Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Isolation of Cancer Stem Cells From Human Prostate Cancer Samples
W tym Artykule
Podsumowanie
The isolation of cancer stem cells (CSCs) directly from human tissues is requisite for their biological characterization. This manuscript describes a methodology for the isolation of prostate CSCs from human tissues, while also providing tips on troubleshooting difficult steps.
Streszczenie
The cancer stem cell (CSC) model has been considerably revisited over the last two decades. During this time CSCs have been identified and directly isolated from human tissues and serially propagated in immunodeficient mice, typically through antibody labeling of subpopulations of cells and fractionation by flow cytometry. However, the unique clinical features of prostate cancer have considerably limited the study of prostate CSCs from fresh human tumor samples. We recently reported the isolation of prostate CSCs directly from human tissues by virtue of their HLA class I (HLAI)-negative phenotype. Prostate cancer cells are harvested from surgical specimens and mechanically dissociated. A cell suspension is generated and labeled with fluorescently conjugated HLAI and stromal antibodies. Subpopulations of HLAI-negative cells are finally isolated using a flow cytometer. The principal limitation of this protocol is the frequently microscopic and multifocal nature of primary cancer in prostatectomy specimens. Nonetheless, isolated live prostate CSCs are suitable for molecular characterization and functional validation by transplantation in immunodeficient mice.
Wprowadzenie
Intratumoral heterogeneity is considered to be a hallmark of cancer1. Indeed, several mechanisms of intratumoral heterogeneity have been described, including genetic mutation and interactions with the microenvironment. In addition, some cancers may contain a cellular hierarchy with a subpopulation of cancer stem cells (CSCs) that exhibits the properties of tumor-initiating capacity and self-renewal in serial transplantation assays2-5. Initially described in hematological6, breast7, and brain8 malignancies, CSCs have also been studied in prostate cancer9-12 as well as other tumor types2-5.
CSCs are generally considered a cellular fraction within a heterogeneous population2-5. Therefore, the functional and molecular characterization of CSCs is contingent upon their enrichment from bulk populations. Accordingly, during the last two decades several methodologies of CSC enrichment have been devised which typically involve the separation of labeled populations by flow cytometry. In addition, a significant consideration in the study of CSCs is that the hierarchical organization of human tissues may be disrupted by experimental manipulations such as serial passaging in culture or immunodeficient mice. As a result, the direct isolation of CSCs from human tissues has emerged as an important methodology in the CSC field.
Prostate cancer is a leading cause of cancer morbidity and mortality in the United States and around the world13. Therefore, the isolation and biological characterization of prostate CSCs is of significant interest. Prostate CSCs have previously been enriched from cell lines, patient derived xenografts, and low passage patient derived suspension cultures9,10,12,14.
We recently reported the isolation of prostate CSCs directly from human surgical samples by virtue of their HLAI-negative cell surface phenotype10. Here we detail the procedures implemented for the isolation of these cells. Prostate tumors are harvested from surgical specimens and made into cell suspensions. Cells are then stained using antibodies against HLAI as well as stromal and viability markers, and CSCs are isolated by fluorescence activated cell sorting (FACS). Isolated CSCs can then be used for assays requiring viable cells.
Protokół
1. Harvesting and Processing of Human Prostate Cancer Tissue from Surgical Specimens
- Prepare two 50 ml polystyrene conical tubes containing 15 ml of Roswell Park Memorial Institute (RPMI) 1640 culture medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
- Pathology service personnel harvest primary and metastatic prostate cancer samples from prostatectomy and palliative surgery specimens, respectively, under an Institutional Review Board approved protocol. Metastatic samples may be obtained from lymph node dissections during primary surgery, the vertebrae during palliative surgical procedures, such as spinal cord decompression, and the lungs during diagnostic removal of single metastatic lesions, among others.
- Harvest excess bulk tissue not needed for clinical diagnostics into the first 50-ml polystyrene conical tube from step 1.1 and store immediately at 4 °C. The harvested tissue should measure at least a 4-5 cm2 in area and 1-2 cm in thickness.
- Laboratory personnel process bulk tissue obtained from the pathology service in preparation for histological examination and generation of cell suspensions.
- Processing of bulk tissues should not exceed 24 hr from surgical resection in order to guarantee maximal cell viability.
- Working in a biosafety cabinet with a sterile scalpel and forceps, take 2-4 mm thick horizontal tissue sections from macroscopic tumor nodules. Immediately store the sections in the second 50 ml polystyrene conical tube from step 1.1.
- When macroscopic nodules are not visualized from prostatectomy samples, collect random 2-4 mm thick horizontal tissue sections from the posterior lobes and transition zone.
- Separate a portion of the surgical tissue using a sterile scalpel and forceps and fix it in 10% formalin prior to generation of cell suspensions. Archive and analyze this material histologically to confirm the presence of prostate cancer tissue.
2. Generation of Cell Suspensions from Tissue Sections
- Working in a sterile biosafety cabinet, transfer a tissue section to a 60 x 15 mm culture dish containing 1 ml of sterile 1x phosphate buffered saline (PBS).
- Mechanically triturate the sample using a sterile scalpel and forceps.
- No enzymatic digestions are in this protocol.
- Transfer the 1 ml suspension into a sterile 50 ml conical tube.
- Add 1 ml to the culture dish and repeat the trituration step until the tissue section is completely dissociated, usually 3-4x.
- Repeat this procedure serially until all tissue sections have been dissociated.
- Resuspend the contents of the 50 ml polystyrene conical tube with a 5 ml serological pipette and vortex at maximum speed (approximately 3,200 rpm) for 1 min.
- Filter the resulting suspension through a 35-μm cell strainer and collect into a second sterile 50 ml polystyrene conical tube.
- Generate a pellet by centrifugation at 450 x g for 10 min.
- Discard the supernatant, resuspend the pellet with 5 ml of red blood cell lysis buffer, and incubate the solution for 5 min at RT. Remove the red blood cell lysis buffer by centrifugation for 5 min at 450 x g at RT.
- Discard the supernatant, resuspend the pellet in 1x PBS supplemented with 5% FBS, and quantify the number of viable (trypan blue-negative) cells using a hemocytometer or automated cell counter. Generate a 1x106 cells/ml suspension and store it on ice for no longer than 1 hr.
- The viability and yield of cells may vary considerably between tumor specimens. In the prostatectomy setting, viability may range between 45-70%, and five 2-4 mm thick horizontal tissue sections from a macroscopic tumor nodule may be expected to yield approximately 3x106 viable cells.
3. Antibody Staining of Cells for FACS
- Label five 15 ml polystyrene conical tubes as shown below. Use CD45 and CD31 antigen labeling to exclude hematopoietic and endothelial elements, respectively.
- Unstained
- IgG2aκ-PE + IgG1κ-FITC
- HLAI-PE + IgG1κ-FITC
- IgG2aκ-PE + CD31-FITC + CD45-FITC
- HLAI-PE + CD45-FITC + CD31-FITC
- Distribute the quantified cell suspension from the previous section (step 2.7) into the five tubes in step 3.1. Add the antibodies at a dilution of 1:250, and incubate the cell suspensions on ice for 30 min.
- Centrifuge the cells at 450 x g for 3 min at 4 °C, and discard the supernatants.
- Wash the cells by resuspending each pellet with sterile 1x PBS supplemented with 10% FBS followed by centrifugation at 450 x g for 3 min at 4 °C.
- Resuspend the cells in a 1 ml solution of 1x PBS containing 4',6-diamidino-2-phenylindole (DAPI) at a concentration of 10 μg/ml.
- Filter the final solution through 35 μm strainer caps into 12 mm x 75 mm polystyrene tubes.
4. Isolation of Prostate CSCs by FACS
- Utilize a flow cytometer for cell sorting.
- Set compensation controls using cells from tubes #1, #2, #3, and #4.
- Create gates that exclude debris and clusters of cells using forward- and side-scatter parameters.
- Establish the FITC and PE gates using the suspensions from tube #3 and tube #4, respectively.
- Gate viable (DAPI-negative) cells using the pacific blue-A channel.
- Using tube #5 discard CD31-positive and CD45-positive cells and collect both HLAI-negative and HLAI-positive populations into sterile 15-ml polystyrene conical tubes containing 2 ml of RPMI supplemented with 10% FBS.
- Centrifuge the sorted cell suspensions at 450 x g for 5 min, and then resuspend the pellets in 200-500 μl of RPMI supplemented with 10% FBS.
- Quantify viable (trypan blue-negative) cells using a hemocytometer or automated cell counter.
Wyniki
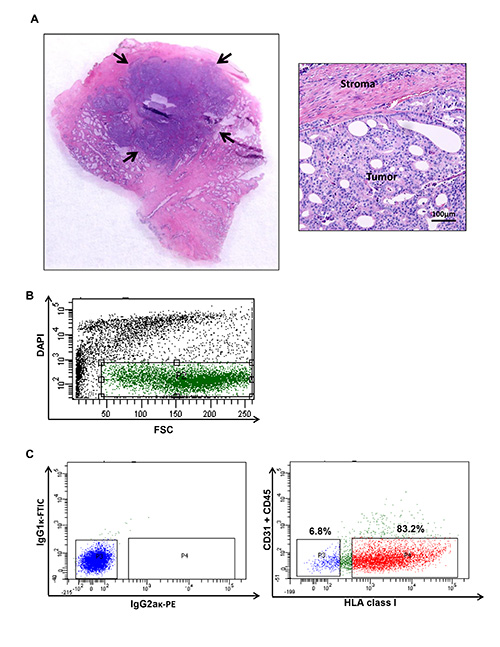
Figure 1. Human prostate cancer tumor harvesting and flow cytometry plot illustrating specific populations from a human prostate cancer. (A) Tumor nodules are harvested to generate a cell suspension. Tumor content of the processed sample is confirmed using conventional Hematoxylin and Eosin staining. (<...
Dyskusje
This protocol describes the isolation of CSCs from fresh human prostate cancer tissues. Several important considerations influence the successful outcome of this protocol.
The recovery of high numbers of viable prostate cancer cells is dependent on careful gross assessment of surgical samples. In our experience, the successful isolation of tumorigenic prostate cells is best assured when macroscopic tumor nodules are observed and processed10.
However, nowa...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This work was supported by the Hariri Family Foundation and the TJ Martell Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| RPMI | Gibco Life Technologies | 11875-093 | |
| Fetal bovine serum | Gibco Life Technologies | 10437-028 | |
| Penicillin Streptomycin | Gibco Life Technologies | 15140-122 | |
| PBS | Corning Cell Gro | 21-031-CM | |
| 60 mm, 15 mm Cell culture dish | Corning | 3295 | |
| 35 µm Cell Strainer | BD Falcon | 352340 | |
| 50 ml conical tube | Crystalgen | 23-2263 | |
| 15 ml conical tube | Crystalgen | 23-2265 | |
| Red blood cell lysing buffer | Sigma | R7757 | |
| HLA class I (W6/32) PE antibody | Abcam | ab43545 | |
| CD31 FITC antibody | eBioscience | 11-0319-42 | |
| CD45 FITC antibody | Abcam | ab27287 | |
| IgG2aκ PE antibody | BD Pharminogen | 555574 | |
| IgG1κ FITC antibody | BD Pharminogen | 551954 | |
| DAPI | Invitrogen | d3571 | |
| 12 mm x 75 mm Polystyrene tubes with cell strainer cap | BD Falcon | 352235 | |
| Vortex Mixer | Crystalgen | CG-BV1000 | |
| 10% Neutral Buffer Formalin | Fisher | RBBP-0480 |
Odniesienia
- Hanahan, D., Weinberg, R. A. Hallmarks of cancer: the next generation. Cell. 144, 646-674 (2011).
- Magee, J. A., Piskounova, E., Morrison, S. J. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 21, 283-296 (2012).
- Nguyen, L. V., Vanner, R., Dirks, P., Eaves, C. J. Cancer stem cells: an evolving concept. Nat. Rev. Cancer. 12, 133-143 (2012).
- Valent, P., et al. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer. 12, 767-775 (2012).
- Visvader, J. E., Lindeman, G. J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 10, 717-728 (2012).
- Lapidot, T., et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367, 645-648 (1994).
- Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A. 100, 3983-3988 (2003).
- Singh, S. K., et al. Identification of human brain tumour initiating cells. Nature. 432, 396-401 (2004).
- Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J., Maitland, N. J. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 65, 10946-10951 (2005).
- Domingo-Domenech, J., et al. Suppression of acquired docetaxel resistance in prostate cancer through depletion of notch- and hedgehog-dependent tumor-initiating cells. Cancer Cell. 22, 373-388 (2012).
- Patrawala, L., Calhoun-Davis, T., Schneider-Broussard, R., Tang, D. G. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 67, 6796-6805 (2007).
- Qin, J., et al. The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell. 10, 556-569 (2012).
- Siegel, R., Naishadham, D., Jemal, A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11-30 (2013).
- Patrawala, L., et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 25, 1696-1708 (2006).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone