Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Reliable Method for Assessing Seed Germination, Dormancy, and Mortality under Field Conditions
W tym Artykule
Podsumowanie
Here we present a protocol for assessing seed survivorship, germination and dormancy under field conditions using buried, labeled seed strips and tetrazolium chloride (TZ) viability testing.
Streszczenie
We describe techniques for approximating seed bank dynamics over time using Helianthus annuus as an example study species. Strips of permeable polyester fabric and glue can be folded and glued to construct a strip of compartments that house seeds and identifying information, while allowing contact with soil leachate, water, microorganisms, and ambient temperature. Strips may be constructed with a wide range of compartment numbers and sizes and allow the researcher to house a variety of genotypes within a single species, different species, or seeds that have experienced different treatments. As opposed to individual seed packets, strips are more easily retrieved as a unit. While replicate packets can be included within a strip, different strips can act as blocks or can be retrieved at different times for observation of seed behavior over time. We used a high temperature glue gun to delineate compartments and sealed the strips once the seed and tags identifying block and removal times were inserted. The seed strips were then buried in the field at the desired depth, with the location marked for later removal. Burrowing animal predators were effectively excluded by use of a covering of metal mesh hardware cloth on the soil surface. After the selected time interval for burial, strips were dug up and seeds were assessed for germination, dormancy and mortality. While clearly dead seeds can often be distinguished from ungerminated living ones by eye, dormant seeds were conclusively identified using a standard Tetrazolium chloride colorimetric test for seed viability.
Wprowadzenie
The overall goal of this method is to reliably assess seed survival over time under field conditions.
Soil seed banks are a reserve of dispersed, viable yet ungerminated seeds distributed either on the soil surface, within surface litter, or within the soil profile, which may persist transiently or for many years1,2. When seed burial methods similar to those presented here were applied to a 17-year study using several dozen species, viable seeds were found in many of the species tested3. Seed dormancy is a block to seed germination until the appropriate combination of conditions for seedling survival arise4. Remaining dormant can allow seeds to survive harsh conditions, such as low winter temperatures, nutrient limitation, or seasonal drought, until an external trigger for dormancy-release allows for germination. Triggers for dormancy-release can vary from exposure to extended cold, compounds left by fire, or physical attack on the seed coat through abrasion or contact with animal stomach acids5. As germination cues can be genera or species specific and often result from past natural selection, maladaptive seed germination is that which occurs at an inappropriate time, and may result in seed or seedling mortality or poor seedling growth. While dormancy has been classified into a number of types based on the mechanisms of dormancy release (e.g., physical dormancy, physiological dormancy),6 seed dormancy remains one of the least understood topics in plant biology. Thus, field studies that allow for assessment of the status of individual seeds or groups of seeds under relevant ecological conditions have higher explanatory power than those that simply rely on standard germination tests in the laboratory.
Exploitation of known seed characteristics can provide insight into the mechanisms of dormancy. Control of seed dormancy is complex, including genetic control of physiological and morphological factors. While a full understanding of the breadth of dormancy mechanisms has yet to be elucidated, a general model has emerged, involving a feedback relationship between the two plant hormones Gibberellic Acid (GA) and Abscisic Acid (ABA)7. In this generalized model for seeds with a physiological component to their dormancy, GA serves as signal for dormancy release, while ABA serves to maintain the dormant state. Maternal genetic effects as well as the maternal growth environment can influence dormancy and other seed traits, such as size, through maternally generated tissues and developmental signals8. Maternally generated external structures (or seed coverings) may maintain dormancy, at times in combination with physiological cues. Since maternally derived seed coverings are controlled by the mother plant's genes, they may not reflect the seed's actual nuclear genetic make-up. We have used the Helianthus annuus achenes from an array of crop-wild hybrid crosses to tease out these maternal vs. embryo genetic effects on seed characteristics9,10. Thus, study designs that include diverse species, cross types, or genotypes can glean information about the ecology and genetics of seed dormancy, germination and survival.
An important example of how seed germination and survival phenotypes can affect population dynamics can be seen in crop-wild hybrid zones. Selection during domestication of cultivated plants eliminates most dormancy and reduces a seed's ability to survive outside of the growing season. Yet gene flow, or hybridization, between the cultivated and wild types in crop-wild hybrid zones can reintroduce crop alleles (or genetic variants) into a wild population, with potential effects on seed bank dynamics. Hybrids between cultivated and wild relatives potentially found in crop-wild hybrid zones may possess a variety of intermediate dormancy phenotypes, with only a few phenotypes expected to survive conditions outside of cultivation (e.g., winter months)11.
The aim of this manuscript is to show how, using the seed burial strip method, we can evaluate germination, dormancy, and survival of a range of seed types at different time periods to investigate their natural variation under field conditions. In our example, we employed sunflower seeds from 15 crop-wild hybrid cross types since we are interested in maternal and embryo genetic effects on seed characteristics.
Protokół
1. Gather Seed from Multiple Species or Controlled Crosses of a Single Species
Note: This example used seed from 15 cross types within the species Helianthus annuus (sunflower) using wild, hybrid, and crop types as the maternal (seed producing) parent.
- At the end of the growing season, collect mature seed heads in labeled bags. Clean seed from chaff and place seed in envelopes labeled with parental cross type in standard format (i.e., maternal x paternal).
- Bulk seed together in large envelopes using equal amounts of seed from an equal number of identical parental cross types. For example, 100 seeds from 10 cross types with the same maternal and paternal parents. Label the envelopes with identifying (cross type) information. These large envelopes are seed masters for use in filling burial strips in Section 2.
| Maternal Parent | |||
| Paternal Parent | Wild: WxW | F1: WxC | Crop: CxC |
| Wild: WxW | 0% WxW | 25% F1xW † | 50% CxW † |
| BC: WxF1 or F1xW | 12.5% WxBC | 37.5% F1xBC | 62.5% CxBC |
| F1: WxC | 25% WxF1 † | 50% F1xF1 | 75% CxF1 † |
| F2: F1xF1 | 25% WxF2 | 50% F1xF2 | 75% CxF2 |
| Crop: CxC | 50% WxC † | 75% F1xC † | 100% CxC |
Table 1. Parental cross types produced from hand pollination. Sunflower crop-wild hybrid cross types were produced with hand-pollination for use in seed burial experiment. For all cross types, the maternal parent listed first and the paternal parent listed second. Cross types marked with † are part of reciprocal cross type pairs with the same % crop alleles but different maternal parents. Table has been previously published in: Pace, B. A. et al. (2015)15.
2. Create Custom Seed Burial Strips
NOTE: For this example, we had three removal date treatments and 15 replicates, so we required 45 strips total. This example uses 15 compartments per strip so, we required enough fine-meshed polyester fabric or mosquito netting to house 15, 7 x 10 cm compartments. See Figure 1.
- Start by cutting a 20 x 105 cm strip of fabric for each seed strip.
- Measure and mark compartments, then fold the fabric in half. Using a high-temperature glue gun, seal one short (10 cm) end. Once the glue cools slightly, but not completely, press the fabric sides together to create a seal.
CAUTION: High-temperature glue and glue gun tips can cause burns if contact with skin is prolonged. - Glue lines from the folded edge to the open short end at each compartment mark. Press the two fabric sides together along the glue lines to ensure seals without holes between compartments. Seeds migrating between compartments contribute to experimental error.
NOTE: Now that the compartments have been created, the unfolded edge should remain open and ready to receive seed and their identifying information (cross type name). - Randomly assign seed cross types to compartments for each strip.
- Fill seed compartments with a uniform number of seeds (20 for each compartment are used in this example). For labels, use industrial permanent markers on cut-to-fit plastic garden darts so that they are able to withstand burial.
NOTE: Alternative labeling methods may be used. - After each compartment is filled with seed and its label, seal the compartment immediately with the high-temperature glue gun. Proceed until the entire seed strip is completely sealed. Label overall strip in the first compartment with replicate, block and/or treatment information.
NOTE: For our study, since we had temporal removals, blocks, and replicate strips within blocks, we included all of this information. - Check for gaps between compartments and spot glue as needed before moving on to the next strip.
- Fill seed compartments with a uniform number of seeds (20 for each compartment are used in this example). For labels, use industrial permanent markers on cut-to-fit plastic garden darts so that they are able to withstand burial.
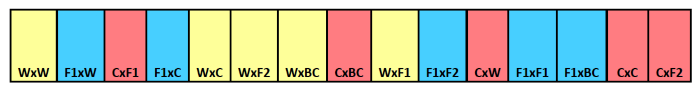
Figure 1. Burial seed strip schematic. Example of burial strip showing cross type identification for individual compartments. Maternal parent is listed first with the paternal parent listed second. Colors indicate different maternal parentage, with yellow for wild, blue for F1 hybrid, and red for crop. Please click here to view a larger version of this figure.
3. Bury Seed Strips in the Field
- Dig plots to the specified depth to house strips. Assign strips to individual blocks in which strips are randomly arranged to allow for removal date to be used as the main plot and compartment identity (in this example, cross type) as the subplot in analysis.
- Cover strips with earth. Cut metal mesh hardware cloth to be 10 cm larger than plots and place over buried strips. Use U-pins to secure edges and cover cloth edges with earth.
NOTE: This covering is for excluding burrowing seed predators. As another option, Mercer et al.13 constructed and buried boxes of hardware cloth that surrounded the seed strips due to intense gopher pressure in their field location.) - Place colored surveyor's flags to distinguish strip location and removal dates to complement clear written records.
4. Excavate Strips and Evaluate Seed
- Dig up seed strips assigned to a given removal date. Maintain the strips in a cool moist environment until they reach the laboratory where seed evaluation will take place. This can be accomplished by wrapping the strips in wet newspaper and placing them in a cooler for transport or overnight shipping.
- Rinse mud and soil from strips in water until strips are clean enough that external debris will not obscure seeds.
- Use scissors to cut open one compartment at a time. Count germinated seeds and remove them. Count clearly dead seeds (e.g., rotting, or putrefied seeds). Place ungerminated seeds on labeled, wet Petri-sized blotter paper to further distinguish ungerminated, dormant, and dead seeds.
- Place Petri dishes with ungerminated seeds into a growth chamber set for standard germination conditions: 25 °C /10 °C 12 hr day/night. Allow seeds one week to germinate. Any that do, count these as viable, but ungerminated since they did not germinate under field conditions.
- After the growth chamber treatment, test remaining ungerminated seeds for viability using Tetrazolium chloride (TZ)13. To accomplish this, make up a percent solution of TZ appropriate for the study species using the AOSA Tetrazolium Testing Handbook14. For this example, a 1% solution is used.
- Cut the ungerminated seeds in half and place one half were the embryo and endosperm are visible in a Petri dish containing enough TZ solution to cover the seed (seeds may float, this is okay).
- Place TZ Petri dishes into an incubator set at 30 °C and wait 3 hr. Note: Higher temperatures will result in a faster reaction, if desired, but overstaining is a risk.
- Remove Petri dishes from the incubator and evaluate. Seeds that have stained red in their embryos are germinable, while unstained seeds are dead. Consult the AOSA Tetrazolium Testing Handbook for more detail on distinguishing marginal cases and particular species.
Wyniki
Cross types with varied maternal parentage and crop allele percentage (Table 1) differed across removal dates in percent germinated, ungerminated, and dead seed (Fig. 2 and 3). Using TZ testing of ungerminated seeds, we found some truly dormant seeds at the second removal (early spring) (Table 2), while all seeds ungerminated by the third removal (spring) were found to be truly dormant.
Dyskusje
Here we present methods for using seed burial strips to observe seed germination, dormancy and mortality of diverse seed stocks at preselected time periods in the field. The advantages of using strips rather than individual packets lie in (1) the speed of strip and compartment construction over creation of individual packets; and (2) the ease and speed of removing multiple compartments in one motion without the danger of omitting a packet or removing one unintentionally. As two of the removal dates in the example present...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by Biotech Risk Assessment Grant Program competitive grand no. 2006-39454-17438 to A. Snow, K. Mercer, and H. Alexander from the United States Department of Agriculture, National Institute of Food and Agriculture. Experiments using this method were conducted at and supported by the University of Kansas Field Station, a research unit of the Kansas Biological Survey and the University of Kansas. The authors would like to thank P. Jourdan and E. Regnier for helpful reviews on earlier versions of this manuscript. Additionally, this work was aided by the contributions of the staff at the University of Kansas Field Station, Waterman Farm at the Ohio State University (OSU), the USDA Ornamental Plant Germplasm Center at OSU, and the Seed Biology Lab in the Department of Horticulture and Crop Science at OSU, especially E. Renze, S. Stieve, A. Evans, and E. Grassbaugh, for technical support.
Materiały
| Name | Company | Catalog Number | Comments |
| Small coin envelopes | Any | ||
| Large coin envelopes | Any | ||
| fine meshed polyester mosquito netting | Any | ||
| high-temperature glue gun | Any | ||
| high-temperature glue stick refills | Any | ||
| Industrial permenant markers | Any | ||
| plastic garden labels | Any | ||
| scissors | Any | ||
| Shovel | Any | ||
| Metal mesh hardward cloth | Any | ||
| Surveyor's flags, multiple colors | Any | ||
| Wet newspaper | Any | ||
| cooler | Any | ||
| blotter paper | Any | ||
| petri dishes | Any | ||
| Temp. controlled growth chamber | Any | ||
| razor blades | Any | ||
| Petri dishes | Any | ||
| Tetrazolium chloride | Any | ||
| water | Any | ||
| heat incubator | Any |
Odniesienia
- Walck, J. L., Baskin, J. M., Baskin, C. C., Hidayati, S. N. Defining transient and persistent seed banks in species with pronounced seasonal dormancy and germination patterns. Seed Sci Res. 15 (3), 189-196 (2005).
- Alexander, H. A., Schrag, A. M. Role of soil seed banks and newly dispersed seeds in population dynamics of the annual sunflower. Helianthus annuus. J Ecol. 91, 987-998 (2003).
- Burnside, O. C., Wilson, R. G., Weiseberg, S., Hubbard, K. Seed longevity of 41 weed species buried 17 years in eastern and western. Weed Sci. 44 (1), 74-86 (1996).
- Baskin, C. C., Baskin, J. M. . Seeds: Ecology, biogeography, and evolution of dormancy and Germination. , (2001).
- Finch-Savage, W. E., Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 171, 501-523 (2006).
- Baskin, J. M., Baskin, C. C. A classification system for seed dormancy. Seed Sci Res. 14 (1), 1-16 (2004).
- Donohue, K., et al. Environmental and genetic influences on the germination of Arabidopsis thaliana in the field. Evolution. 54 (4), 740-757 (2005).
- Roach, D. A., Wulff, R. D. Maternal effects in plants. Ann Rev Ecol Syst. 18, 209-235 (1987).
- Pace, B. A., Alexander, H. M., Emry, J. D., Mercer, K. L. Seed fates in crop-wild hybrid sunflower: crop allele and maternal effects. Evol Appl. 8 (2), 121-132 (2015).
- Alexander, H. M., Emry, D. J., Pace, B. A., Kost, M. A., Sparks, K. A., Mercer, K. L. Roles of maternal effects and nuclear genetic composition change across the life cycle of crop-wild hybrids. Am J Bot. 101 (7), 1176-1188 (2014).
- Stewart, N. C., Matthew, J., Halfhill, D., Warwick, S. I. Transgene introgression from genetically modified crops into their wild relatives. Genetics. 4, 806-817 (2003).
- Mercer, K. L., Shaw, R. G., Wyse, D. L. Increased germination of diverse crop-wild hybrid sunflower seeds. Ecol Appl. 16, 845-854 (2006).
- Delouche, J. C., Still, T. W., Rapset, M., Lienhard, M. The tetrazolium test for seed viability. Mississippi Sta Uni Ag Exp Sta Techn Bull. 51, 1-63 (1962).
- Association of Official Seed Analysts. . Tetrazolium Testing Handbook. , (2010).
- Alexander, H. M., Emry, D. J., Pace, B. A., Kost, M. A., Sparks, K. A., Mercer, K. L. Roles of maternal effects and nuclear genetic composition change across the life cycle of crop-wild hybrids. Am J Bot. 10 (7), 1176-1188 (2014).
- Weiss, A. N., Primer, S. B., Pace, B. A., Mercer, K. L. Maternal effects and embryo genetics: germination and dormancy of crop-wild sunflower hybrids. Seed Sci Res. 23, 241-255 (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone