- After electrophoresis, the gel is stained with Coomassie blue for 10 min, and destained for ~1 hr.
7. Dynamic Light Scattering (DLS)
- Nanodiscs are purified by gel filtration chromatography as in step 2.4 in TSG10 buffer using a Superdex 200 HR 10/300 column at a flow rate of 0.1 ml/min. Fractions containing nanodiscs are pooled and concentrated to ~10 mg/ml with an Amicon centrifugal filter.
- The sample is filtered twice (0.22 μm filter) prior to analysis by DLS using a DynaPro nanostar instrument (Wyatt Technology) in a 1 μl inner volume quartz cuvette. Data is fitted using the DYNAMICS software (Wyatt Technology) to estimate the diameter and molecular weight of the particles.
8. ATPase Measurements
- The ATPase activity of MalFGK2-reconstituted nanodiscs is determined using a colorimetric assay8.
- 1 μM of purified discs and 1 mM ATP are mixed together with increasing amounts of MalE and incubated at 37 °C for 20 min. The release of inorganic phosphate is measured at 660 nm.
- The amount of phosphate released is compared to a standard curve generated with a Phosphorous Standard solution.
9. Representative Results
The nanodiscs are purified by gel filtration chromatography (Figure 2A, left). The chromatogram shows that the majority of the reconstituted discs (black trace) elute as a single peak, whereas discs made with excess lipids (red trace) elute in the void volume and as a series of broad peaks. The quality of the nanodiscs is further analyzed by native gel electrophoresis and dynamic light scattering spectroscopy (DLS). Properly reconstituted discs migrate as a sharp band on the gel whereas those reconstituted in the presence of an excess of lipids migrate as a smear (Figure 2A, right). Analysis by DLS shows that the disc population is homogeneous with an average diameter of 11.4 nm (Figure 2B). The reconstituted discs have an apparent molecular weight of 215 kDa based on the DLS approximation. Samples reconstituted in the presence of excess lipids display widely distributed radii around 100 nm, which is typical for non-homogeneous samples.
The quality of the MalFGK2 complex is assessed by native-gel electrophoresis and its activity by ATPase measurements (Figure 3). The maltose binding protein MalE binds with a high-affinity to the MalFGK2 transporter9, 10. Using non-denaturing gel electrophoresis, it is possible to detect a complex between MalE and MalFGK2 (Figure 3A). The stimulation of the MalK2 ATPase activity by MalE is shown in Figure 3B.
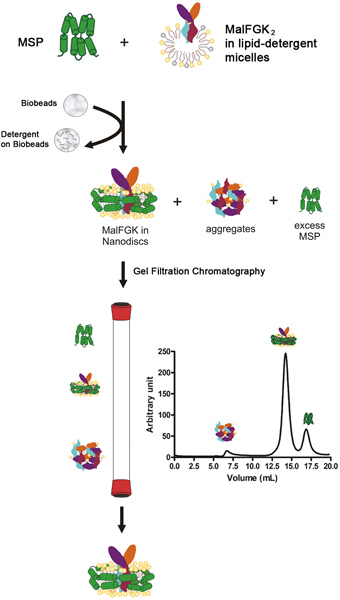
Figure 1. Typical flowchart for the reconstitution protocol.
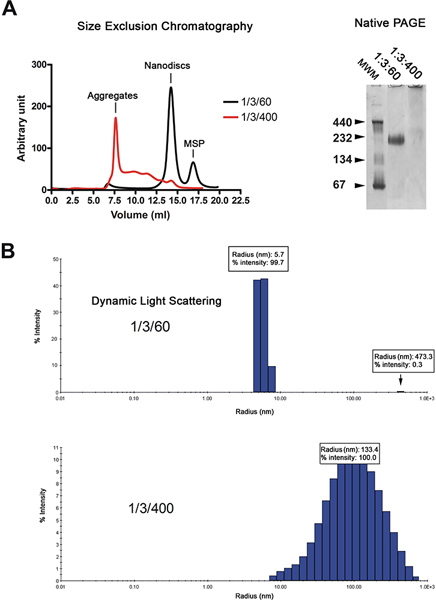
Figure 2. Quality control of the nanodisc preparation. A. Gel filtration analysis (Superdex 200 HR 10/300 column) of the nanodiscs reconstituted at low lipid ratio (1/3/60; black trace) or high lipid ratio (1/3/400; red trace). Native gel electrophoresis of the same disc preparation. Molecular weight markers in kDa are indicated. B. Dynamic light scattering analysis of the same disc preparation. Click here to view larger figure.

Figure 3. Analysis of the MalFGK2-nanodisc particles. A. Gel shift analysis of MalE incubated with increasing amounts of MalFGK2-nanodisc particles. B. ATPase activity of the MalFGK2-nanodisc particles as a function of MalE concentration.
Dyskusje
We describe a simple procedure for the reconstitution of the maltose transporter into nanodiscs. The transporter is ATPase active and the interaction with the soluble binding partner MalE can be recreated (Figure 3). The successful reconstitution of the transporter into nanodiscs open the way for additional biophysical and biochemical analysis. Of particular interest will be the systematic analysis the MalK ATPase and maltose transport activity in detergent, liposome and nanodiscs. ABC transporters c...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by the Canadian Institute of Health Research. CSC was funded by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada. FD is a Tier II Canada Research Chair.
Materiały
| Name | Company | Catalog Number | Comments | ||||||||||||||||||||||||||||||
| Amicon Ultra-4 50K centrifugal filter | Millipore | UFC805008 | Follow manufacturer's protocol for proper use | ||||||||||||||||||||||||||||||
| Bio-Beads SM-2 Adsorbent | Bio-Rad | 152-3920 | |||||||||||||||||||||||||||||||
| E. coli total lipids | Avanti Polar Lipids | 100500C | Dissolved in chloroform, handle as appropriate for an organic solvent | ||||||||||||||||||||||||||||||
| Ni sepharose HP resin | GE Healthcare | 17-5268-01 | |||||||||||||||||||||||||||||||
| Phosphorous standard solution | Sigma-Aldrich | P3869 | |||||||||||||||||||||||||||||||
| pMSP1D1 | Addgene | 20061 | |||||||||||||||||||||||||||||||
| Superdex 200 HR 10/300 | GE Healthcare | 17-5172-01 | |||||||||||||||||||||||||||||||
| Table I. Specific reagents. | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Odniesienia
- Denisov, I. G., Ginkova, Y. V., Lazarides, A. A., Sligar, S. G. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477-3487 (2004).
- Boldog, T., Grimme, S., Li, M., Sligar, S. G., Hazelbauer, G. L. Nanodiscs separate chemoreceptor oligomeric states and reveal their signaling properties. Proc. Natl. Acad. Sci. USA. 103, 11509-11514 (2006).
- Bass, B. J., Denisov, I. G., Sligar, S. G. Homotropic cooperativity of monomeric cytochrome P450 3A4 in a nanoscale native bilayer environment. J. Biol. Chem. 282, 7066-7076 (2007).
- Alami, M., Dalal, K., Lelj-Garolla, B., Sligar, S. G., Duong, F. Nanodiscs unravel the interaction between the SecYEG channel and its cytosolic partner SecA. EMBO J. 26, 1995-2004 (2007).
- Mi, L. -. Z., Grey, M. J., Nishida, N., Walz, T., Lu, C., Springer, T. A. Functional and structural stability of the epidermal growth factor receptor in detergent micelles and phospholipid nanodiscs. Biochemistry. 47, 10314-10323 (2008).
- Schägger, H., Cramer, W. A., von Jagow, G. Analysis of molecular masses and oligomeric states of protein complexes by blue native electrophoresis and isolation of membrane protein complexes by two-dimensional native electrophoresis. Anal. Biochem. 217, 220-230 (1994).
- Dalal, K., Duong, F. Reconstitution of the SecY translocon in Nanodiscs. Methods Mol. Biol. 619, 145-156 (2010).
- Lanzetta, P. A., Alvarez, L. J., Reinach, P. S., Candia, O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100, 95-97 (1979).
- Davidson, A. L., Dassa, E., Orelle, C., Chen, J. Structure, function and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72, 317-364 (2008).
- Bordignon, E., Grote, M., Schneider, E. The maltose ATP-binding cassette transporter in the 21st century-towards a structural dynamic perspective on its mode of action. Mol. Microbiol. 77, 1354-1366 (2010).
- Alvarez, F. J., Orelle, C., Davidson, A. L. Functional reconstitution of an ABC transporter for use in electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 132, 9513-9515 (2010).
- Ritchie, T. K., Grinkova, Y. V., Bayburt, T. H., Denisov, I. G., Zolnerciks, J. K., Atkins, W. M., Sligar, S. G. Reconstitution of membrane proteins in phospholipid bilayer Nanodiscs. Methods Enzymol. 464, 211-231 (2009).
- Glück, J. M., Koenig, B. W., Willbold, D. Nanodiscs allow the use of integral membrane proteins as analytes in surface plasmon resonance studies. Anal. Biochem. 408, 46-52 (2011).
- Wan, C. -. P. L., Chiu, M. H., Wu, X., Lee, S. K., Prenner, E. J., Weers, P. M. M. Apolipoprotein-induced conversion of phosphatidylcholine bilayer vesicles into nanodisks. Biochim. Biophys. Acta (BBA). 1808, 606-613 (2011).
- Nath, A., Trexler, A. J., Koo, P. K., Miranker, A. D., Atkins, W. M., Rhoades, E. Single-molecule fluorescence spectroscopy using phospholipid bilayer Nanodiscs. Methods Enzymol. 472, 89-117 (2010).
- Denisov, I. G., Sligar, S. G. Cytochromes P450 in Nanodiscs. Biochim. Biophys. Acta. 1814, 223-229 (2011).
- Zhang, X. X., Chan, C. S., Bao, H., Fang, Y., Foster, L. J., Duong, F. Nanodiscs and SILAC-based mass spectrometry to identify a membrane protein interactome. J. Proteome Res. , (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone