Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Analysis of Histone Antibody Specificity with Peptide Microarrays
W tym Artykule
Podsumowanie
This manuscript describes methods for applying peptide microarray technology to specificity profiling of antibodies that recognize histones and their post-translational modifications.
Streszczenie
Post-translational modifications (PTMs) on histone proteins are widely studied for their roles in regulating chromatin structure and gene expression. The mass production and distribution of antibodies specific to histone PTMs has greatly facilitated research on these marks. As histone PTM antibodies are key reagents for many chromatin biochemistry applications, rigorous analysis of antibody specificity is necessary for accurate data interpretation and continued progress in the field. This protocol describes an integrated pipeline for the design, fabrication and use of peptide microarrays for profiling the specificity of histone antibodies. The design and analysis aspects of this procedure are facilitated by ArrayNinja, an open-source and interactive software package we recently developed to streamline the customization of microarray print formats. This pipeline has been used to screen a large number of commercially available and widely used histone PTM antibodies, and data generated from these experiments are freely available through an online and expanding Histone Antibody Specificity Database. Beyond histones, the general methodology described herein can be applied broadly to the analysis of PTM-specific antibodies.
Wprowadzenie
Genomic DNA is elegantly packaged inside the eukaryotic cell nucleus with histone proteins to form chromatin. The repeating subunit of chromatin is the nucleosome, which consists of 147 base pairs of DNA wrapped around an octameric core of histone proteins – H2A, H2B, H3, and H41. Chromatin is broadly organized into loosely packed euchromatin and tightly packed heterochromatin domains. The degree of chromatin compaction regulates the extent to which protein machineries can access the underlying DNA to carry out fundamental DNA-templated processes like replication, transcription, and repair.
Key regulators of genome accessibility in the context of chromatin are PTMs on the unstructured tail and core domains of histone proteins2,3. Histone PTMs function directly by influencing the structure of chromatin4 and indirectly through the recruitment of reader proteins and their associated macromolecular complexes that have chromatin remodeling, enzymatic, and scaffolding activities5. Studies of histone PTM function over the past two decades overwhelmingly suggest these marks play key roles in regulating cell fate, organismal development, and disease initiation/progression. Fueled by advances in mass spectrometry-based proteomic technology, more than 20 unique histone PTMs on more than 80 distinct histone residues have been discovered6. Notably, these modifications often occur in combinations, and consistent with the "histone code" hypothesis, numerous studies suggest that reader proteins are targeted to discrete regions of chromatin through recognition of specific combinations of histone PTMs7,8,9. A key challenge moving forward will be to assign functions to the growing list of histone PTMs and to determine how specific combinations of histone PTMs orchestrate the dynamic functions associated with chromatin.
Antibodies are the lynchpin reagents for the detection of histone PTMs. As such, more than 1,000 histone PTM-specific antibodies have been commercially developed for use in chromatin biochemistry research. With the rapid development of high-throughput DNA sequencing technology, these reagents are being used extensively by individual investigators and large-scale epigenomics "roadmap" initiatives (e.g., ENCODE and BLUEPRINT) in ChIP-seq (chromatin immunoprecipitation coupled with next-generation sequencing) pipelines to generate high-resolution spatial maps of histone PTM distribution genome-wide10,11. However, recent studies have shown that the specificity of histone PTM antibodies can be highly variable and that these reagents exhibit unfavorable properties such as off-target epitope recognition, strong positive and negative influence by neighboring PTMs, and difficulty discriminating the modification order on a particular residue (e.g., mono-, di-, or tri-methyllysine)12,13,14,15,16,17,18. Therefore, rigorous quality control of histone PTM-specific antibody reagents is necessary to accurately interpret data generated with these valuable reagents.
Microarray technology enables the simultaneous interrogation of thousands of macromolecular interactions in a high-throughput, reproducible, and miniaturized format. For this reason, a variety of microarray platforms have been created to analyze protein-DNA19,20, protein-protein 21, and protein-peptide interactions22. Indeed, histone peptide microarrays have emerged as an informative discovery platform for chromatin biochemistry research, enabling high-throughput profiling of the writers, erasers, and readers of histone PTMs15,23,24, and also for the analysis of histone antibody specificity17,25. Beyond their application in chromatin and epigenetics research, histone peptide arrays have potential utility as a diagnostic/prognostic test for systemic lupus erythematosus and other autoimmune diseases where anti-chromatin autoantibodies are generated26,27.
Here, we describe an integrated pipeline that we have developed for designing, fabricating, and querying histone peptide microarrays to generate specificity profiles for antibodies that recognize histones and their PTMs. The pipeline is facilitated by ArrayNinja, an open-source, interactive software application that we recently developed, which integrates the design and analysis stages of microarray experiments28. ArrayNinja works best in Google Chrome. Briefly, a robotic contact microarray printer is used to deposit a library of biotin-conjugated histone peptides at defined positions on streptavidin-coated glass microscope slides. Arrays can then be used in a competitive and parallel assay format to interrogate antibody-epitope interactions (Figure 1). The peptide library consists of hundreds of unique synthetic peptides harboring PTMs (lysine acetylation, lysine/arginine methylation, and serine/threonine phosphorylation) alone and in relevant combinations largely derived from proteomics datasets. Methods for peptide synthesis and validation are detailed elsewhere23. Data generated from our ongoing histone PTM antibody screening efforts utilizing this array platform are archived on a public web resource, the Histone Antibody Specificity Database (www.histoneantibodies.com). Notably, histone peptide microarrays fabricated with variations of this protocol have also been used extensively to characterize the activity of histone PTM reader domains8,29,30,31,32,33,34,35,36,37 and more recently to profile histone PTM writer and eraser activities24.

Figure 1: Cartoon Depiction of the Stepwise Procedure for Antibody Screening on a Histone Peptide microarray. Biotinylated histone peptides harboring defined post-translational modifications (red and blue circles) are co-printed with biotin-fluorescein on streptavidin-coated glass. Positive interactions are visualized as red fluorescence. Please click here to view a larger version of this figure.
Protokół
1. Installing and Running ArrayNinja
- Download and install Oracle Virtual Box from www.virtualbox.org.
- Download and uncompress the ArrayNinja virtual machine (VM) from http://research.vai.org/Tools/arrayninja.
- Open Virtual Box and add the ArrayNinja VM by clicking 'Machine,' 'Add,' and select arrayninja.vbox from the folder where ArrayNinja VM was saved.
- Start ArrayNinja by selecting it inside Virtual Box and clicking the green 'start' arrow.
- Virtual Box will open a new window and display a message that the VM can be accessed by navigating the web browser to localhost:2080. NOTE: A containerized version of ArrayNinja is also available via hub.docker.com/r/bradley.dickson/arrayninja/
2. Designing the Array Slide and Source Plate Layout
- Under the 'Plan a slide layout' heading on the ArrayNinja interface, click on the link that corresponds to the microarray printer being used.
NOTE: ArrayNinja has been programmed to mimic the robotic movement of two commonly used microarray printers (see Table 1). Compatibility with the other arrayers can be configured upon request. - Click inside the 'Load plate' dialog box, type "empty", and click 'enter.' See Figure 2 for a screenshot of the ArrayNinja design module.
- Adjust the spot diameter to 275 µm and spot spacing to 375 µm. Adjust the remaining settings (Plate Blocks/plate row, Total plate Rows, replicates, features in y, Super Arrays, SuperA fudge; see Figure 2) to customize how the features will appear on the microarray slide.
NOTE: The spot diameter is determined by the size of the microarray pin. As these settings are adjusted, the cartoon slide will update in real-time. Use this cartoon to preview how each setting is modifying the final slide layout. - After the layout of features on the slide is finalized, mouse over each unique feature and enter the feature identifier in the pop-up dialog box.
NOTE: Feature identifiers can consist of numbers, letters, or combinations. This is only required for unique features, and a dialog box will not appear when replicates are selected. - After all unique features have been assigned an identifier, click 'populate.' Enter a name for the slide layout and click 'print your plate' to save. A new page will open displaying the number of 384-well source plates needed to fabricate the chosen slide design and a table mapping the physical location of each feature to be loaded in the source plate(s).
NOTE: Remember this name, as it will be used to recall this design when analyzing microarray data (see section 6.2). Clicking 'print your plate' saves the layout within ArrayNinja.

Figure 2: ArrayNinja Design Module. A screen shot of the ArrayNinja design module is shown in the dotted line. The control panel (top) shows all of the parameters that can be altered on the microarray printer. As these parameters are adjusted, the cartoon image of the slide layout (bottom left) updates in real time. After the layout is set, the user can mouse over individual spots to enter unique feature identifiers. ArrayNinja constructs from this user input a map of the position of each feature in the source plate(s) (bottom right) needed to fabricate a specified microarray slide layout. Please click here to view a larger version of this figure.
3. Fabricating Microarrays
- Preparing the Source Plate
- Use the map generated with ArrayNinja in section 2.5 to create the 384-well source plate(s).
NOTE: Detailed descriptions of peptides queried on this platform can be found elsewhere17. - Deposit 1 - 2 µL of each feature (e.g., biotinylated histone peptide) into the correct well of the 384-well source plate(s).
NOTE: Biotinylated histone peptides are typically deposited from 200 - 400 µM stock solutions, which equates to 10 to 25-fold molar excess of peptide to streptavidin binding sites in a single array spot. This is calculated using the equation:
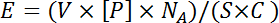
where V is the volume delivered by a pin, [P] is the concentration of the feature being printed, NA is Avogadro's number, and S is the surface area of a spot. C is the coverage of the slide, expressed as the number of streptavidin molecules per unit area multiplied by three (the average number of available streptavidin binding sites). V and C are obtained by the respective manufacturer's. Other features may require different concentrations depending on the size of the biomolecule where crowding may be a concern. A range of printing concentrations for each new type of feature should be tested empirically to determine the optimum printing concentration. - Dilute each feature 10-fold with 1x printing buffer supplemented with 1% bovine serum albumin (BSA). Spin the source plate(s) at 500 x g for 2 min at room temperature.
NOTE: The inclusion of fluorescein-labeled biotin (5 µg/µL) in the printing buffer is recommended as a spotting control and as a visual aid to facilitate proper array alignment during analysis (see Figure 4).
- Use the map generated with ArrayNinja in section 2.5 to create the 384-well source plate(s).
- Printing Protocol (Figure 3A – B).
- Prepare the arrayer by emptying the waste collection container and filling the wash solution container and humidifier container with sterile distilled water.
- Enter the parameters used to design the slide in ArrayNinja (section 2 and Figure 2) into the microarray printer control program.
- Using the microarray printer control program, set the washing procedure for a 1 s wash with one immersion. Set the post-wash settings to re-dip the pins 5 times following each wash. Set the humidity to 60%.
NOTE: The optimal wash configuration may vary depending on the microarray printer being used. The optimal post-wash pin dip configuration may vary depending on the microarray printer being used. - Insert functionalized slides (e.g., streptavidin-coated glass) into the substrate platens and place all platens into the platen elevator. Insert the source plate(s) into the plate holder(s) and place into the source plate elevator.
- Click 'print'. Monitor the printing process for a few rounds of feature deposition to ensure all wash and dip settings are correct. When the print run is complete, remove the substrate platens from the arrayer.
NOTE: When large print runs prohibit completion of blocking steps within one day, printed slides can be incubated in a humidified chamber at 4 °C overnight. Incubate the slides next to a small beaker of water inside a cardboard box sealed with plastic wrap. - Block the slides with blocking buffer for 30 min at room temperature with mixing.
- Wash the slides 2 x 10 min at room temperature in phosphate buffered saline (PBS), pH 7.6 with mixing. Dry the slides by spinning in a microarray slide centrifuge for 30 s at room temperature.
NOTE: For processing a large number of slides at once, a high-throughput microscope slide washing chamber can be used, allowing 50 slides to be washed in parallel. - For slides designed to be partitioned with wax, proceed to section 4.1. For all other designs, store slides at 4 °C protected from light and moisture.
NOTE: Printed biotinylated histone peptides are stable for at least 6 months when stored this way.
4. Partitioning Microarray Slides
- Hydrophobic Wax Pen (Figure 3C)
- Apply wax around the areas that contain features using a wax pen. Allow wax to air dry for 5 min before proceeding to section 5.
NOTE: After blocking, the array spots can be very difficult to visualize by eye. The slide design from ArrayNinja can be printed to scale and used as a guide for applying wax.
- Apply wax around the areas that contain features using a wax pen. Allow wax to air dry for 5 min before proceeding to section 5.
- Silicon gasket (Figure 3D)
- Peel the clear film off the back of the array gasket and place adhesive side down onto the microarray slide.
- Hold the gasket in place for 5 s prior to proceeding to section 5.
- Wax Imprint (Figure 3E)
- For slides designed to be partitioned by wax, print a test slide on plain glass using 10% BSA. Use this test slide to optimize the wax imprinter guides or array settings to ensure all features will be within the wax-mold chambers.
- Heat the microarray wax imprinter to 85 °C until all the wax is completely melted, approximately 30 min.
- Insert the slide with the printed side facing down and push the slide all the way to the right guide on the microarray imprinter. Pull up the lever to bring the mold into contact with the surface of the slide. Hold for 2 s.
NOTE: The hold time can be altered to achieve optimal wax border thickness. - Quickly remove the slide and visually inspect the wax borders to ensure all wells are enclosed and that the borders are not so thick that they encroach on the spots. Store slides at 4 °C protected from moisture and light.
NOTE: The hold time can be increased or decreased to obtain thicker or thinner borders.
Figure 3: Microarray Fabrication. (A) Histone peptide microarray fabrication on streptavidin-coated microscope slides using a contact microarray printer. (B) Microarrays fabricated with 3 subarrays of a 48 x 48 grid of peptide features. Separation of (C) 3 subarrays with a hydrophobic wax pen, (D) 2 subarrays with a silicon adhesive, and (E) 48 subarrays with a wax imprint. All microarrays shown are fabricated using 25 x 75 mm microscope slides. Please click here to view a larger version of this figure.
5. Hybridizing a Histone PTM Antibody with a Peptide Microarray
- Prepare hybridization buffer (PBS, pH 7.6, 5% BSA, 0.1% Tween-20).
- Equilibrate the slide in hybridization buffer using a hybridization vessel. Completely cover the entire slide in hybridization buffer and incubate for 30 min at 4 °C on an orbital shaker at low speed.
- Prepare a solution containing diluted histone PTM antibody in hybridization buffer.
NOTE: In the example data, both histone Antibody #1 and Antibody #2 were diluted 1:1,000 in hybridization buffer. A dilution range similar to that used for immunoblotting is recommended as a starting point. - Incubate the array with the antibody solution for 1 h at 4 °C. Remove the antibody solution and wash the array 3 times for 5 min at 4 °C with cold PBS, pH 7.6.
- Prepare a 1:5,000 - 1:10,000 dilution of fluorescent dye-conjugated secondary antibody in hybridization buffer.
- Incubate the array with secondary antibody solution for 30 min at 4 °C protected from light. Remove the secondary antibody solution and wash the microarray slide 3 times for 5 min at 4 °C with PBS, pH 7.6. Dip the microarray in a 50-mL conical tube containing 0.1x PBS, pH 7.6 to remove excess salt at room temperature. Dry the slide in a microarray slide centrifuge at room temperature.
- Scan the slide with a microarray scanner at 25 µm resolution or higher, following the microarray scanner manufacturer's recommended scanning protocol.
NOTE: If fluorescein-labeled biotin tracer is present, scan both the green channel (ex: 488 nm, em: 509 nm) and the channel that corresponds to the fluorescent dye-conjugated secondary antibody, typically red (ex: 635 nm, em: 677 nm). The goal of scanning is to obtain single channel .tif files that can be merged into a single .png file (as described in section 6.1).
6. Analysis of Microarray Data using ArrayNinja
- Preparing a merged microarray image
NOTE: The goal of this section is to create a .png image file that merges the two single-channel .tif files (obtained in section 5). This is the only image format compatible with the ArrayNinja analysis module. The following instructions represent one possible way to obtain a merged image file. However, other solutions are available (e.g., the freeware ImageJ).- From the command line in a bash terminal of a computer with the freeware ImageMagick installed, navigate to the folder that contains the single channel .tif files and copy/paste the following steps, hitting 'enter' between each step (6.1.4 - 6.1.7).
NOTE: File names in capital letters (e.g., RED_CHANNEL) should be replaced with the file name of the microarray slide images. - If necessary, first invert the images using the command 'convert INPUT.tif -negate OUTPUT.tif'. This is required if the scanner saves .tif files with the signal in white and background in black.
- convert -depth 16 RED_CHANNEL.TIF -clone 0 -channel GB -evaluate set 0 -delete 0 out.png 2>error.file.
- convert - depth 16 CONTROL_CHANNEL.TIF -clone 0 -channel RB -evaluate set 0 -delete 0 outa.png 2>error.file.
- convert -depth 16 CONTROL_CHANNEL.TIF -clone 0 -channel RG -evaluate set 0 -delete 0 outB.png 2>error.file.
- convert out.png outa.png outB.png -set colorspace RGV -combine merged.png.
NOTE: A file named 'merged.png' will be saved in the same folder as the original .tif files.
- From the command line in a bash terminal of a computer with the freeware ImageMagick installed, navigate to the folder that contains the single channel .tif files and copy/paste the following steps, hitting 'enter' between each step (6.1.4 - 6.1.7).
- Quantifying data using ArrayNinja
- Open ArrayNinja and click on the appropriate microarray printer link under "To quantify images which have a known source plate." Type the name of the saved slide design (step 2.5) in the "load plate" dialog box and click 'enter.' See Figure 4 for a screenshot of the ArrayNinja analysis module.
- Click 'Choose File' and navigate to and select the 'merged.png' file created in section 6.1. The merged image will load as well as a grid for the slide layout.
- Use the sliders at the bottom of the ArrayNinja control panel to adjust the contrast, brightness, and midpoint.
NOTE: These adjustments are for visualization purposes only and have no impact on quantification. - Select "resolution" and enter the value that matches the resolution of the scanned image. Use the "margin side" and "margin top" to move the grid into alignment with the spots on the array. Adjust as needed to get the grid as closely aligned over each spot as possible.
- Click 'Spot Seek' and wait until the button returns to the original grey color.
NOTE: This can be repeated several times to center the grid circles on individual spots. The Seek function relaxes the grid toward each spot to fine-tune the alignment. - Change the "Super Arrays" value to "1" to work up each sub array panel individually (if desired). If using a 4 x 12 wax imprint slide layout (Figure 3E), use the "iPin" "jPin" and "subA" controls to turn off features to analyze any desired combination of 4 x 12 wells. For example, to analyze the top left and top right wells as replicates of each other, enter "1 4" into iPin, "1 1" into jPin and "1 4" into subA. Press 'enter'.
- Hover the mouse over individual spots to view the identity of that feature.
- Click 'Toggle zoom' to review features more carefully. Select background reference spots by pressing the 'R' key while the mouse is over a spot. Reference spots will be highlighted in orange.
NOTE: In "toggle zoom" mode, a magnified image of the feature that the mouse is over is displayed in the upper right hand corner. A detailed discussion of additional background correction features in ArrayNinja are discussed elsewhere28. - Toggle off spots (with widely varied spot morphology or debris that would affect accurate quantification) by pressing the 'A' key while hovering the mouse over the affected spots. Inactivated spots will turn white.
- Click 'populate' followed by 'Submit' to quantify the spots.
NOTE: A new tab will open and display a bar graph of data normalized to the brightest spot average. A table below the bar graph contains both the normalized and raw data values. This table can be copied into a spreadsheet for archiving and further analysis.
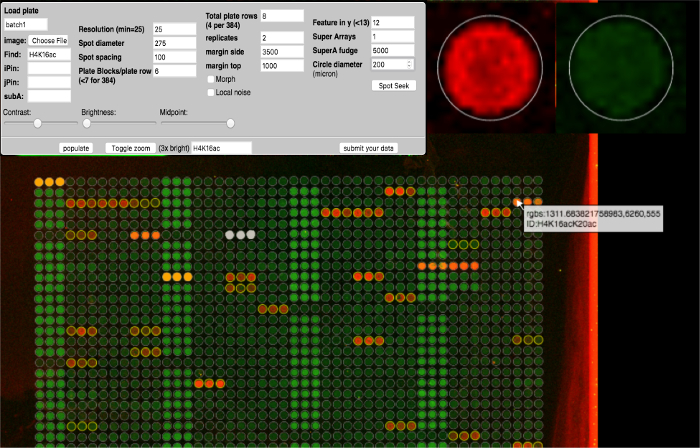
Figure 4: ArrayNinja Analysis Module. A screen shot of the ArrayNinja analysis module is shown. The control panel (top left) shows all of the parameters that can be adjusted to visualize the array, find spots, and align a grid over the array image. Hovering the mouse over a feature shows a zoomed-in view (top right) and displays a popup that contains the identification information associated with that feature (bottom). Reference spots selected for background correction are orange. Features to be excluded from downstream analysis are white. ArrayNinja contains a text-based search feature that highlights matching features in yellow, as shown in the example for H4K16. Please click here to view a larger version of this figure.
Wyniki
This protocol has been used to design and fabricate a peptide microarray platform for the analysis of histone PTM antibody specificity. The array queries a library of more than 300 unique peptide features (20 - 40 residues in length) representing many of the known combinations of PTMs found on core and variant histone proteins38. This pipeline has been a workhorse for the screening of many widely used and commercially available histone PTM antibodies, and full data...
Dyskusje
Antibody reliability in biomedical research applications is paramount46,47. This is especially true in chromatin biochemistry given the position of antibodies as key tools for the majority of techniques developed to characterize the abundance and distribution of histone PTMs. The protocol presented here details an optimized pipeline for the design, fabrication, and use of peptide microarrays to analyze histone PTM antibody specificity. This pipeline has been used...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported in part by the Van Andel Research Institute and a research grant from the National Institutes of Health (CA181343) to S.B.R.
Materiały
| Name | Company | Catalog Number | Comments |
| Printing Buffer | ArrayIt | PPB | |
| BSA | Omnipure | 2390 | |
| Streptavidin-coated glass microscope slides | Greiner Bio-one | 439003-25 | |
| polypropylene 384 well plate | Greiner Bio-one | 784201 | |
| Biotin-fluorescein | Sigma | 53608 | |
| contact microarray printer | Aushon | 2470 | Aushon 2470 Microarray Printer |
| contact microarray printer | Gene Machines | OmniGrid 100 | OmniGrid Microarray Printer |
| PBS | Invitrogen | 14190 | |
| Blocking Buffer | ArrayIt | SBB | |
| Hydrophobic wax pen | Vector Labs | H-4000 | ImmEdge Hydrophobic Barrier PAP Pen |
| Silicon Gasket | Grace Bio-labs | 622511 | |
| Hybridization Vessel | Thermo Scientific | 267061 | or similar vessel |
| Fluorescent-dye conjugated secondary antibody | Life Technologies | A-21244 | Alexa Fluor 647 (anti-rabbit) |
| Fluorescent-dye conjugated secondary antibody | Life Technologies | A-21235 | Alexa Fluor 647 (anti-mouse) |
| Wax Imprinter | ArrayIt | MSI48 | |
| Tween-20 | Omnipure | 9490 | |
| Microarray Scanner | Innopsys | InnoScan 1100AL | or equivalent microarray scanner |
| EipTitan Histone Peptide Microarray | Epicypher | 112001 | |
| AbSurance Pro Histone Peptide Microarray | Millipore | 16668 | |
| MODified Histone Peptide Array | Active Motif | 13001 | |
| Histone Code Peptide Microarrays | JPT | His_MA_01 | |
| Wax | Royal Oak | GulfWax | for wax imprinter |
| Humidified Microarray Slide Hybridization Chamber | VWR | 97000-284 | |
| High throughput microscope slide washing chamber | ArrayIt | HTW | |
| Microscope slide centrifuge | VWR | 93000-204 | |
| Antibody 1 | Abcam | 8898 | |
| Antibody 2 | Millipore | 07-473 | |
| Biotinylated histone peptide | EpiCypher | 12-0001 | Example peptide. Similar peptides with various modifications are available from several commercial sources. |
| ImageMagick | https://www.imagemagick.org/script/index.php | ||
| ArrayNinja | https://rothbartlab.vai.org/tools/ |
Odniesienia
- van Steensel, B. Chromatin: constructing the big picture. EMBO J. 30 (10), 1885-1895 (2011).
- Kouzarides, T. Chromatin modifications and their function. Cell. 128 (4), 693-705 (2007).
- Rothbart, S. B., Strahl, B. D. Interpreting the language of histone and DNA modifications. Biochim Biophys Acta. 1839 (8), 627-643 (2014).
- Shogren-Knaak, M., Ishii, H., Sun, J. -. M., Pazin, M. J., Davie, J. R., Peterson, C. L. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 311 (5762), 844-847 (2006).
- Musselman, C. A., Lalonde, M. -. E., Côté, J., Kutateladze, T. G. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol. 19 (12), 1218-1227 (2012).
- Huang, H., Sabari, B. R., Garcia, B. A., Allis, C. D., Zhao, Y. SnapShot: Histone Modifications. Cell. 159 (2), 458 (2014).
- Strahl, B. D., Allis, C. D. The language of covalent histone modifications. Nature. 403 (6765), 41-45 (2000).
- Rothbart, S. B., Krajewski, K., et al. Association of UHRF1 with methylated H3K9 directs the maintenance of DNA methylation. Nat Struct Mol Biol. 19 (11), 1155-1160 (2012).
- Wang, Z., Zang, C., et al. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 40 (7), 897-903 (2008).
- Stunnenberg, H. G., Hirst, M. The International Human Epigenome Consortium: A Blueprint for Scientific Collaboration and Discovery. Cell. 167 (5), 1145-1149 (2016).
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 489 (7414), 57-74 (2012).
- Egelhofer, T. A., Minoda, A., et al. An assessment of histone-modification antibody quality. Nat Struct Mol Biol. 18 (1), 91-93 (2011).
- Bock, I., Dhayalan, A., Kudithipudi, S., Brandt, O., Rathert, P., Jeltsch, A. Detailed specificity analysis of antibodies binding to modified histone tails with peptide arrays. Epigenetics. 6 (2), 256-263 (2011).
- Busby, M., Xue, C., et al. Systematic comparison of monoclonal versus polyclonal antibodies for mapping histone modifications by ChIP-seq. Epigenetics Chromatin. 9, 49 (2016).
- Fuchs, S. M., Krajewski, K., Baker, R. W., Miller, V. L., Strahl, B. D. Influence of combinatorial histone modifications on antibody and effector protein recognition. Curr Biol. 21 (1), 53-58 (2011).
- Kungulovski, G., Jeltsch, A. Quality of histone modification antibodies undermines chromatin biology research. F1000Research. 4, 1160 (2015).
- Rothbart, S. B., Dickson, B. M., et al. An Interactive Database for the Assessment of Histone Antibody Specificity. Mol Cell. 59 (3), 502-511 (2015).
- Rothbart, S. B., Lin, S., et al. Poly-acetylated chromatin signatures are preferred epitopes for site-specific histone H4 acetyl antibodies. Sci Rep. 2, 489 (2012).
- Berger, M. F., Bulyk, M. L. Universal protein-binding microarrays for the comprehensive characterization of the DNA-binding specificities of transcription factors. Nat Protoc. 4 (3), 393-411 (2009).
- Hu, S., Wan, J., et al. DNA methylation presents distinct binding sites for human transcription factors. eLife. 2, e00726 (2013).
- Moore, C. D., Ajala, O. Z., Zhu, H. Applications in high-content functional protein microarrays. Curr Opin Chem Biol. 30, 21-27 (2016).
- MacBeath, G., Schreiber, S. L. Printing proteins as microarrays for high-throughput function determination. Science. 289 (5485), 1760-1763 (2000).
- Rothbart, S. B., Krajewski, K., Strahl, B. D., Fuchs, S. M. Peptide microarrays to interrogate the "histone code" . Methods Enzymol. 512, 107-135 (2012).
- Cornett, E. M., Dickson, B. M., et al. Substrate Specificity Profiling of Histone-Modifying Enzymes by Peptide Microarray. Methods Enzymol. 574, 31-52 (2016).
- Nady, N., Min, J., Kareta, M. S., Chédin, F., Arrowsmith, C. H. A SPOT on the chromatin landscape? Histone peptide arrays as a tool for epigenetic research. Trends Biochem Sci. 33 (7), 305-313 (2008).
- Dieker, J., Berden, J. H., et al. Autoantibodies against Modified Histone Peptides in SLE Patients Are Associated with Disease Activity and Lupus Nephritis. PLoS ONE. 11 (10), (2016).
- Price, J. V., Tangsombatvisit, S., et al. "On silico" peptide microarrays for high-resolution mapping of antibody epitopes and diverse protein-protein interactions. Nat Med. 18 (9), 1434-1440 (2012).
- Dickson, B. M., Cornett, E. M., Ramjan, Z., Rothbart, S. B. ArrayNinja: An Open Source Platform for Unified Planning and Analysis of Microarray Experiments. Methods Enzymol. 574, 53-77 (2016).
- Gatchalian, J., Fütterer, A., et al. Dido3 PHD modulates cell differentiation and division. Cell Rep. 4 (1), 148-158 (2013).
- Cai, L., Rothbart, S. B., et al. An H3K36 methylation-engaging Tudor motif of polycomb-like proteins mediates PRC2 complex targeting. Mol Cell. 49 (3), 571-582 (2013).
- Rothbart, S. B., Dickson, B. M., et al. Multivalent histone engagement by the linked tandem Tudor and PHD domains of UHRF1 is required for the epigenetic inheritance of DNA methylation. Genes Dev. 27 (11), 1288-1298 (2013).
- Ali, M., Rincón-Arano, H., et al. Molecular basis for chromatin binding and regulation of MLL5. Proc Natl Acad Sci U S A. 110 (28), 11296-11301 (2013).
- Kinkelin, K., Wozniak, G. G., Rothbart, S. B., Lidschreiber, M., Strahl, B. D., Cramer, P. Structures of RNA polymerase II complexes with Bye1, a chromatin-binding PHF3/DIDO homologue. Proc Natl Acad Sci U S A. 110 (38), 15277-15282 (2013).
- Klein, B. J., Piao, L., et al. The histone-H3K4-specific demethylase KDM5B binds to its substrate and product through distinct PHD fingers. Cell Rep. 6 (2), 325-335 (2014).
- Kim, H. -. S., Mukhopadhyay, R., et al. Identification of a BET family bromodomain/casein kinase II/TAF-containing complex as a regulator of mitotic condensin function. Cell Rep. 6 (5), 892-905 (2014).
- Greer, E. L., Beese-Sims, S. E., et al. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Rep. 7 (1), 113-126 (2014).
- Andrews, F. H., Tong, Q., et al. Multivalent Chromatin Engagement and Inter-domain Crosstalk Regulate MORC3 ATPase. Cell Rep. 16 (12), 3195-3207 (2016).
- Sidoli, S., Lin, S., Karch, K. R., Garcia, B. A. Bottom-Up and Middle-Down Proteomics Have Comparable Accuracies in Defining Histone Post-Translational Modification Relative Abundance and Stoichiometry. Anal Chem. 87 (6), 3129-3133 (2015).
- Tsukada, Y., Ishitani, T., Nakayama, K. I. KDM7 is a dual demethylase for histone H3 Lys 9 and Lys 27 and functions in brain development. Genes Dev. 24 (5), 432-437 (2010).
- Tachibana, M., Sugimoto, K., Fukushima, T., Shinkai, Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J Biol Chem. 276 (27), 25309-25317 (2001).
- Wu, H., Chen, X., et al. Histone methyltransferase G9a contributes to H3K27 methylation in vivo. Cell Res. 21 (2), 365-367 (2011).
- Koch, C. M., Andrews, R. M., et al. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 17 (6), 691-707 (2007).
- Okitsu, C. Y., Hsieh, J. C. F., Hsieh, C. -. L. Transcriptional Activity Affects the H3K4me3 Level and Distribution in the Coding Region. Mol Cell Biol. 30 (12), 2933-2946 (2010).
- Zentner, G. E., Tesar, P. J., Scacheri, P. C. Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 21 (8), 1273-1283 (2011).
- Garske, A. L., Oliver, S. S., et al. Combinatorial profiling of chromatin binding modules reveals multisite discrimination. Nat Chem Biol. 6 (4), 283-290 (2010).
- Baker, M. Reproducibility crisis: Blame it on the antibodies. Nature. 521 (7552), 274-276 (2015).
- Bradbury, A., Plückthun, A. Reproducibility: Standardize antibodies used in research. Nature. 518 (7537), 27-29 (2015).
- Nguyen, U. T. T., Bittova, L., et al. Accelerated chromatin biochemistry using DNA-barcoded nucleosome libraries. Nat Methods. 11 (8), 834-840 (2014).
- Frank, R. Spot-synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron. 48 (42), 9217-9232 (1992).
- Hilpert, K., Winkler, D. F. H., Hancock, R. E. W. Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion. Nat Protoc. 2 (6), 1333-1349 (2007).
- Kudithipudi, S., Kusevic, D., Weirich, S., Jeltsch, A. Specificity analysis of protein lysine methyltransferases using SPOT peptide arrays. J Vis Exp. (93), e52203 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone