Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
The Mouse Isolated Perfused Kidney Technique
W tym Artykule
Podsumowanie
The mouse isolated perfused kidney (MIPK) is a technique for keeping a mouse kidney under ex vivo conditions perfused and functional for 1 hr. The buffers and surgical technique are described in detail.
Streszczenie
The mouse isolated perfused kidney (MIPK) is a technique for keeping a mouse kidney under ex vivo conditions perfused and functional for 1 hr. This is a prerequisite for studying the physiology of the isolated organ and for many innovative applications that may be possible in the future, including perfusion decellularization for kidney bioengineering or the administration of anti-rejection or genome-editing drugs in high doses to prime the kidney for transplantation. During the time of the perfusion, the kidney can be manipulated, renal function can be assessed, and various pharmaceuticals administered. After the procedure, the kidney can be transplanted or processed for molecular biology, biochemical analysis, or microscopy.
This paper describes the perfusate and the surgical technique needed for the ex vivo perfusion of mouse kidneys. Details of the perfusion apparatus are given and data are presented showing the viability of the kidney's preparation: renal blood flow, vascular resistance, and urine data as functional, transmission electron micrographs of different nephron segments as morphological readouts, and western blots of transport proteins of different nephron segments as molecular readout.
Wprowadzenie
The isolated perfusion of organs has been the subject of an ongoing effort among physiologists for many decades1. The technique enables the function of the organ, without systemic influences such as blood pressure, hormones, or nerves, to be studied. Carl Eduard Loebell is considered to be the first to have described the successful perfusion of an isolated kidney, in 18492. Since then, the perfusion apparatus has undergone significant refinement. Frey and Gruber introduced an artificial lung for oxygenation and pulsatile pumps for continuous perfusion2. While early researchers mainly studied the kidneys of large mammals-namely, pigs2 and dogs3-the first report of the use of rat kidneys, by Weiss et al., was a milestone in the study of small-mammal-organ perfusion4. Schurek et al. reported the necessity of adding mammalian erythrocytes to the perfusate if sufficient renal tubular oxygenation was to be achieved5. Critical for long-term experiments was the introduction of continuous dialysis of the buffer by the same research group6. In 2003, Schweda et al. were the first to report a functional mouse isolated perfused kidney (MIPK)7, later refined by Rahgozar et al.18 and Lindell et al.14.
While technically more challenging than the rat isolated perfused kidney, the use of the MIPK bears the advantage of enabling the use of a wide array of genetically altered mice. This paper presents the details of the authors' method for perfusing isolated mouse kidneys for 1 hr. The method allows for the continuous assessment of renal flow rate, vascular resistance, hormone release, blood gas analysis, urine analysis, and the application of drugs. Following the procedure, kidneys could be processed for molecular and biochemical analysis, be fixed for microscopy, or transplanted into a recipient mouse (Figure 1).

Figure 1: Overview of Possible Input/Output to the Isolated Perfused Kidney. BGA: Blood gas analysis. Please click here to view a larger version of this figure.
This technique likely will receive increasing attention over coming years, as many innovative applications are being discussed with the dawn of prolonged normothermic kidney perfusion prior to transplantation (with or without the application of anti-rejection or genome-editing drugs)8,9, 10, 11, the bioengineering of whole kidneys from decellularized scaffolds12, and the application of high doses of fluorescent dyes for multiphoton imaging13. It is also an ideal model with which to study the role of specific genes during acute kidney injury14.
A step-by-step protocol is given to allow other laboratories to perform isolated mouse kidney perfusion successfully. First, the composition and preparation of the buffer is specified. Then, the surgery is described in detail and the critical steps are shown. Third, data is presented that are representative of a successful preparation: renal blood flow, vascular resistance, glomerular filtration rate , and fractional electrolyte excretion-all as functional measurements of viability-and transmission electron micrographs of the morphology of different nephron segments of perfused kidneys fixed after 1 hr of perfusion.
Protokół
All procedures involving animals described in this manuscript were conducted according to Swiss law and approved by the veterinary administration of the Canton of Zurich, Switzerland.
1. Buffer Preparation
- Prepare solutions 1 - 4 and the antidiuretic hormone (ADH) solution (Table 1).
- Prepare the dialysis buffer (Table 1).
NOTE: This is the buffer used as the dialysis buffer during the perfusion. Later, the erythrocytes will be diluted in this buffer to form the final perfusate. - Erythrocyte preparation.
- Dilute 250 ml of human erythrocyte concentrate (tested material obtained from the local blood bank) to 500 ml with dialysis buffer. Centrifuge at 2,000 x g for 8 min. Remove the buffer, being careful not to remove any erythrocytes. Repeat 3x.
- Prepare the albumin (bovine serum albumin; BSA) buffer.
- In 200 ml of dialysis buffer, dissolve 44 g of BSA using a stir bar. Filter the solution with filter paper.
- Prepare the perfusate.
- Filter the erythrocytes from step 1.3.1 through filter paper into the BSA buffer. Fill up to a total volume of 800 ml with dialysis buffer.
NOTE: This is the final perfusate. The hematocrit should now be between 8 and 12%. The perfusate can be stored for up to 12 hr at 4 °C.
- Filter the erythrocytes from step 1.3.1 through filter paper into the BSA buffer. Fill up to a total volume of 800 ml with dialysis buffer.
2. Initiating Dialysis and Oxygenation
- Turn on the water bath surrounding the larger buffer reservoir, smaller buffer reservoir, and the moist chamber (a small, double-walled chamber brought to 37 °C and 100% humidity to later hold the kidney) to 37 °C (Figure 2).
- Fill the larger buffer reservoir with the dialysis buffer and the smaller reservoir with the perfusate.
- Turn on the 5% CO2/95% O2 gas inflow to the dialysis buffer.
- Switch on continuous dialysis of the perfusate against the dialysis buffer. Take care to use low-flux dialysis tubing. Proceed to Step 3.

Figure 2: Schematic Drawing of the Perfusion Circuit. Scheme shows the main components of the perfusion circuit and the direction of buffer flow. All components surrounded by dark blue are kept at 37 °C with a water bath/thermostat. 1: Dialysis buffer of at least 3 times the volume of the perfusion buffer is continuously bubbled with 95% O2/5% CO2. 2: Dialysis buffer and perfusion buffer are continuously dialyzed against each other in a dialysis tube by a roller pump. 3: Due to this dialysis, the perfusion buffer is enriched with 9% O2/5% CO2 and electrolyte levels are kept constant throughout perfusion. 4: A roller pump propels the perfusion buffer toward the kidney. 5: A windkessel removes peristaltic waves and acts as a bubble trap. 6: Pressure transducer (connected to 4. (roller pump) to keep continuous pressure while allowing freely alternating flow). 7: Throughout perfusion, the kidney remains in a moist chamber for 100% air humidity and 37 °C kidney temperature. Please click here to view a larger version of this figure.
3. Surgical Procedure Part 1 (for a diagram of all ligatures, see Figure 3)
Note: Perform all ligatures using 5-0 surgical thread.
- Anesthetize a mouse by intraperitoneal injection (10 µl/g of body weight, 20 mg/ml ketamine and 1 mg/ml xylazine dissolved in 0.9% NaCl). Confirm sufficient depth of anesthesia by testing for absence of rear-foot reflexes.
- Fix the mouse in a supine position in the moist chamber. Protect the eyes with vet ointment. Place a 1 ml syringe below the spine to elevate the lumbar vessels.
- Perform a median laparotomy from the pubic crest to the sternum opening first the skin, then the abdominal muscles, with scissors.
- Remove the intestine and place it on the left side of the mouse lateral from the abdomen.
- Free the bladder from connective tissue and explore both ureters and the urethra.
- Place a ligature around the left ureter (ligature I). Close it.
- Place a ligature around the urethra (ligature II). Close it.
- Place a "lasso" ligature around the whole bladder (ligature III).
- Incise the bladder 1 mm.
- Cannulate the opening with 2 cm PE 50 tubing.
- Close ligature III around the tubing.
- Cut the left ureter and urethra distal from the ligatures. The bladder is now attached to the right ureter only and freely moving.
- Clear the abdominal aorta of connective tissue and fat.
- Place an abdominal mid-aorta ligature (ligature IV).
- Place a ligature around the aorta below the diaphragm between the superior mesenteric artery and the coeliac trunk (ligature V).
- Place a ligature around the superior mesenteric artery (ligature VI).
- Place an aortic ligature directly below the right and above the left renal artery (ligature VII).
- Place a ligature around the caudal vein package (cava) (ligature VIII). Proceed to Step 4.
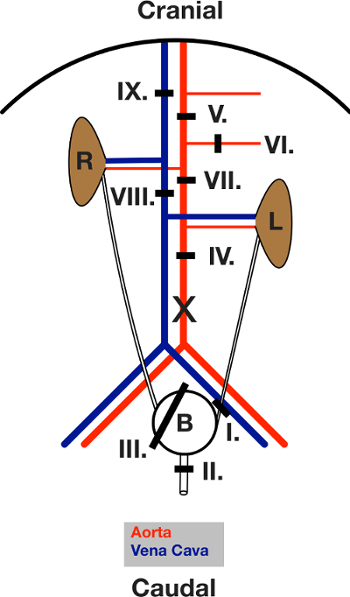
Figure 3: Schematic Drawing of the Ligatures placed during Surgery. View of the open abdomen after the laparotomy. The intestine is moved out to the left. L and R indicate the left and right kidney. The black lines show the area of the respective ligature. Ligatures are first placed and then closed, in the sequence given in the text. X marks the location of the incision for aorta cannulation. Please click here to view a larger version of this figure.
4. Priming of the Perfusion Circuit
- Start the rotary pump and fill the tubing with perfusate. Take care to empty all air bubbles from it.
- Fill the windkessel device to approximately mid-level with perfusate.
- Calibrate the pressure transducer to 0 mm Hg when all tubing is filled and flow is 0. Keep the perfusion needle at kidney level during this time.
- Keep flow at a constant minimal level (0.6 ml/min) and proceed to Step 5.
5. Surgical Procedure Part 2
- Place a clamp between ligature IV and the branching of the left renal artery.
- Make a small incision in the aorta caudal of ligature IV, taking care to not cut the dorsal wall.
- Dilate the opening in the aorta with a vessel dilator.
- Cannulate the aorta with a needle (2 cm long, pulled PE 50), pushing the tip just to the clamp.
- Open the clamp.
- Push the tip of the needle cranially until it reaches the junction of the right kidney artery and the aorta.
- Close ligature VII.
- Close ligature IV.
- Open the chest with scissors by dissecting the diaphragm. With a single cut, separate the aorta, vena cava, heart and vegetative nerves. With this step, the animal is sacrificed via rapid exsanguination under continuous deep anesthesia.
- Start pressure control of the perfusion pump. Maintain the mean pressure between 80 and 100 mmHg.
- Close ligature V.
- Close ligature VI.
- Close ligature VIII.
- Free the right kidney from connective tissue and its embedding into the adipose capsule with scissors.
- Cut the aorta proximally to ligature V.
- Cut the superior mesenteric artery distally to ligature VI.
- Cut the kidney-supporting vessel bundle out, taking care to not cut into the vessels themselves.
- Cut the liver at the connection to the kidney. Take care to free the kidney, but leave a small part of the liver adherent to it, so that the vena cava is kept open by it.
- Take the kidney bundle out of the mouse. Remove the mouse from the moist chamber.
- Place a "lasso" ligature around the connection of liver and kidney (ligature IX).
- Cannulate the vena cava with a venous line (2 cm PE 50).
- Close ligature IX. Venous outflow through the venous line should immediately start.
- Close the moist chamber.
6. Downstream Analyses
- During the following hour, continuously monitor blood flow and intravascular pressure15. Collect venous outflow, which can be used for analysis of, for example, renal renin release7. Collect urine for analysis of electrolyte concentration and glomerular filtration rate 14. After 1 hr of perfusion, kidneys can be snap-frozen for western blotting or be fixed for imaging approaches16.
Wyniki
With the method described, isolated mouse kidneys can remain viable for at least 1 hr. We tested the tissue viability after 1 hr of continuous perfusion with functional (renal blood flow and vascular resistance, blood gas analysis of venous outflow, glomerular filtration rate, urinary fractional Na+ and K+ excretion, and urine osmolality) and morphological (transmission electron microscopy, TEM) methods in four kidneys of wildtype C57Bl/6 mice. Additionally, western ...
Dyskusje
The mouse isolated perfused kidney is a tool for studying kidney function in a controlled environment ex vivo for 1 hr, bridging the gap between in vivo experiments in intact animals, which may be flawed by the impact of numerous systemic factors, and in vitro experiments in isolated nephron segments or cultured cells, which necessarily neglect the impact of intact organ structure on function. There is, to the authors' knowledge, no alternative technique with which to perform this specific ...
Ujawnienia
The authors have no competing financial interests and nothing else to disclose.
Podziękowania
The authors would like to thank Hans-Joachim Schurek for invaluable scientific advice. The authors would like to thank Monique Carrel and Michèle Heidemeyer for excellent technical assistance, David Penton Ribas and Nourdine Faresse for a critical reading of the manuscript and Carsten Wagner and Jürg Biber for the NaPi-2a antibody. This work was supported by the Swiss National Centre for Competence in Research "Kidney.CH" and by a project grant (310030_143929/1) from the Swiss National Science Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Perfusion Circuit: | |||
| Moist chamber 834/8 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2901 | |
| Cannular with basket and side port | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2947 | |
| Thermostat TC120-ST5 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-4544 | |
| ISM 827/230V Roller Pump Reglo Analogue | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-0114 | |
| Reservoir jacketed for buffer solution 1 L | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3438 | |
| Reservoir jacketed for buffer solution 0.5 L | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3436 | |
| Pressure Transducer APT300 | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3862 | |
| TAM-D Plugsys Transducer | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-1793 | |
| SCP Plugsys servo controller | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-2806 | |
| Windkessel | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3717 | |
| HSE-USB data acquisition | Harvard Apparatus/Hugo Sachs Elektronik GmbH | 73-3330 | |
| Low-Flux Dialysator Diacap Polysulfone | B.Braun | 7203525 | |
| PE-Tubing for aorta cannulation 1.19 mm I.D. x 1.70 mm O.D. | Scientific Commodities Inc. | BB31695-PE/8 | |
| Name | Company | Catalog Number | Comments |
| Buffer reagents: | |||
| Aminoplasmal 10% | B.Braun | 134518064 | |
| Sodium pyruvate | Sigma-Aldrich | P2256-25G | |
| L-Glutamic acid monosodium salt hydrate | Sigma-Aldrich | G1626-100G | |
| L-(-)-Malic acid sodium salt | Sigma-Aldrich | M1125-25G | |
| Sodium-L-Lactate | Sigma-Aldrich | L7022-10G | |
| alpha-Ketoglutaric acid sodium salt | Sigma-Aldrich | K1875-25G | |
| NaCl | Sigma-Aldrich | 31434-1KG-R | |
| NaHCO3 | Sigma-Aldrich | S5761-5KG | |
| KCl | Sigma-Aldrich | 60130-1KG | |
| Urea | Sigma-Aldrich | U5378-500G | |
| Creatinine | Sigma-Aldrich | C4255-10G | |
| Ampicillin | Roche | 10835242001 | |
| MgCl2 * 6H2O | Sigma-Aldrich | M2393-500G | |
| D-Glucose | Sigma-Aldrich | G8270-1KG | |
| CaCl2 * 6H2O | Riedel-de-Haën | 12074 | |
| NaH2PO4 | Sigma-Aldrich | S9638-500G | |
| Na2HPO4 | Sigma-Aldrich | S0876-500G | |
| Antidiuretic Hormone dDAVP | Sigma-Aldrich | V2013-1MG | |
| FITC-Inulin | Sigma-Aldrich | ||
| Filter used for erythrocyte filtration | Macherey-Nagel | MN 615 | |
| BGA Analysis: | |||
| ABL 80 flex | Radiometer Medical ApS | ||
| Electron Microscope: | |||
| Philips CM100 TEM | FEI |
Odniesienia
- Carrel, A., Lindbergh, C. A. The culture of whole organs. Science (New York, N.Y.). 81 (2112), 621-623 (1935).
- Skutul, K. . Über Durchströmungsapparate Pflüger's Archiv. 123 (4-6), 249-273 (1908).
- Nizet, A. The isolated perfused kidney: possibilities, limitations and results. Kidney Int. 7 (1), 1-11 (1975).
- Weiss, C., Passow, H., Rothstein, A. Autoregulation of flow in isolated rat kidney in the absence of red cells. Am J Physiol. 196 (5), 1115-1118 (1959).
- Schurek, H. J., Kriz, W. Morphologic and functional evidence for oxygen deficiency in the isolated perfused rat kidney. Lab Invest. 53 (2), 145-155 (1985).
- Stolte, H., Schurek, H. J., Alt, J. M. Glomerular albumin filtration: a comparison of micropuncture studies in the isolated perfused rat kidney with in vivo experimental conditions. Kidney Int. 16 (3), 377-384 (1979).
- Schweda, F., Wagner, C., Krämer, B. K., Schnermann, J., Kurtz, A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. AJP Renal Physiol. 284 (4), F770-F777 (2003).
- Moers, C., Smits, J. M., et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 360 (1), 7-19 (2009).
- Nicholson, M. L., Hosgood, S. A. Renal transplantation after ex vivo normothermic perfusion: the first clinical study. Am J Transplant. 13 (5), 1246-1252 (2013).
- Worner, M., Poore, S., Tilkorn, D., Lokmic, Z., Penington, A. J. A low-cost, small volume circuit for autologous blood normothermic perfusion of rabbit organs. Art Org. 38 (4), 352-361 (2014).
- Kaths, J. M., Spetzler, V. N., et al. Normothermic Ex Vivo Kidney Perfusion for the Preservation of Kidney Grafts prior to Transplantation. J Vis Exp. (101), e52909 (2015).
- Song, J. J., Guyette, J. P., Gilpin, S. E., Gonzalez, G., Vacanti, J. P., Ott, H. C. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nature Med. 19 (5), 646-651 (2013).
- Hall, A. M., Crawford, C., Unwin, R. J., Duchen, M. R., Peppiatt-Wildman, C. M. Multiphoton imaging of the functioning kidney. JASN. 22 (7), 1297-1304 (2011).
- Lindell, S. L., Williams, N., Brusilovsky, I., Mangino, M. J. Mouse IPK: A Powerful Tool to Partially Characterize Renal Reperfusion and Preservation Injury. Open Transplant J. 5, 15-22 (2011).
- Wagner, C., De Wit, C., Kurtz, L., Grünberger, C., Kurtz, A., Schweda, F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 100 (4), 556-563 (2007).
- Czogalla, J., Vohra, T., Penton, D., Kirschmann, M., Craigie, E., Loffing, J. The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflüger's Archiv. , (2016).
- Poosti, F., et al. Precision-cut kidney slices (PCKS) to study development of renal fibrosis and efficacy of drug targeting ex vivo. Dis Model Mech. 8 (10), 1227-1236 (2015).
- Rahgozar, M., Guan, Z., Matthias, A., Gobé, G. C., Endre, Z. H. Angiotensin II facilitates autoregulation in the perfused mouse kidney: An optimized in vitro model for assessment of renal vascular and tubular function. Nephrology. 9 (5), 288-296 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone