Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Trans-vivo Delayed Type Hypersensitivity Assay for Antigen Specific Regulation
W tym Artykule
Podsumowanie
We describe a valuable diagnostic assay that could potentially be used to decide the withdrawal of immunosuppression after transplant without elevated risk of graft rejection. The assay uses the principles of Delayed Type Hypersensitivity and provides accurate assessment of both donor specific effector and regulatory immune responses mounted by recipients.
Streszczenie
Delayed-type hypersensitivity response (DTH) is a rapid in vivo manifestation of T cell-dependent immune response to a foreign antigen (Ag) that the host immune system has experienced in the recent past. DTH reactions are often divided into a sensitization phase, referring to the initial antigen experience, and a challenge phase, which usually follows several days after sensitization. The lack of a delayed-type hypersensitivity response to a recall Ag demonstrated by skin testing is often regarded as an evidence of anergy. The traditional DTH assay has been effectively used in diagnosing many microbial infections.
Despite sharing similar immune features such as lymphocyte infiltration, edema, and tissue necrosis, the direct DTH is not a feasible diagnostic technique in transplant patients because of the possibility of direct injection resulting in sensitization to donor antigens and graft loss. To avoid this problem, the human-to-mouse "trans-vivo" DTH assay was developed 1,2. This test is essentially a transfer DTH assay, in which human peripheral blood mononuclear cells (PBMCs) and specific antigens were injected subcutaneously into the pinnae or footpad of a naïve mouse and DTH-like swelling is measured after 18-24 hr 3. The antigen presentation by human antigen presenting cells such as macrophages or DCs to T cells in highly vascular mouse tissue triggers the inflammatory cascade and attracts mouse immune cells resulting in swelling responses. The response is antigen-specific and requires prior antigen sensitization. A positive donor-reactive DTH response in the Tv-DTH assay reflects that the transplant patient has developed a pro-inflammatory immune disposition toward graft alloantigens.
The most important feature of this assay is that it can also be used to detect regulatory T cells, which cause bystander suppression. Bystander suppression of a DTH recall response in the presence of donor antigen is characteristic of transplant recipients with accepted allografts 2,4-14. The monitoring of transplant recipients for alloreactivity and regulation by Tv-DTH may identify a subset of patients who could benefit from reduction of immunosuppression without elevated risk of rejection or deteriorating renal function.
A promising area is the application of the Tv-DTH assay in monitoring of autoimmunity15,16 and also in tumor immunology 17.
Protokół
1. Preparation of Lymphocytes
- Collect blood into ACD (Acid Citrate Dextrose) tubes.
- Isolate PBMC from fresh human peripheral blood using Lymphocyte Separation Medium according to standard methods.
- Wash the PBMC three times with PBS to remove contaminating platelets. Platelets were found to interfere with trans-vivo DTH assay. Maximal allowable platelet contamination of PBMC preparation is ≤1x107/injection.
- If there is a noticeable red blood cell contamination, perform lysis of red cell using ACK lysis buffer after first wash. Remove ACK buffer by washing 2 times with PBS.
2. Preparation of Alloantigen
- Isolate PBMC from the donor peripheral blood using the procedure shown above.
- Resuspend donor PBMC in PBS at a concentration of 120x106 cells/ml (4x106 cells / 30 μl).
- Add 1 μM PMSF to the mixture to prevent protein degradation.
- Sonicate the cell suspension using seven 1-second pulses with a 2 mm-probe sonicator. (Note: Keep the material cold and avoid excessive bubbles. If foaming occurs, let the cell suspension sit on ice for a 2-3 min.)
- Verify the disruption of >90% of the cells using a hemocytometer.
- Centrifuge the mixture at 14,000 rpm at 4 °C for 20 min in refrigerated microfuge.
- Transfer supernatant to a new 2.0 ml safe-lock tube and determine protein concentration.
3. Cell Preparation for Injections
- For each injection aliquot 7x106 PBMC into 2.0 ml safe-lock tubes.
- Centrifuge at 6,000 rpm for a minute in microfuge and remove the supernatant.
- Resuspend the cells in PBS with or without antigens. Adjust the injection volume to 35 μl with PBS. The following scheme is used for injections:
Negative Control: PBMC + PBS
Positive Control: PBMC + TT/DT (25 μg/injection)
Experimental Antigen Specific Response: PBMC + test Ag (4-8 μg/injection)
Experimental Antigen Specific Regulation: PBMC + test Ag + TT/DT
4. Pre-measurement, Injection, and Post-measurement
- Anesthetize CB17 SCID mouse with isoflurane. Measure rear footpad thickness using a spring-loaded caliper. Put caliper at the center of the footpad, with one edge touching the last walking pad of the foot, to provide a benchmark to keep the measurement site consistent. Footpad thickness is recorded when gauge reading has stabilized.
- Slowly inject cell suspensions subcutaneously into the footpads of the mice using ½ cc insulin syringes with 28-gauge needle. Perform injection with the needle pointing toward the toes, and the bevel facing up. (Note: make sure there is no leakage).
- 18-24 hr after injection, anesthetize mouse with isoflurane and repeat measurement of footpad swelling.
- Subtract the thickness of each footpad prior to injection from the post injection value to obtain the footpad swelling value, expresses the data in units of 10-4 inches. Calculate the antigen-specific net swelling by subtracting control footpad swelling (PBMC+PBS) from the footpad swelling values obtained from the treatments (PBMC + donor alloantigen, TT/DT, or donor alloantigen + TT/DT). A positive control response to recall antigen TT/DT of ≥ 25x10-4 inches over background response to PBS is required for the test to be considered valid.
- Determine the inhibition of recall responses in the presence of donor antigens by comparing the net swelling of each injection using the following formula:
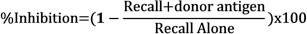
Wyniki
1. Evaluation of renal transplant recipients for donor antigen-specific response using Tv-DTH assay
To test the donor-reactive cellular immunity in renal transplant recipients we injected PBMC from these patients with donor antigens alone or with a recall antigen tetanus toxoid (TT). As a positive control cells were injected with TT alone. We observe three main patterns of delayed type hypersensitivity in transplant recipients (Figure 1). All patients responded strongly to Recall...
Dyskusje
The trans-vivo DTH assay is a novel diagnostic test with a potential clinical application in assessing cell-mediated responses in transplant, cancer and autoimmune patients. It is valuable because it is not only useful in monitoring recall T effector responses, but also it can detect T regulatory responses. A reliable way to detect human DTH regulation might predict safety of immunosuppression withdrawal in patients who are candidates for monotherapy or tolerance trials.
The Tv-DTH is very sen...
Ujawnienia
The authors of this manuscript have no conflicts of interest to disclose.
Podziękowania
The authors would like to acknowledge the contributions of A.M. VanBuskirk to the development of our understanding of the regulated DTH response in transplant recipients. This work was supported NIH grants PO1AI084853 and R01AI066219-06,and by the EU-sponsored One Study.
Materiały
| Name | Company | Catalog Number | Comments |
| ACD tube for blood collection | BD | 02-684-26 | |
| Lymphocyte Separation Medium | Cellgro | 25-072-CV | |
| Dulbecco's Phosphate-Buffered Saline | Cellgro | 21-031-CM | Without calcium & magnesium |
| ACK Lysis Buffer | BioWhittaker | 10-548E | |
| TT/DT or EBV | Sanofi Pasteur Inc./ Meridian Life Science, Inc. | TT/DT 25 μg/injection EBV 8 μg/injection | |
| Protease inhibitor PMSF | Sigma-Aldrich | 78830 | |
| Eosine for cell count | Sigma-Aldrich | E-6003 | |
| Alloantigen | Purified HLA antigens, synthetic allopeptides can be used instead of donor cell-free lysates | ||
| 50 ml sterile centrifuge tubes | Fisher Scientific | 06-443-18 | |
| 10 ml pipettes and pipettor | BD Falcon | 13-675-20 | |
| 2 ml safe-lock tubes | Costar | 3213 | |
| 1000 μl, 100 μl , 10 μl pipettes with sterile tips | |||
| Hemocytometer | Fisher | 02-671-10 | |
| Full size centrifuge and microfuge | Beckman Coulter/Eppendorf | ||
| 1/2cc or 1cc insulin syringes | Becton Dickinson | 14-826-79 | 28 gauge |
| Vibracell sonicator | Divtech Equipment Co. Sonocs Materials Inc | 2 mm probe | |
| Dial thickness gauge | Mitutoyo, Japan | ||
| SCID mice | Harlan | ||
| Isoflurane | Piramal Healthcare | Inhalant anesthesia |
Odniesienia
- Carrodeguas, L., et al. Trans vivo analysis of human delayed-type hypersensitivity reactivity. Hum. Immunol. 60, 640-651 (1999).
- VanBuskirk, A. M., et al. Human allograft acceptance is associated with immune regulation. J. Clin. Invest. 106, 145-155 (2000).
- Burlingham, W. J., Jankowska-Gan, E. Mouse strain and injection site are crucial for detecting linked suppression in transplant recipients by trans-vivo DTH assay. Am. J. Transplant. 7, 466-470 (2007).
- Burlingham, W. J., et al. Loss of tolerance to a maternal kidney transplant is selective for HLA class II: Evidence from trans-vivo DTH and alloantibody analysis. Human Immunology. 61, 1395-1402 (2000).
- Geissler, F., et al. Human liver allograft acceptance and the 'tolerance assay': In vitro anti-donor T cell assays show hyporeactivity to donor cells but, unlike DTH, fail to detect linked suppression. Transplantation. 72, 571-580 (2001).
- Jankowska-Gan, E., et al. Human liver allograft acceptance and the 'tolerance assay'. II. donor HLA-A, -B but not DR antigens are able to trigger regulation of DTH. Hum. Immunol. 63, 862 (2002).
- Cai, J., et al. Minor H Antigen HA-1-specific Regulator and Effector CD8+ T Cells, and HA-1 Microchimerism, in Allograft Tolerance. J. Exp. Med. 199, 1017-1023 (2004).
- Rodriguez, D. S., et al. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am. J. Transplant. 4, 537-543 (2004).
- Xu, Q., et al. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J. Immunol. 178, 3983-3995 (2007).
- Derks, R. A., Jankowska-Gan, E., Xu, Q., Burlingham, W. J. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J. Immunol. 179, 3443-3451 (2007).
- Jankowska-Gan, E., et al. Successful reduction of immunosuppression in older renal transplant recipients who exhibit donor-specific regulation. Transplantation. 88, 533-541 (2009).
- Jankowska-Gan, E., et al. Pretransplant immune regulation predicts allograft outcome: bidirectional regulation correlates with excellent renal transplant function in living-related donor-recipient pairs. Transplantation. 93, 283-290 (2012).
- Knechtle, S. J., et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. Am. J. Transplant. 9, 1087-1098 (2009).
- Haynes, L. D., et al. Donor-specific indirect pathway analysis reveals a B-cell-independent signature which reflects outcomes in kidney transplant recipients. Am. J. Transplant. 12, 640-648 (2012).
- Burlingham, W. J., et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J. Clin. Invest. 117, 3498-3506 (2007).
- Bobadilla, J. L., et al. TH-17, Monokines, Collagen Type V, and Primary Graft Dysfunction in Lung Transplantation. Am. J. Respir. Crit. Care Med. 177 (6), (2008).
- Olson, B. M., et al. Human prostate tumor antigen-specific CD8+ regulatory T cells are inhibited by CTLA-4 or IL-35 blockade. J. Immunol. 189, 5590-5601 (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone