Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Proteoliposome-Based Efflux Assay to Determine Single-molecule Properties of Cl- Channels and Transporters
W tym Artykule
Podsumowanie
Proteoliposomes are used to study purified channels and transporters reconstituted in a well-defined biochemical environment. An experimental procedure to measure efflux mediated by these proteins is illustrated. The steps to prepare proteoliposomes, perform the recordings, and analyze data to quantitatively determine the functional properties of the reconstituted protein are described.
Streszczenie
The last 15 years have been characterized by an explosion in the ability to overexpress and purify membrane proteins from prokaryotic organisms as well as from eukaryotes. This increase has been largely driven by the successful push to obtain structural information on membrane proteins. However, the ability to functionally interrogate these proteins has not advanced at the same rate and is often limited to qualitative assays of limited quantitative value, thereby limiting the mechanistic insights that they can provide. An assay to quantitatively investigate the transport activity of reconstituted Cl- channels or transporters is described. The assay is based on the measure of the efflux rate of Cl- from proteoliposomes following the addition of the K+ ionophore valinomycin to shunt the membrane potential. An ion sensitive electrode is used to follow the time-course of ion efflux from proteoliposomes reconstituted with the desired protein. The method is highly suited for mechanistic studies, as it allows for the quantitative determination of key properties of the reconstituted protein, such as its unitary transport rate, the fraction of active protein and the molecular mass of the functional unit. The assay can also be utilized to determine the effect of small molecule compounds that directly inhibit/activate the reconstituted protein, as well as to test the modulatory effects of the membrane composition or lipid-modifying reagents. Where possible, direct comparison between results obtained using this method were found to be in good agreement with those obtained using electrophysiological approaches. The technique is illustrated using CLC-ec1, a CLC-type H+/Cl- exchanger, as a model system. The efflux assay can be utilized to study any Cl- conducting channel/transporter and, with minimal changes, can be adapted to study any ion-transporting protein.
Wprowadzenie
In last two decades the ability to overexpress and purify membrane transport proteins has dramatically increased: ion channels, primary and secondary transporters are now routinely purified from heterologous expression systems as well as natural sources. New approaches to monitor expression, improve and facilitate the extraction and enhance stability of these proteins are constantly being developed 1-5. These technological breakthroughs have been instrumental in triggering the explosion of atomic-level structural information on membrane proteins which, in turn, enhanced our understanding of the structural bases of their function. In contrast, our ability to probe the functional properties of the purified proteins did not increase at the same rate, so that in some cases high resolution structural information is accompanied by qualitative functional data, thus limiting our ability to quantitatively test structure-based predictions. Hence, the development of quantitative and generalizable functional assays is a key step towards the elucidation of the mechanistic underpinnings of membrane protein function.
Here we describe an efflux assay that can be used to quantitatively determine key functional properties of purified and reconstituted Cl- channels and transporters. The principles underlying the assay can be generalized to a variety of transport systems, as well as to non ion-transporting proteins. Liposomes are reconstituted with purified Cl- channel/transporters in the presence of a large Cl- gradient (Figure 1A, B). Cl- efflux is initiated by the addition of an ionophore to allow for counter-ion flux, in our case the K+ ionophore valinomycin, which shunt the voltage established by the Cl- gradient and set the initial membrane potential to the equilibrium potential of K+6,7. Without the ionophore no significant net Cl- efflux occurs, as it is prevented by the generation of a transmembrane potential. The data is quantitatively described by two measurable parameters (Figure 1C): τ, the time constant of Cl- efflux, and f0, the fraction of liposomes not containing an active protein. From τ and f0 the unitary Cl- transport rate, the fraction of active proteins and the molecular mass of the active complex can be derived 8. The technique is illustrated here using proteoliposomes reconstituted with CLC-ec1, a well characterized CLC-type H+/Cl- exchanger of known structure and function. This assay is readily generalized to channels or transporters with different ionic selectivity or whose activity depends on the presence of voltage and/or ligands. Furthermore, this assay can be used to determine whether small molecules directly affect the reconstituted protein, to quantitate the effects of these compounds and how membrane composition or lipid-modifying reagents affect the function of the reconstituted channels and transporters.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Lipid Preparation
- Aliquot the desired amount lipids into a clear glass tube. Use E. coli polar lipid extract, but most lipid compositions can be used. If the lipids are in powder form, resuspend them in chloroform to a concentration of 20 mg/ml. Dry the lipids at RT under N2 gas until all solvent has evaporated.
- Resuspend the lipids in pentane and dry it again a steady stream of gas N2 to remove leftover traces of chloroform. Dry until all solvent has evaporated. While drying, gently rotate the tube so that the lipid is distributed in a uniform film covering the bottom third of the tube rather than a forming a dense mass at the bottom.
- Add the reconstitution buffer containing detergent (300 mM KCl, 50 mM citric acid, 25 mM K2HPO4, pH 4.5, 35 mM CHAPS) to dissolve the lipids. Use other mild detergents for this step if the protein is poorly tolerant to CHAPS.
- Resuspend the lipids to clarity using a water bath sonicator. Immerse the tube in the center of the water sonicator bath at maximum power in short (30 sec-1 min) pulses with short (30 sec-1 min) pauses. The solution should become clear.
NOTE: The final degree of clarity achieved depends on the specific lipid composition used. Some batch-to-batch variation in this step, even among nominally identical lipids, is also possible. Residual haze is due to light scattering by large particles in the suspension, which can be removed by centrifugation. - Incubate the resuspended lipids at RT for 20-60 min.
2. Proteoliposome Formation
NOTE: Several strategies can be employed to insert the detergent-solubilized protein into liposomes. For CLC-ec1 dialysis works well and is therefore the method of choice 6,9,10.
- Add the purified protein to the desired protein-to-lipid (P/L) ratio (expressed in µg of Protein/mg Lipid). The choice of P/L ratio depends on the purpose of the experiment.
- If the goal is to assess the activity of the protein of interest, reconstitute at high P/L’s to maximize the signal, as each liposome contains multiple copies of the protein. To quantify the activity and/or unitary transport rate of the protein, reconstitute in a Poisson dilution regime at low P/L’s, so that each vesicle contains on average 1 protein. See Step 7 and Discussion for a more in depth analysis of when to use each regime.
- To determine the optimal P/L values for the various regimens, perform a complete titration of the activity as a function of protein concentration 8,11-13. However, for any protein for which the molecular weight (MW, expressed in kDa) of the active unit is known the following P/L values can be used as initial guesstimates:
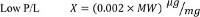

- Load the protein and lipid mixture in a pre-wetted dialysis tube or a cassette. Place the dialysis device in a beaker containing 500-1,000x the volume of the lipid reconstitution buffer (i.e., 1 L buffer for 1 ml of lipid resuspension) under gentle stirring.
- Change buffer 3-4 times at intervals >3 hr. At every solution change, wash the cassette/tubing and the beaker with distilled water and fill the beaker with fresh reconstitution buffer. Include 1-2 O/N intervals for the solution change. The exact number and duration of the solution changes depends on the exact lipid and detergents used.
- After the last step is complete, remove the liposomes from the dialysis vessel, aliquot them in tubes and flash freeze them in liquid N2 or freeze at -80 °C.
3. Recording Set-up
NOTE: The recording set-up (Figure 2A) consists of two chambers (flat bottomed cylinders, ~3-4 ml volume), a Cl- (see below) electrode, a pH meter with an analog or digital electrical output, a digitizer, and a computer with an appropriate acquisition software.
- Connect the Cl- electrode to the pH meter, the output of the pH meter to the digitizer which is connected to the computer. Place the reference electrode in one chamber and the recoding wire in the other (Figure 2B).
NOTE: The polarity of the wires is correct if ΔVCal (see Step 6.4 and 7.2) is positive. - Place the chambers on a stir plate with a stir bar in the recording chamber (Figure 1B). Make sure the stir bar does not touch the electrode.
- Connect the chambers using an agar bridge (Figure 2B) (100 mM KCl and 2% Agarose). Store the Agar bridges in 100 mM KCl.
4. Preparation of the Cl- Electrode
- Take an old and -possibly- non-functioning pH electrode. Break the glass coating to completely reveal the silver wires.
- Carefully remove the coating from the silver wires using a scalpel blade. Clean the wires using copious amounts of H2O and EtOH.
- Place in a saturated solution of FeCl3 (or bleach) until wires are covered with a uniform dark coat of AgCl.
5. Preparation of Unilamellar Vesicles
- The night before the experiments, swell 1 g of Sephadex G-50 beads in 15 ml of external buffer (1 mM KCl, 150 mM K2SO4, 25 mM citric acid, pH 4.5) with gentle shaking (do not stir) for at least 3 hr at RT before use. Each experiment requires ~3 ml of swollen beads. Keep the swollen beads at 4 °C for a few days at most.
- While the liposomes are thawing at RT, pour in each column ~3 ml of swollen G-50 beads. Let them dry by gravity flow at RT; this usually takes 1-2 min.
- Prepare unilamellar vesicles by extruding the proteoliposomes 11 times through a Mini-Extruder using a 0.4 m Teflon cutoff.
- Place the column in a plastic round-bottomed tube. Spin the columns to remove excess solution. Centrifuge for 20-30 sec at 1,400 x g in a clinical centrifuge.
- Discard flow-through, place column in a 13 x 100 mm glass tube and add 100 µl of the extruded vesicles to the column. Spin column for 1 min at 500 x g in a clinical centrifuge.
- Collect ~200 µl of flow-through that will be added to the recording chamber in step 6.5.
6. Efflux Measurement
- Place 2 ml of 100 mM KCl in the reference (ground) chamber and 1.8 ml of the external buffer (1 mM KCl, 150 mM K2SO4, 25 mM citric acid, pH 4.5) to the recording chamber. The slight osmotic imbalance between the internal and external solutions does not affect
- Start the acquisition program. Let the baseline equilibrate, this may take a few minutes.
- Once the signal reaches a stable baseline (the liposomes are ready and the columns dry), start recording (Figure 3A).
- Add 15 µl of a 10 mM KCl solution to calibrate the system (Figure 2A).
- Add liposomes. A small jump might be visible due to incomplete removal of external Cl- from the proteoliposomes (Figure 3A). Wait till the baseline stabilizes.
- Add Valinomycin (1 µl at 1 mg/ml in EtOH) to initiate efflux (Figure 3A).
- Let the efflux run its time course till it plateaus (Figure 3A).
- Add 40 µl of 1.5 M β-octylglucoside (β-OG) prepared in the external buffer to dissolve all liposomes (Figure 3A). End the recording.
7. Data Analysis
- Export the trace file from in an ascii or text format and import it to the analysis program of choice.
- Measure the Voltage at the following points (Figure 3A):
At the beginning of the recording (V0);
After addition of the calibrating pulse (Vcal);
After addition of the liposomes (Vlipo);
At the end of the efflux (Vfin);
After addition of detergent (Vtot);
Baseline the voltages so that V0=0. - Use the Nernst-Plank equation to determine the experimental value of
 by measuring
by measuring

Where VolCal is the volume of the calibration pulse in µl, 1,800 is the chamber volume at the beginning of the experiment expressed in µl, [Cl]cal/in are the Cl- concentrations of the calibration pulse and of the external buffer in mM and ΔVcal is the jump in mV measured after the calibration pulse.
NOTE: This empirical determination of α serves three purposes: it ensures that the system is responding properly, offers a measure of the consistency of the instrument’s response between experiments as well as to check that no mistakes were made in the solution making. - Calculate the Cl concentrations after each step as follows:



The factor 3/400 comes from the dilution of the 15 µl calibration pulse into the 2,000 µl final volume. - Calculate the changes in [Cl] at each step, ΔCllipo/fin/tot.
- Convert V(t) in Cl(t) with

Directly determine the [Cl] values at the critical points and compare them to the calculated values as an internal control. - Normalize the trace so that the Clrellipo=0 and Clreltot=1

- Fit the efflux time course to the following equation:

Where L is the rate of Cl- leak that is experimentally determined by measuring Cl- efflux from protein-free liposomes prepared in the same conditions and τ is the time constant of the process. - Calculate v, the initial velocity:

Where ΔClfin is expressed in millimolar and V is the volume of the chamber in μl. - Calculate the transport rate/conductance

Where p is the P/L, MW is the molecular weight of the active complex expressed in kDa
Access restricted. Please log in or start a trial to view this content.
Wyniki
We describe a detailed and robust protocol to measure Cl- transport mediated by purified CLC-ec1, a prokaryotic CLC-type H+/Cl- exchanger, reconstituted in liposomes. A schematic representation of the experiment is shown in Figure 3. Proteoliposomes reconstituted with purified CLC-ec1 and containing high internal Cl- are immersed in a bath solution containing low Cl-. Under these conditions net Cl- efflux is prevented by the buildup of pos...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
We have described a detailed protocol to measure Cl- transport mediated by purified anion-selective channels or transporters reconstituted in liposomes. The example used was the prokaryotic H+/Cl- exchanger CLC-ec1. However, the methodology can be readily adapted to study channels gated by ligands 12,13,15, voltage 11,12, or sporting different anionic selectivity 15,16 by replacing the Ag:AgCl electrode with one suitable for the ion under consideration. El...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors declare no competing financial interests.
Podziękowania
This work was supported by NIH grant GM085232 and an Irma T. Hirschl/ Monique Weill-Caulier Scholar Award (to A.A.).
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Liposomicator, Avanti Polar Lipids Inc. | Avanti Polar Lipids Inc. | 610200 | |
| IEC Centra CL2 Benchtop | Thermo Scientific | ||
| Orion Research Model 701A Digital pH-mV meter | These can be found on Ebay. | ||
| Non-functional pH probe | Any pH meter probe with silver wires will work. The glass/plastic coating needs to be removed and the wires cleaned. | ||
| DI-710 Data Logger | DATAQ instruments | ||
| WinDAQ acquisition software | DATAQ instruments | ||
| Pierce Disposable Plastic Columns, Gravity-flow, 2ml | Pierce (Thermo Scientific) | 29922 | |
| KIMAX Culture Tubes, Disposable, Borosilicate Glass | Kimble Chase | 73500-13100 | |
| Extruder Set With Holder/Heating Block | Avanti Polar Lipids Inc. | 610000 | |
| Computer |
Odniesienia
- Kawate, T., Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure. 14, 673-681 (2006).
- Drew, D., et al. GFP-based optimization scheme for the overexpression and purification of eukaryotic membrane proteins in Saccharomyces cerevisiae. Nat Protocols. 3, 784-798 (2008).
- Hattori, M., Hibbs, R. E., Gouaux, E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure. 20, 1293-1299 (2012).
- Almo, S. C., Love, J. D. Better and faster: improvements and optimization for mammalian recombinant protein production. Curr Opin Struct Biol. 26, 39-43 (2014).
- Xiao, S., Shiloach, J., Betenbaugh, M. J. Engineering cells to improve protein expression. Curr Opin Struct Biol. 26, 32-38 (2014).
- Accardi, A., Miller, C. Secondary active transport mediated by a prokaryotic homologue of CLC Cl- channels. Nature. 427, 803-807 (2004).
- Nguitragool, W., Miller, C. Uncoupling of a CLC Cl-/H+ exchange transporter by polyatomic anions. J. Mol. Biol. 362, 682-690 (2006).
- Walden, M., et al. Uncoupling and turnover in a Cl-/H+ exchange transporter. J. Gen. Physiol. 129, 317-329 (2007).
- Accardi, A., Kolmakova-Partensky, L., Williams, C., Miller, C. Ionic currents mediated by a prokaryotic homologue of CLC Cl- channels. J. Gen. Physiol. 123, 109-119 (2004).
- Basilio, D., Noack, K., Picollo, A., Accardi, A. Conformational changes required for H(+)/Cl(-) exchange mediated by a CLC transporter. Nat Struct Mol Biol. 21, 456-463 (2014).
- Lee, S. Y., Letts, J. A., MacKinnon, R. Functional reconstitution of purified human Hv1 H+ channels. Journal of Molecular Biology. 387, 1055-1060 (2009).
- Terashima, H., Picollo, A., Accardi, A. Purified TMEM16A is sufficient to form Ca2+ activated Cl- channels. Proc Natl Acad Sci U S A. 110, 19354-19359 (2013).
- Malvezzi, M., et al. Ca2+-dependent phospholipid scrambling by a reconstituted TMEM16 ion channel. Nature Communications. 4, 2367(2013).
- Picollo, A., Malvezzi, M., Houtman, J. C., Accardi, A. Basis of substrate binding and conservation of selectivity in the CLC family of channels and transporters. Nat Struct Mol Biol. 16, 1294-1301 (2009).
- Eckford, P. D., Li, C., Ramjeesingh, M., Bear, C. E. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem. 287, 36639-36649 (2012).
- Stockbridge, R. B., et al. Fluoride resistance and transport by riboswitch-controlled CLC antiporters. Proc Natl Acad Sci U S A. 109, 15289-15294 (2012).
- Menon, I., et al. Opsin is a phospholipid flippase. Curr Biol. 21, 149-153 (2011).
- Nimigean, C. M., Miller, C. Na+ block and permeation in a K+ channel of known structure. J Gen Physiol. 120, 323-335 (2002).
- Picollo, A., Xu, Y., Johner, N., Bernèche, S., Accardi, A. Synergistic substrate binding determines the stoichiometry of transport of a prokaryotic H(+)/Cl(-) exchanger. Nat Struct Mol Biol. 19, 525-531 (2012).
- Tsai, M. F., Fang, Y., Miller, C. Sided functions of an arginine-agmatine antiporter oriented in liposomes. Biochemistry. 51, 1577-1585 (2012).
- Heginbotham, L., Kolmakova-Partensky, L., Miller, C. Functional reconstitution of a prokaryotic K+ channel. J Gen Physiol. 111, 741-749 (1998).
- Lundbaek, J. A., Collingwood, S. A., Ingólfsson, H. I., Kapoor, R., Andersen, O. S. Lipid bilayer regulation of membrane protein function: gramicidin channels as molecular force probes. J R Soc Interface. 7, 373-395 (2010).
- Lim, H. H., Stockbridge, R. B., Miller, C. Fluoride-dependent interruption of the transport cycle of a CLC Cl(-)/H(+) antiporter. Nat Chem Biol. 9, 712-715 (2013).
- Stockbridge, R. B., Robertson, J. L., Kolmakova-Partensky, L., Miller, C. A family of fluoride-specific ion channels with dual-topology architecture. Elife. 2, e01084(2013).
- Nimigean, C., Shane, T., Miller, C. A cyclic nucleotide modulated prokaryotic K+ channel. J Gen Physiol. 124, 7(2004).
- Brohawn, S. G., del Mármol,, J,, MacKinnon, R. Crystal Structure of the Human K2P TRAAK, a Lipid- and Mechano-Sensitive K+ Ion Channel. Science. 335, 436-441 (2012).
- Jayaram, H., Robertson, J. L., Wu, F., Williams, C., Miller, C. Structure of a slow CLC Cl-/H+ antiporter from a cyanobacterium. Biochemistry. 50, 788-794 (2011).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone