Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Measuring and Modeling Contractile Drying in Human Stratum Corneum
W tym Artykule
Podsumowanie
This article describes a method of quantifying the dynamic drying behavior and mechanical properties of stratum corneum by measuring spatially resolved in-plane drying displacements of circular tissue samples adhered to an elastomer substrate. This technique can be used to measure how different chemical treatments alter drying and tissue mechanical properties.
Streszczenie
Stratum corneum (SC) is the most superficial skin layer. Its contact with the external environment means that this tissue layer is subjected to both cleansing agents and daily variations in ambient moisture; both of which can alter the water content of the tissue. Reductions in water content from severe barrier dysfunction or low humidity environments can alter SC stiffness and cause a build-up of drying stresses. In extreme conditions, these factors can cause mechanical rupture of the tissue. We have established a high throughput method of quantifying dynamic changes in the mechanical properties of SC upon drying. This technique can be employed to quantify changes in the drying behavior and mechanical properties of SC with cosmetic cleanser and moisturizer treatments. This is achieved by measuring dynamic variations in spatially resolved in-plane drying displacements of circular tissue samples adhered to an elastomer substrate. In-plane radial displacements acquired during drying are azimuthally averaged and fitted with a profile based on a linear elastic contractility model. Dynamic changes in drying stress and SC elastic modulus can then be extracted from the fitted model profiles.
Wprowadzenie
The outer most layer of the epidermis, or stratum corneum (SC) consists of cohesive corneocyte cells surrounded by a lipid rich matrix1,2. The composition and structural integrity of SC is essential for maintaining correct barrier functionality3, which prevents invasion from microorganisms and resists both mechanical forces and excessive water loss4. The capacity of personal care products to maintain or degrade skin barrier function is of great interest to skin healthcare and the cosmetic industry5. The daily application of personal care products is known to alter the mechanical properties of the SC6,7,8. For example, surfactants contained in cosmetic cleansers can cause significant increases in the elastic modulus and a build-up of drying stresses in SC, increasing the tissue's propensity to crack7,9. Glycerol contained in nearly all cosmetic moisturizers can soften SC and decrease the build-up of drying stresses8,10,11, reducing the likelihood of tissue rupture.
The method detailed in this article is capable of quantifying the dynamic drying behavior and mechanical properties of SC drying in controlled environments7,8. Previously, this technique has been demonstrated to be capable of elucidating the effect of different cosmetic products on changes in the dynamic drying behavior and mechanical properties of SC tissue. This is achieved by quantifying drying-induced shrinkage of human SC tissue adhered to a soft elastomer substrate, fitting drying displacements with a simple contractility model, and then extracting the elastic modulus and drying stress from the fitted profile. When testing of multiple SC samples is required, this method offers a more rapid alternative to uniaxial tensometry, utilizes significantly less tissue and provides more physiologically relevant drying by preventing evaporation from the sample underside.
Access restricted. Please log in or start a trial to view this content.
Protokół
An exempt approval (3002-13) to carry out research using de-identified tissue samples pursuant to the Department of Health and Human Services regulations, 45 CFR 46.101(b)(4) was granted. Full thickness skin is received from elective surgery. In this article, the tissue source is 66-year-old Caucasian female breast.
1. Preparation of Elastomer Coated Coverslips
- In a 20 mL glass vial, mix 0.107 g of Sylgard 184 curing agent with 5.893 g base. The total mixture mass is 6 g with a base to curing agent ratio of 55:1.
- After mixing with a glass rod to ensure homogeneity, place the glass vial in a vacuum chamber and degas to remove all bubbles.
- Place a glass cover-slip (55 mm x 25 mm) in the center of a spin coater. Add ~1 mL of the mixture onto the center of the cover-slip. Use a 5,000 µL pipette with the end cut off with scissors. Spin coat the cover-slip at 2,000 rpm for 60 s.
- Repeat this process to create 5-6 substrates.
- Cure the cover-slips in an oven for 12 h at 60 °C.
- Use a razor blade to partially remove the elastomer film from a sacrificial substrate. Use an indelible marker to mark the topside of the elastomer film and the exposed glass.
- Mount the sample on an inverted microscope and use a remote focus accessory to record the difference in z-height between the focal planes of the two marks. This corresponds to the elastomer substrate thickness, h.
2. Preparation of the Stratum Corneum
- Use a water bath or heated stir plate to heat a glass beaker half filled with deionized water (DW) to 60 °C. Inside a biological safety cabinet, immerse the full thickness human skin in the water for 4 min.
- Immediately transfer the skin sample into a beaker containing DW cooled to <10 °C for 4 min. Half filling the beaker minimizes splashing of biohazardous material.
- Remove the skin from the beaker, place in a Petri dish, and gently isolate the epidermis using a pair of bent nosed tissue tweezers.
- Place the isolated epidermis basal side down in a Petri dish lined with gauze. Ensure the basal layer is fully in contact with the gauze.
- Soak the gauze in a 0.25% (wt/vol) type IX-S porcine pancreas trypsin solution dissolved in 0.1 M phosphate buffered saline for 6-8 h at room temperature. Add only enough trypsin in the container to wet the gauze.
- Lift the gauze with tissue tweezers and float it in a container partially filled with DW. Gently pull the SC to separate it from the gauze.
- Wash the stratum corneum 3-4 times in DW to remove residual epidermal tissue that remains attached to the SC.
- Float the isolated SC in a solution of 0.4% glycine max(soybean) trypsin inhibitor in DW. Use a plate shaker to agitate the tissue for 10 min.
- Float SC in a Petri dish partially filled with DW. Use a plate shaker to agitate the tissue for 10 min.
- Dry the isolated SC sheet on an ultra-fine plastic mesh for 48 h at room temperature (25 °C, 40% relative humidity (R.H.)).
- Separate the SC from the mesh and cut out individual circular R=3 mm radius samples using a circular hole punch. Mark the center of the outmost face with a small spiral mark using an indelible marker. This provides a visual cue for recognizing the topside of the SC.
NOTE: The indelible mark should be applied in the center of the sample, where the drying deformations will be smallest. This will minimize the impact of the marker on recorded drying displacement profiles.
3. Sample Treatment and Deposition
- Agitate SC samples for 30 min in 15 mL DW containing 90 µL fluorescent marker beads (505/515 nm, 1 µm diameter, carboxylate-modified). This deposits beads onto the SC surface
NOTE: While deposition of large numbers of beads on the SC may marginally slow drying relative to samples without beads present12, it will maximize the spatial resolution of the in-plane deformation fields that can be subsequently obtained. The choice of bead volume added should therefore be made ad hoc. - Remove SC samples and place them in a Petri dish partially filled with DW.
- Partially immerse a substrate in the DW at a shallow angle of 15-30°.
- Pin an edge of the floating SC sample at the contact line between the substrate and water interface. Vertically withdrawing the substrate from the water will smoothly laminate the SC sample to the substrate without wrinkles or entrapped air bubbles.
- Repeat step 3.4 to place up to 6 SC samples onto each substrate. Leave at least a 2-3 mm gap between samples and avoid sample lamination close to the substrate edge. This prevents drying of one sample influencing drying displacements in another.
- Dry the mounted SC samples in laboratory conditions for 60 min. This allows residual water between the SC and substrate to evaporate and ensures complete tissue adhesion.
NOTE: At this juncture, SC samples can be treated with a chemical or cosmetic formulations7,8 by placing the substrates upside down in a desired solution for a requisite period of time. Repeat step 3.6 once the treatment step is performed. After drying, incomplete adhesion of SC samples to the substrate can be verified using transmitted light microscopy. Trapped bubbles under the SC sample or delaminated edges will form clear contrast variations in the sample with well-defined edges. - Create a humidity chamber by placing a Petri dish partially filled with water into a hermetically sealed container.
- Place substrates into the chamber for 24 h to equilibrate to a relative humidity of 99%. Do not place the substrates into the Petri dish.
4. Microscope Environmental Control
- Achieve control of environmental conditions through a humidity control system connected to a microscope mountable perfusion chamber. Details of the humidity control system are provided in German et al. (2013)7 and Liu and German (2015)8.
- Mount the substrate on the microscope, place the perfusion chamber over the substrate and seal edges of perfusion chamber to the elastomer using vacuum grease.
- Once mounted, equilibrate internal air to 99% R.H. prior to experimentation. This prevents evaporation of water prior to experimentation. Once imaging in sections 5 or 7 has begun, reduce internal air humidity to desired value.
NOTE: In this article, SC samples are dried to 25% R.H.
5. Imaging in Plane Drying Displacements
- Acquire images of SC samples using an inverted microscope with 1X objective lens. Excite fluorescent beads using a light engine with FITC filter (503-530 nm emission bandpass). Multiple samples can be imaged sequentially throughout drying using an automated x-y stage.
- Record fluorescent and transmitted light images using a digital CCD camera at a resolution of 1,392 x 1,040 pixels. The field of view of each image is 8.98 x 6.71 mm, allowing a single image to capture a full SC sample. Take images with a frequency of 10 min for 16 h.
6. Substrate Preparation for Thickness Measurement
- In a chemical fume hood, place 1 mL silane (3-aminopropyltriethoxysilane; ≥98%) in some small plastic cap. Place elastomer substrates from section 1 and the cap in a sealed container for 5 h. Do not allow substrates to come directly in contact with the silane.
- Add 5 mg of EDC (N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride; ≥99%) in a 1.5 mL tube. Add 500 µL of DW to the EDC. Agitate the solution for 10 s with a vortex mixer.
- Add 0.076 g sodium tetraborate and 0.1 g boric acid to 20 mL DW. Mix using a magnetic stirrer at 70 °C (1 h). Add boric acid until the pH is 7.4.
- Add 20 mL of borate buffer to a 50 mL centrifuge tube. Add 60 µL of 1 µm beads (535/575 nm, carboxylate-modified) to the borate buffer. Finally, add 200 µL of EDC solution to bottle. Shake the tube to mix bead solution and then pour into a 10 cm diameter Petri dish.
- Remove the silanated substrates from the container and place them elastomer film-side down into the bead solution. Do so slowly to prevent bubbles from becoming trapped. Two substrates will fit in each Petri dish.
- Leave substrates to float in the bead solution for 45 min.
- Use tweezers to remove the substrates from the bead solution, then rinse in DW to remove unbound beads.
- Air dry the substrates. Blowing compressed air over the elastomer film surface reduces the formation of water spots.
- Seal the substrates in an opaque box to prevent photo-bleaching of the beads until SC sample deposition.
7. Imaging Thickness of SC
- Deposit SC samples on to a substrate using section 3. However, perform step 3.1 without adding fluorescent beads to the DW. Additionally, apply a 5 µL drop of undiluted fluorescent marker bead solution (505/515 nm, 0.1 µm diameter) to the surface of each deposited SC sample with a pipette before completing step 3.6.
- Establish measurements of SC thickness using the microscope with 40X objective lens. Measure the thickness of SC samples over time using a remote focus accessory to record the difference in z-height between the two bead layer focal planes located at the SC-substrate interface and the topside of the SC.
- Measure the thickness of 3 regions of each SC sample over a 3 h drying period. The thickness of SC samples reaches a steady state value within this time frame8.
8. Quantifying and Modeling Tissue Deformation
- Use particle image velocimetry13 to obtain spatially resolved in-plane drying displacements from the fluorescent images at each recorded time step.
- Use MATLAB to obtain azimuthally averaged radial and azimuthal displacement profiles from the displacement field of each radially symmetric SC sample.
NOTE: An example dataset (entitled 'd.mat') and MATLAB code (entitled 'PIV-processing.m') that performs both this step and step 8.3 has been provided in the supplemental information. - Fit radial displacement profiles to a model7,8,14,15,16 describing drying SC as a shrinking linear elastic circular disk of time varying thickness, hSC, radius, R, and elastic modulus, ESC, adhered to a deformable elastic substrate with elastic modulus, E. Assume SC has a well-defined and constant Poisson's ratio, νSC = 0.47,8. Obtain best fits using a minimum least squares approach.
NOTE: The model used for fitting describes radial displacements in terms of modified Bessel functions are:
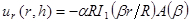 (1)
(1)
with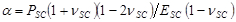 ,
,  and
and 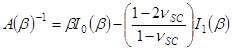
The term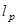 corresponds to a penetration depth given by,
corresponds to a penetration depth given by,
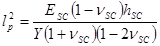 .
.
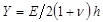 denotes a substrate rigidity parameter; valid when sample sizes are much greater than the substrate thickness. Here, the parameters,
denotes a substrate rigidity parameter; valid when sample sizes are much greater than the substrate thickness. Here, the parameters, 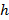 and
and 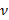 respectively denote the substrate thickness Poisson's ratio. The Poisson's ratio of the silicone elastomer substrate17 is ν = 0.5.
respectively denote the substrate thickness Poisson's ratio. The Poisson's ratio of the silicone elastomer substrate17 is ν = 0.5. - Obtain model parameters α and β at each time step from the least squares fit of Equation (1) to the radial displacement profile.
- Employ the fitting parameter β to obtain SC elastic modulus, ESC, using the expression,
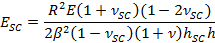
- Use fitting parameter α to obtain the time varying contractile drying stress, PSC, using the expression,
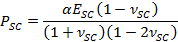
- Employ the fitting parameter β to obtain SC elastic modulus, ESC, using the expression,
Access restricted. Please log in or start a trial to view this content.
Wyniki
Figure 1(a) shows a representative fluorescent image of an SC sample coated with fluorescent beads (section 3). The corresponding transmitted light image of the sample is shown in Figure 1(b) overlaid with a quiver plot of spatially resolved drying displacements that form after 16 h drying at 25% R.H. Due to the circular symmetry of the samples, these displacements can be azimuthally averaged. Figure 1(c) shows radial (ur, soli...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In this article, we describe a technique that can be used to measure the dynamic drying behavior and mechanical properties of human SC. Previous studies have demonstrated that this technique can be used to quantify the effects of environmental conditions and chemical products commonly used in cosmetic cleansers and moisturizers on the dynamic drying behavior of SC7,8. There are a number of key steps in the protocol. Firstly, SC swells notably with water content; ...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors have no acknowledgements.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| Silicone elastomer base | Dow-Corning | 1064291 | |
| Silicone elastomer Curing Agent | Dow-Corning | 1015311 | |
| FluoSpheres Carboxylate 0.1 µm yellow green fluorescent 505/515 | Thermo Fisher | F8803 | |
| FluoSpheres Carboxylate 1 µm yellow green fluorescent 505/515 | Thermo Fisher | F8823 | |
| FluoSpheres Carboxylate 1 µm nile red fluorescent 535/575 | Thermo Fisher | F8819 | |
| Trypsin from porcine pancreas | Sigma-Aldrich | T6567 | |
| Trypsin inhibitor type II-s | Sigma-Aldrich | T9128 | |
| (3-aminopropyl)triethoxysilane | Sigma-Aldrich | 440140 | |
| Sodium tetraborate | Sigma-Aldrich | 221732 | |
| Boric acid | Sigma-Aldrich | B0294 | |
| Phosphate buffered saline | Sigma-Aldrich | P7059 | |
| N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride | Sigma-Aldrich | E7750 | |
| Vortexer mixer | VWR | 58816-123 | |
| 6 mm diameter hole punch | Sigma-Aldrich | Z708860 | |
| SOLA 6-LCR-SB | Lummencor light engine | No.3526 | |
| Cfi Plan Achro Uw 1X Objective | Nikon Plan UW | MRL00012 | |
| CFI Plan Fluor 40X Oil Objective 1.3 na - 0.20 mm wd | Nikon Plan Fluor | MRH01401 | |
| Nikon Eclipse Ti-U inverted microscope | Nikon | MEA53200 | |
| Clara-E Camera | Andor | DR-328G-C02-SIL | |
| Remote Focus Attachment E-RFA Ergo Design | Nikon | 99888 | |
| Ti-S-E Motorized Stage | Nikon | MEC56110 |
Odniesienia
- Van Hal, D., Jeremiasse, E., Junginger, H. E., Spies, F., Bouwstra, J. Structure of fully hydrated human stratum corneum: a freeze-fracture electron microscopy study. J. Invest. Dermatol. 106 (1), 89-95 (1996).
- Norlén, L., Al-Amoudi, A. Stratum corneum keratin structure, function, and formation: the cubic rod-packing and membrane templating model. J. Invest. Dermatol. 123 (4), 715-732 (2004).
- Liu, X., Cleary, J., German, G. K. The global mechanical properties and multi-scale failure mechanics of heterogeneous human stratum corneum. Acta Biomater. , (2016).
- Geerligs, M. Skin layer mechanics. , Technische Universiteit Eindhoven. (2010).
- Farage, M. S., Miller, K. W., Maibach, H. I. Textbook of Aging Skin. , (2010).
- Levi, K., Kwan, A., Rhines, A. S., Gorcea, M., Moore, D. J., Dauskardt, R. H. Emollient molecule effects on the drying stresses in human stratum corneum. Br. J. Dermatol. 163 (4), 695-703 (2010).
- German, G. K., Pashkovski, E., Dufresne, E. R. Surfactant treatments influence drying mechanics in human stratum corneum. J. Biomech. 46 (13), 2145-2151 (2013).
- Liu, X., German, G. K. The effects of barrier disruption and moisturization on the dynamic drying mechanics of human stratum corneum. J. Mech. Behav. Biomed. Mater. 49 (13), 80-89 (2015).
- Levi, K., Weber, R. J., Do, J. Q., Dauskardt, R. H. Drying stress and damage processes in human stratum corneum. Int. J. Cosmet. Sci. 32 (4), 276-293 (2010).
- Levi, K., et al. Effect of glycerin on drying stresses in human stratum corneum. J. Dermatol. Sci. 61, 129-131 (2011).
- Fluhr, J. W., Darlenski, R., Surber, C. Glycerol and the skin: holistic approach to its origin and functions. Br. J. Dermatol. 159 (1), 23-34 (2008).
- German, G. K., et al. Heterogeneous drying stresses in stratum corneum. Biophys. J. 102 (11), 2424-2432 (2012).
- Willert, C. E., Gharib, M. Digital particle image velocimetry. Exp. Fluids. 10 (4), 181-193 (1991).
- Mertz, A. F., et al. Scaling of traction forces with the size of cohesive cell colonies. Phys. Rev. Lett. 108 (19), 1-5 (2012).
- Banerjee, S., Marchetti, M. C. Substrate rigidity deforms and polarizes active gels. Euro Phys. Lett. 96 (2), 28003(2011).
- Edwards, C. M., Schwarz, U. S. Force localization in contracting cell layers. Phys. Rev. Lett. 107 (12), 128101(2011).
- Cesa, C., et al. Micropatterned silicone elastomer substrates for high resolution analysis of cellular force patterns. Rev. Sci. Instrum. 78 (3), 34301(2007).
- Wu, K. S., Van Osdol, W. W., Dauskardt, R. H. Mechanical And Microstructural Properties Of Stratum Corneum. Mater. Res. Soc. 724, 27-33 (2002).
- Yuan, Y., Verma, R. Measuring microelastic properties of stratum corneum. Colloids Surf. B. 48 (1), 6-12 (2006).
- Christensen, M. S., Hargens, C. W., Nacht, S., Gans, E. H. Viscoelastic properties of intact human skin: instrumentation, hydration effects, and the contribution of the stratum corneum. J Invest. Dermatol. 69 (3), (1977).
- Pailler-Mattei, C., Bec, S., Zahouani, H. In vivo measurements of the elastic mechanical properties of human skin by indentation tests. Med. Eng.Phys. 30 (5), 599-606 (2008).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone