Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Simple and Effective Administration and Visualization of Microparticles in the Circulatory System of Small Fishes Using Kidney Injection
W tym Artykule
Podsumowanie
This article demonstrates the principles of a quick, minimally invasive injection of fluorescent microparticles into the circulatory system of small fishes and the in vivo visualization of the microparticles in fish blood.
Streszczenie
The systemic administration of micro-size particles into a living organism can be applied for vasculature visualization, drug and vaccine delivery, implantation of transgenic cells and tiny optical sensors. However, intravenous microinjections into small animals, which are mostly used in biological and veterinary laboratories, are very difficult and require trained personnel. Herein, we demonstrate a robust and efficient method for the introduction of microparticles into the circulatory system of adult zebrafish (Danio rerio) by injection into the fish kidney. To visualize the introduced microparticles in the vasculature, we propose a simple intravital imaging technique in fish gills. In vivo monitoring of the zebrafish blood pH was accomplished using an injected microencapsulated fluorescent probe, SNARF-1, to demonstrate one of the possible applications of the described technique. This article provides a detailed description of the encapsulation of pH-sensitive dye and demonstrates the principles of the quick injection and visualization of the obtained microcapsules for in vivo recording of the fluorescent signal. The proposed method of injection is characterized by a low mortality rate (0-20%) and high efficiency (70-90% success), and it is easy to institute using commonly available equipment. All described procedures can be performed on other small fish species, such as guppies and medaka.
Wprowadzenie
The administration of micro-size particles into an animal organism is an important task in such areas as drug and vaccine delivery1, vasculature visualization2, transgenic cell implantation3, and tiny optical sensor implantation4,5. However, the implantation procedure for microscale particles into the vascular system of small laboratory animals is difficult, especially for delicate aquatic organisms. For popular research specimens like zebrafish, it is advised that these procedures be clarified using video protocols.
Intracardiac and capillary microinjections require trained personnel and unique microsurgery facilities for the delivery of microobjects into zebrafish blood. Previously, a retro-orbital manual injection3 was suggested as an easy and effective method for the administration of whole cells. However, in our experience, because of the small area of the eye capillary network, it takes much practice to achieve the desired outcome from this technique.
Herein, we describe a method for robust and efficient microparticle implantation into the circulatory system by manual injection directly into the kidney tissue of adult zebrafish, which is rich in capillaries and renal vessels. This technique is based on the video protocol for cell transplantation into the zebrafish kidney6, but the traumatic and time-consuming microsurgical steps were eliminated. The proposed method is characterized by low mortality (0-20%) and high efficiency (70-90% success), and it is easy to institute using commonly available equipment.
An important part of the proposed protocol is the visualization of the implanted microparticles (if they are fluorescent or colorized) in the gill capillaries, which allows for the verification of the injection quality, a rough relative assessment of the number of injected particles, and the detection of the spectral signal for physiological measurements directly from the circulating blood. As an example of the possible applications of the described technique, we demonstrate the protocol for in vivo measurements of zebrafish blood pH using a microencapsulated fluorescent probe, SNARF-1, originally suggested in Borvinskaya et al. 20175.
Protokół
All experimental procedures were conducted in accordance with the EU Directive 2010/63/EU for animal experiments and have been approved by the Animal Subjects Research Committee of Institute of Biology at Irkutsk State University.
1. Fabrication of Microcapsules
NOTE: Microcapsules carrying a fluorescent dye are prepared using a layer-by-layer assembly of oppositely charged polyelectrolytes7,8. All procedures were performed at room temperature.
- To synthesize porous CaCO3 microcores enclosing the fluorescent dye, mix 2 mL of the SNARF-1-dextran solution (most polymer-bound fluorescent dye such as FITC-BSA can be used) at a concentration of ~2 mg/mL with 0.6 mL each of 1 mol/L solutions of CaCl2 and Na2CO3 under fast stirring.
NOTE: Pay attention to the different sensitivities of fluorescent dyes to photobleaching; if a light-sensitive fluorescent probe (like SNARF-1) is used, the manipulation and storage of the microparticles must be performed with as little light as possible. - After 5-10 s of agitation, transfer the suspension to 2 mL microcentrifuge tubes and centrifuge for 15 s at 10,000-12,000 g to pellet CaCO3 microcores.
- Discard the supernatant, wash the cores with ~2 mL of deionized water, and resuspend the pellet by shaking.
- Repeat the centrifugation-washing procedure three times in total. After the last centrifugation, discard the supernatant.
- Incubate the microcores for 1 min in an ultrasonic bath to reduce their aggregation.
CAUTION: Do not forget to protect ears with headphones. - To deposit the first polymeric layer on the templates, resuspend the cores in ~2 mL of a 4 mg/mL solution of poly(allylamine hydrochloride) (PAH) in 1 mol/L NaCl.
- Keep the microcores in the solution for ~5 min with constant shaking.
- After 15 s of centrifugation, discard the supernatant with the unbound PAH. Wash the covered microcores with deionized water at least 3 times through multiple centrifugation and washing steps. After the last centrifugation, discard the supernatant.
- Incubate the microcores for 1 min in an ultrasonic bath to reduce their aggregation.
NOTE: If the applied fluorescent dye is cationic, start from poly(sodium 4-styrenesulfonate) (PSS) in 1 mol/L NaCl (see step 1.7).
- Repeat step 1.6 with ~2 mL of a 4 mg/mL solution of PSS (also containing 1 mol/L NaCl) to deposit the second polymeric layer on the templates.
- Repeat steps 1.6 and 1.7 six times to deposit 12 polymeric layers.
NOTE: It is not recommended to take a long break (~12 h or more) in the procedure until ~3-5 layers have been deposited because CaCO3 microcores without coverage may tend to recrystallize. Note that PSS coverage causes a higher aggregation of the microcores, and the long pause is advisable only when PAH or poly-L-lysine grafted with polyethylene glycol (PLL-g-PEG) is the outmost layer. - Incubate the covered microcores in 2 mg/mL PLL-g-PEG (~1 mL per microtube) for at least 2 h.
- Wash the microcores with water via sequential centrifugation and resuspension steps. After the last centrifugation, discard the supernatant.
- To obtain hollow microcapsules, dissolve the CaCO3 templates by adding 2 mL of 0.1 mol/L ethylenediaminetetraacetic acid (EDTA) solution (adjusted to pH 7.1 with NaOH) to the covered microcores.
- After ~5 min of incubation, centrifuge the microcapsules for 45 s and discard the supernatant with the EDTA.
- Repeat steps 1.10-1.10.1 twice.
- Wash the microcapsules with 0.9% NaCl three times through multiple centrifugation steps within 45 s followed by washing steps. After the last centrifugation step, discard the supernatant.
NOTE: The final microcapsule solution for injection must be kept sterile (for example by adding ampicillin, 0.1 mg/mL), and the media should be biocompatible with the object of investigation (isotonic media with neutral pH). - Estimate the concentration of the prepared microcapsules in a hemocytometer under a fluorescence microscope. Take a series of pictures of the microcapsules, measure the diameter of about a hundred microcapsules using ImageJ9 or equivalent software, and investigate the size distribution using a histogram.
- Store the obtained encapsulated probe in the dark.
NOTE: After several washings in sterile 0.9% NaCl, the microcapsules can be stored for months at 4 °C. Complete drying of the microcapsules during storage is not recommended.
2. Preparation of Optical Setup and Calibration of Microencapsulated SNARF-1
Note: Rough pH measurements with microencapsulated SNARF-1 can be made using images in two channels of a fluorescent microscope7, but in this protocol a one-channel fluorescent microscope connected to a fiber spectrometer was applied.
- Place the required set of fluorescence filters to the fluorescent microscope according to the characteristics of the applied fluorescent dye and turn on the fluorescent lamp.
- Pull out the lever to the eyepieces.
CAUTION: Excess light can damage the spectrometer matrix. Thus, make sure that the lever is in the "eyepiece" mode when the spectrometer is not used. - Connect one end of the optical fiber to the spectrometer and the other end to a collimator. Using adapters, place the collimator in the focus of the camera tube or other available port of the fluorescent microscope.
- Turn on the spectrometer. Run the spectrometer control program and prepare the spectrometer for measurements.
- Pull out the lever to the eyepieces.
- For calibration of the microcapsule batch, place ~5 µL of the microcapsule suspension (~10 000 microcapsules per µL in deionized water) on a microscope slide, and dry the drop in a dark place (for example, in a thermostat at 35 °C).
- To calibrate the spectral characteristics of the microencapsulated SNARF-1, use a series of buffers with different pH values in the range ~6-9. Drop ~10 µL of a buffer onto the dried microcapsules with SNARF-1-dextran and cover it with a coverslip.
- Place the glass slide on the microscope stage. Locate the microcapsules using a ×40 objective.
- Turn the microscope lever to the camera port. Register their fluorescence with the spectrometer.Turn the lever back to the eyepieces.
NOTE: Make sure the spectral signal is far beyond the background level, and ensure that the microcapsules in the field of view are not in a bubble (by switching to a lower magnification if necessary). Avoid prolonged illumination of the same microcapsules. SNARF-1 is sensitive to photobleaching. - Repeat step 2.2.3 for different microcapsules 10-15 times.
- Calculate the fluorescence peak ratios (for example, using R or Scilab) for all registered spectra and determine the regression line between the median ratio (for each buffer) and medium pH using the following formula:
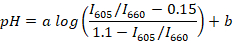
NOTE: SNARF-1 has a spectrum with two peaks corresponding to the emission of the protonated and deprotonated dye, and the ratio between the peaks is responsive to the pH of the medium. In this study, the ratio between the fluorescence intensity for 605 and 660 nm is used. These wavelengths are chosen depending on the filter set used. a and b are coefficients to be determined by non-linear regression (for example, using R). Values 0.15 and 1.1 are, respectively, the minimum and maximum values of I605/I660 observed during the calibration. - Collect about 10 µL of fish blood from approximately 5 adult animals. Place fish into a Petri dish with 1 µL/mL water suspension of clove oil for anesthesia and wait until the animal turns on its side and stops responding to pinches of the fin (usually ~2-3 min). Transfer the fish on a glass slide. Cut off the fish tail with a lancet and collect approximately 2 µL of fish blood from the tail vein.
NOTE: To prevent blood clotting, treat the incision with heparin (5000 U/mL) and use heparinized glass capillaries and microcentrifuge tubes to collect the blood.- Drip about 10 µL of blood with a pipette onto the tip of the microelectrode and determine the pH using a pH-meter.
- Drop the blood onto a slide with dried microcapsules and register the ratio of the fluorescence intensity as described for the calibration buffers (steps 2.2 - 2.3).
- Adjust the linear coefficient of the calibration curve to make the curve match the measurements in the fish blood (for more details see Borvinskaya et al. 20175).
3. Preparation for Injection
- Release the steel needle from the tip of the insulin pen (or syringe) by removing the plastic with a sharp lancet.
NOTE: Any thin needle (Ø0.33 mm or less) or glass capillary (usually Ø1 mm) can be prepared for microinjection10,11. - Insert the needle halfway into the glass microcapillary; quickly and gently solder it using a gas torch.
- Connect the glass microcapillary to the microinjector and flush it with sterile water three times. Ensure that the liquid flows through the needle.
- Fill the system with distilled water.
NOTE: Make sure there are no bubbles in the system.
4. Injection
- Resuspend the prepared suspension of microcapsules (in sterile 0.9% NaCl, or any other media used for the injections, with a concentration of 0.5 to 6 million microcapsules per microliter) using the ultrasonic bath for 1 min.
NOTE: Since the microcapsules tend to precipitate, during the following injection, shake the vial with the microcapsules mechanically (using a rotor) or manually every few minutes to resuspend them and prevent their aggregation. - Place the fish into a Petri dish with anesthetic (0.1 mL/L of clove oil suspended in water) for ~2-3 min. Wait until the fish turns on its side and stops responding to a light pinch of the fin.
- Using a spoon, transfer the fish out of the anesthetic and gently place it on a damp sponge in a lateral position with the head towards the left (for right-handed person) or towards the right (for left-handed person).
- Just before the injection, suck 1-2 mm of air into the glass capillary connected with microinjector. Then, load it with approximately 2 µL of the dispersed microcapsules.
NOTE: Before the injection, the microcapsule solution must be adjusted to the temperature at which the fish are kept. - Gently stabilize the body of the fish on the sponge with non-dominant hand.
- Find the lateral line of the fish. Mentally select a segment that extends from the operculum to the end of the abdominal cavity. Find the middle of this segment. Put the needle 1 mm lower in the ventral direction.
- With a scraping movement, gently move the fish scales aside, and make a puncture. Insert the needle into the body at an angle of 45° to the table surface.
- Push the needle toward the spine until it carefully rests against it.
- Release approximately 1 µL of the microcapsules' suspension into the kidney, and slowly withdraw the needle.
NOTE: To find the proper puncture site more easily, it is useful to practice finding the trunk kidney by transilluminating the fishes using a bottom light, as shown in Figure 2A and 2B.
- Rinse the fish from head to tail with a stream of water to remove any spilled microcapsules at the injection site.
5. In Vivo Visualization
- Use the dissection scissors to remove the gill cover from the fish head and denude the fish gills. Rinse the gills with water.
- Using a spoon, transfer the fish to a microscope slide, and place it on the stage of the fluorescent microscope.
NOTE: Make sure that the gills of the fish do not dry out during successive procedures. To avoid this, periodically moisten them with water using a Pasteur pipette (approximately every 1-2 min). - Darken the room and using low magnification (x10 objective) inspect the gills to find the fluorescent microcapsules.
NOTE: When the procedure is used for introduction of some fluorescent particles into fish circulatory system, it is recommended to inspect gills of several individuals for unexpected fluorescent particles before injections. Gills of wild-type zebrafish do not have autofluorescence, but in some cases sporadic fluorescent particles (like food pieces or unicellular symbionts) may be present on gills. If necessary, such particles can be recognized based on their specific shape (for example, food pieces have irregular shape, unlike spherical microcapsules) or fluorescence spectrum (i.e., color).- Switch the lens to a higher magnitude (×40 objective), and position a microcapsule or a group of microcapsules in the center of the field of view.
- Turn the lever to the port with a connected spectrometer. Record the spectral signal.
- Turn the lever back to the eyepiece.
- Repeat the measurements for different microcapsules several times.
- Transfer the fish to the aquarium with proper aeration for recovery.
NOTE: With minimal practice, it is possible to perform the injection and signal recording at an approximate rate of 2-3 min per fish. The measurement can be repeated for one individual several times with the use of repeated low, harmless doses of anesthesia or another method of fixation. For long-term observation, use a system with continuous anesthesia12.
Wyniki
The obtained results come from one of the three main categories of the presented protocol: the formation of fluorescent microparticles by encapsulation of a fluorescent dye (Figure 1), the kidney injection of microcapsules with further visualization in gill capillaries (Figure 2 and 3) and, finally, the in vivo spectral recording of SNARF-1 fluorescence to monitor blood pH leve...
Dyskusje
To demonstrate the injection of microparticles into the zebrafish kidney, we used semi-permeable microcapsules loaded with an indicator dye. Thus, the protocol contains instructions for the fabrication of the microcapsules using the layer-by-layer assembly of oppositely charged polyelectrolytes7,8,15,16,17,18 (
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Authors greatly acknowledge the help of Bogdan Osadchiy and Evgenii Protasov (Irkutsk State University, Russia) in preparation of the video protocol. This research was supported by the Russian Science Foundation (#15-14-10008) and the Russian Foundation for Basic Research (#15-29-01003).
Materiały
| Name | Company | Catalog Number | Comments |
| SNARF-1-dextran, 70000 MW | Thermo Fisher Scientific | D3304 | Fluorescent probe. Any other appropriate polymer-bound fluorescent dye can be used as a microcapsule filler |
| Albumin-fluorescein isothiocyanate conjugate (FITC-BSA) | SIGMA | A9771 | Fluorescent probe |
| Rhodamine B isothiocyanate-Dextran (RITC-dextran) | SIGMA | R9379 | Fluorescent probe |
| Calcium chloride | SIGMA | C1016 | CaCO3 templates formation |
| Sodium carbonate | SIGMA | S7795 | CaCO3 templates formation |
| Poly(allylamine hydrochloride), MW 50000 (PAH) | SIGMA | 283215 | Cationic polymer |
| Poly(sodium 4-styrenesulfonate), MW 70000 (PSS) | SIGMA | 243051 | Anionic polymer |
| Poly-L-lysine [20 kDa] grafted with polyethylene glycol [5 kDa], g = 3.0 to 4.5 (PLL-g-PEG) | SuSoS | PLL(20)-g[3.5]-PEG(5) | Final polymer to increase the biocompatibility of microcapsules |
| Sodium chloride | SIGMA | S8776 | To dissolve applied polymers |
| Water Purification System | Millipore | SIMSV0000 | To prepare deionized water |
| Magnetic stirrer | Stegler | For CaCO3 templates formation | |
| Eppendorf Research plus pipette, 1000 µL | Eppendorf | Dosing solutions | |
| Eppendorf Research plus pipette, 10 µL | Eppendorf | Dosing solutions | |
| Pipette tips, volume range 200 to 1000 µL | F.L. Medical | 28093 | Dosing solutions |
| Pipette tips, volume range 0.1-10 μL | Eppendorf | Z640069 | Dosing solutions |
| Mini-centrifuge Microspin 12, High-speed | BioSan | For microcapsule centrifugation-washing procedure | |
| Microcentrifuge tubes, 2 mL | Eppendorf | Z666513 | Microcapsule synthesis and storage |
| Shaker Intelli-mixer RM-1L | ELMY Ltd. | To reduce microcapsule aggregation | |
| Ultrasonic cleaner | To reduce microcapsule aggregation | ||
| Head phones | To protect ears from ultrasound | ||
| Ethylenediaminetetraacetic acid | SIGMA | EDS | To dissolve the CaCO3 templates |
| Monosodium phosphate | SIGMA | S9638 | Preparation of pH buffers |
| Disodium phosphate | SIGMA | S9390 | Preparation of pH buffers |
| Sodium hydroxide | SIGMA | S8045 | To adjust the pH of the EDTA solution and buffers |
| Thermostat chamber | To dry microcapsules on glass slide | ||
| Hemocytometer blood cell count chamber | To investigate the size distribution and concentration of the prepared microcapsules | ||
| Fluorescent microscope Mikmed 2 | LOMO | In vivo visualization of microcapsules in fish blood | |
| Set of fluorescent filters for SNARF-1 (should be chosen depending on the microscope model; example is provided) | Chroma | 79010 | Visualization of microcapsules with fluorescent probes |
| Fiber spectrometer QE Pro | Ocean Optics | Calibration of microcapsules under microscope | |
| Optical fiber QP400-2-VIS NIR, 400 μm, 2 m | Ocean Optics | To connect spectrometer with microscope port | |
| Collimator F280SMA-A | Thorlabs | To connect spectrometer with microscope port | |
| Glass microscope slide | Fisherbrand | 12-550-A3 | Calibration of microcapsules under microscope |
| Coverslips, 22 x 22 mm | Pearl | MS-SLIDCV | Calibration of microcapsules under microscope |
| Glass microcapillaries Intra MARK, 10 µL | Blaubrand | BR708709 | To collect fish blood |
| Clove oil | SIGMA | C8392 | Fish anesthesia |
| Lancet No 11 | Apexmed international B.V. | P00588 | To cut the fish tail and release the steel needle from the tip of insulin autoinjector |
| Heparin, 5000 U/mL | Calbiochem | L6510-BC | For treating all surfaces that come in contact with fish blood during fish blood collection |
| Seven 2 Go Pro pH-meter with a microelectrode | Mettler Toledo | To determine fish blood pH | |
| Insulin pen needles Micro-Fine Plus, 0.25 x 5 mm | Becton, Dickinson and Company | For injection procedure. Any thin needle (Ø 0.33 mm or less) is appropriate | |
| Glass capillaries, 1 x 75 mm | Hirschmann Laborgeräte GmbH & Co | 9201075 | For injection procedure |
| Gas torch | To solder steel needle to glass capillary | ||
| Microinjector IM-9B | NARISHIGE | For precise dosing of microcapsules suspension | |
| Petri dishes, 60 mm x 15 mm, polystyrene | SIGMA | P5481 | For manipulations with fish under anesthesia |
| Plastic spoon | For manipulations with fish under anesthesia | ||
| Damp sponge | For manipulations with fish under anesthesia | ||
| Dissection scissors | Thermo Scientific | 31212 | To remove the gill cover from the fish head |
| Pasteur pipette, 3.5 mL | BRAND | Z331767 | To moisten fish gills |
Odniesienia
- Rivas-Aravena, A., Sandino, A. M., Spencer, E. Nanoparticles and microparticles of polymers and polysaccharides to administer fish vaccines. Biol. Res. 46 (4), 407-419 (2013).
- Yashchenok, A. M., Jose, J., Trochet, P., Sukhorukov, G. B., Gorin, D. A. Multifunctional polyelectrolyte microcapsules as a contrast agent for photoacoustic imaging in blood. J. Biophotonics. 9 (8), 792-799 (2016).
- Pugach, E. K., Li, P., White, R., Zon, L. Retro-orbital injection in adult zebrafish. J. Vis. Exp. (34), e1645 (2009).
- Gurkov, A., Shchapova, &. #. 1. 0. 4. 5. ;., Bedulina, D., Baduev, B., Borvinskaya, E., Timofeyev, M. Remote in vivo stress assessment of aquatic animals with microencapsulated biomarkers for environmental monitoring. Sci. Rep. 6, e36427 (2016).
- Borvinskaya, E., Gurkov, A., Shchapova, E., Baduev, B., Shatilina, Z., Sadovoy, A., et al. Parallel in vivo monitoring of pH in gill capillaries and muscles of fishes using microencapsulated biomarkers. Biol. Open. 6 (5), 673-677 (2017).
- Diep, C. Q., Davidson, A. J. Transplantation of cells directly into the kidney of adult zebrafish. J. Vis. Exp. (51), e2725 (2011).
- Kreft, O., Javier, A. M., Sukhorukov, G. B., Parak, W. J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 17 (42), 4471-4476 (2007).
- Sadovoy, A., Teh, C., Korzh, V., Escobar, M., Meglinski, I. Microencapsulated bio-markers for assessment of stress conditions in aquatic organisms in vivo. Laser Phys. Lett. 9 (7), 542-546 (2012).
- Ferreira, T., Rasband, W. S. . ImageJ User Guide - Version 1.44. , (2012).
- Poland, R. S., Bull, C., Syed, W. A., Bowers, M. S. Rodent brain microinjection to study molecular substrates of motivated behavior. J. Vis. Exp. (103), e53018 (2015).
- Liu, L., Duff, K. A technique for serial collection of cerebrospinal fluid from the cisterna magna in mouse. J. Vis. Exp. (21), e960 (2008).
- Johnston, L., Ball, R. E., Acuff, S., Gaudet, J., Sornborger, A., Lauderdale, J. D. Electrophysiological recording in the brain of intact adult zebrafish. J. Vis. Exp. (81), e51065 (2013).
- Gerlach, G. F., Schrader, L. N., Wingert, R. A. Dissection of the adult zebrafish kidney. J. Vis. Exp. (54), e2839 (2011).
- McKee, R. A., Wingert, R. A. Zebrafish renal pathology: Emerging models of acute kidney injury. Curr Pathobiol Rep. 3 (2), 171-181 (2015).
- Donath, E., Sukhorukov, G. B., Caruso, F., Davi, S. A., Möhwald, H. Novel hollow polymer shells by colloid-templated assembly of polyelectrolytes. Angew. Chem. Int. Ed. 37 (17), 2201-2205 (1998).
- Antipov, A. A., Shchukin, D., Fedutik, Y., Petrov, A. I., Sukhorukov, G. B., Möhwald, H. Carbonate microparticles for hollow polyelectrolyte capsules fabrication. Colloids Surf. A. 224, 175-183 (2003).
- Gaponik, N., Radtchenko, I. L., Gerstenberger, M. R., Fedutik, Y. A., Sukhorukov, G. B., Rogach, A. L. Labeling of biocompatible polymer microcapsules with near-infrared emitting nanocrystals. Nano Lett. 3 (3), 369-372 (2003).
- Volodkin, D. V., Larionova, N. I., Sukhorukov, G. B. Protein encapsulation via porous CaCO3 microparticles templating. Biomacromolecules. 5 (5), 1962-1972 (2004).
- Tzaneva, V., Perry, S. F. A Time differential staining technique coupled with full bilateral gill denervation to study ionocytes in fish. J. Vis. Exp. (97), e52548 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone