Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Obtaining Cancer Stem Cell Spheres from Gynecological and Breast Cancer Tumors
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
The aim of this methodology is to identify cancer stem cells (CSC) in cancer cell lines and primary human tumor samples with the sphere-forming protocol, in a robust manner, using functional assays and phenotypic characterization with flow cytometry and Western blot.
Streszczenie
Cancer stem cells (CSC) are a small population with self-renewal and plasticity which are responsible for tumorigenesis, resistance to treatment and recurrent disease. This population can be identified by surface markers, enzymatic activity and a functional profile. These approaches per se are limited, due to phenotypic heterogeneity and CSC plasticity. Here, we update the sphere-forming protocol to obtain CSC spheres from breast and gynecological cancers, assessing functional properties, CSC markers and protein expression. The spheres are obtained with single-cell seeding at low density in suspension culture, using a semi-solid methylcellulose medium to avoid migration and aggregates. This profitable protocol can be used in cancer cell lines but also in primary tumors. The tridimensional non-adherent suspension culture thought to mimic the tumor microenvironment, particularly the CSC-niche, is supplemented with epidermal growth factor and basic fibroblast growth factor to ensure CSC signaling. Aiming for robust identification of CSC, we propose a complementary approach, combining functional and phenotypic evaluation. Sphere-forming capacity, self-renewal and sphere projection area establish CSC functional properties. Additionally, characterization comprises flow cytometry evaluation of the markers, represented by CD44+/CD24- and CD133, and Western blot, considering ALDH. The presented protocol was also optimized for primary tumor samples, following a sample digestion procedure, useful for translational research.
Wprowadzenie
Cancer populations are heterogeneous, with cells presenting different morphologies, proliferation and invasion capacity, due to differential gene expression. Among these cells, a minority population exists named cancer stem cells (CSC)1, which have the capacity for self-renewal, recapitulating the heterogeneity of the primary tumor niche and producing aberrantly differentiating progenitors that do not respond adequately to homeostatic controls2. CSC properties can be directly translated in clinical practice, given the association with events, such as tumorigenicity or resistance to chemotherapy3. The identification of CSC can lead to the development of targeted therapies that may include blockage of surface markers, promotion of CSC differentiation, blocking of CSC signaling pathway components, niche destruction, and epigenetic mechanisms4.
The isolation of CSC has been performed in cells lines and in samples of primary tumors5,6,7,8. The functional profile described for CSC includes clonogenic capacity, side population and tumorosphere formation9. The CD44high/CD24low phenotype has been consistently associated with breast CSC, which has proved to be tumorigenic in vivo and has been already associated with epithelial to mesenchymal transition5,10. High ALDH activity has also been associated with stemness and epithelial to mesenchymal transition (EMT) in several types of solid tumors11. ALDH expression has been associated with resistance to chemotherapy and to CSC phenotype in vitro12,13,14,15,16. Several other markers have been linked to CSC properties in different types of tumors, such as CD133, CD49f, ITGA6, CD1663,4 and others, as described in Table 1.
The tumorspheres consist of a three-dimensional model for the study and expansion of CSC. In this model, the cell suspensions from cell lines and from blood or tumor samples are cultivated in a medium supplemented with growth factors, namely epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF), without fetal bovine serum and in non-adherent conditions17. Inhibition of cell adhesion results in death by anoikis of differentiated cells18. Spheres are derived from the clonal growth of an isolated cell. For this purpose, the cells are distributed at low density to avoid cell fusion and aggregation19. Another strategy includes the use of semisolid methylcellulose20.
The sphere-forming protocol gained popularity in CSC isolation and expansion, due to time and cost and technical, profitable, and reproducible reasons21,22. Despite some reserves on the extent of which sphere formation reflects CSC, there is a propensity of stem cells to grow in non-adherent conditions with the characteristic phenotype, which resembles the native microenvironment21. None of the methods available for isolation of CSC from solid tumors has complete efficiency, highlighting the importance of developing more specific markers or combinations of methodologies and markers.
In this protocol, we detail the isolation of CSC with the sphere-forming protocol, with the principle of single-cell growth in non-adherent conditions and the capacity to produce a differentiated phenotype. A schematic representation of this procedure is represented in Figure 1. We also describe the characterization with surface markers and ALDH expression for CSC, both for breast and gynecological tumor cells lines and samples of primary tumors.
Protokół
This protocol was performed complying with the ethical guidelines of the Coimbra Hospital and Universitary Center (CHUC) Tumor Bank, and was approved by CHUC's Ethics Committee for Health and by the Portuguese National Data Protection Commission.
1. Sphere-forming Protocol and Derived Adherent Populations from Continuous Cell Cultures
NOTE: Perform all procedures under strict sterile conditions.
- Preparation of non-adherent suspension culture flasks or plates by coating the growth surface with poly(2-hydroxyethyl-methacrylate (poly-HEMA)
- Prepare a 15 mg/mL solution by stirring poly-HEMA in absolute ethanol at 65 °C. Coat cell culture flasks or plates with 50 μL/cm2.
- Leave to dry at 37 °C in a drying oven. If necessary, wrap the plates and store at room temperature.
- Preparation of the sphere culturing media (SCM)
- Prepare a 2% solution of methylcellulose in ultrapure water and sterilize in the autoclave. Methylcellulose tends to be easier to solubilize by cooling; therefore disperse the powder in water at 80 °C and stir until cooled23.
- Prepare a two-times concentrated solution of SCM (stock solution). SCM working solution contains DMEM-F12, supplemented with 100 mM putrescine, 1% insulin, transferrin, selenium and 1% antibiotic-antimycotic solution (10,000 U/mL penicillin, 10 mg/mL streptomycin and 25 μg/mL amphotericin B).
- To prepare the SCM, mix equal volumes of the SCM stock solution with the 2% solution of methylcellulose.
- Complete the medium immediately prior to use by adding 10 ng/mL epidermal growth factor (EFG) and 10 ng/mL basic fibroblast growth factor (bFGF).
- If more fastidious cell lines are in use, supplement the medium with 0.4% bovine serum albumin, which might be an advantage.
- Start with a flask of MCF7 or HCC1806 breast cancer or ECC-1 or RL95-2 endometrial cancer cells (or other cancer cell line of choice) with 80% to 90% confluence.
- Discard the cell culture media, wash with phosphate buffered saline solution (PBS) and detach the cells with trypsin-EDTA (1 to 2 mL for a 75 cm2 cell culture flask).
- Add cell culture media (2 to 4 mL for a 75 cm2 cell culture flask) and centrifuge at 200 x g for 5 min to discard enzymes.
- Suspend the pellet in a known volume of cell culture media and pipette up and down to ensure a single cell suspension. For this purpose, a 40 μm cell strainer can be used.
- Count the cells in the hemocytometer and calculate the cell concentration of the cell suspension. Take advantage of this step to ensure observation of a single cell suspension. Careful cell counting is essential to accurately quantify the effects of treatments.
- Suspend the determined amount of cell suspension in SCM complete medium and transfer to poly-HEMA coated dishes. As a reference value for seeding density, consider 500 to 2000 cells/cm2.
NOTE: Optimization of seeding density and time of culture for each cell line is highly recommended24. - Incubate at 37 °C and 5% CO2 for 2 days without disturbing the plates.
- Re-establish the concentration of growth factors by adding 10 ng/mL EFG and 10 ng/mL bFGF to the cell culture media. Repeat this step every two days.
- Incubate at 37 °C and 5% CO2 until 5 days after plating (this can vary from 3 to 12 days according to the cell line) to obtain spheres, which present the morphology of suspension ball-shaped cell colonies.
- Use or collect the spheres, by pipetting, for the experiments.
- To obtain derived adherent populations, place the spheres into standard culture conditions, respective of the cell line used. 1 to 2 days later, it is possible to observe a monolayer of cells growing around adherent spheres, which presents a morphology similar to the cell line of origin.
2. Sphere-forming Protocol from Human Tumor Samples
NOTE: The use of human samples for research purposes must comply with each country's legislation, and to be approved by the Ethics Committee of the Institutions involved.
- Prepare the transport media containing DMEM/F12, supplemented with 10% fetal bovine serum (FBS) and 2% antibiotic-antimycotic solution (10,000 U/mL penicillin, 10 mg/mL streptomycin and 25 μg/mL amphotericin B).
- Prepare the digestion media containing DMEM/F12, supplemented with 10% FBS, 1% antibiotic antimycotic solution, 1 mg/mL type IV collagenase and 100 μg/mL DNAse I.
- Prepare the enzyme inactivation media containing DMEM/F12, supplemented with 10% FBS and 1% antibiotic-antimycotic solution (10,000 U/mL penicillin, 10 mg/mL streptomycin and 25 μg/mL amphotericin B).
- Prepare the SCM as described in section 1.2.
- Obtain the sample during the macroscopic study of the operative piece as soon as possible after surgical removal.
- Place the samples in transport media and transfer them to the laboratory for where processing. Sample processing should begin within 1 h following collection to improve the success rate of the procedure. Apply caution in sample collection. Handle the samples carefully. Avoid the use of necrotic or cauterized zones.
- Under the sterile flow chamber, transfer the sample to a dish and cut into smaller pieces (around 1 mm3) with a scalpel.
- Incubate the human tissue in a tube with digestion media in a rotating shaker up to 180 min, at 37 °C. Identify this tube as Tube A.
- Replace the enzyme solution every 15 min.
- Collect the digestion media (without removing any tissue fragments) and transfer it through a 40 μm cell strainer to a new tube half-filled with enzyme inactivation media. Maintain this tube at room temperature and identify it as Tube B.
- Add new digestion media to Tube A and return it to the rotating shaker at 37 °C.
- At each collection, check cell viability using the trypan blue exclusion method.
- Repeat this procedure for 180 min or until cell count is significantly lower.
- Incubate the tissue fragments in Tube A in a second digestion solution containing equal parts of accutase and trypsin-EDTA, stirring for 10 min at 37 °C.
- Add the enzyme inactivation media to Tube A and filter the contents through a 40 μm cell strainer into Tube B.
- Centrifuge the cell suspension in Tube B at 200 x g for 10 min.
- Suspend the pellet in SCM and check cell concentration using a hemocytometer.
- Suspend the determined amount of cell suspension in SCM and transfer to poly-HEMA coated dishes (see step 1.1) with a seeding density of 4000 cells/cm2.
- Incubate at 37 °C and 5% CO2 for 2 days without disturbing the plates.
- Re-establish the concentration of growth factors by adding 10 ng/mL EFG and 10 ng/mL bFGF to the cell culture media.
NOTE: You must do this every two days. - Incubate at 37 °C and 5% CO2 until 5 days after plating (this can vary up to 12 days) to obtain spheres, which present the morphology of suspension ball-shaped cell colonies.
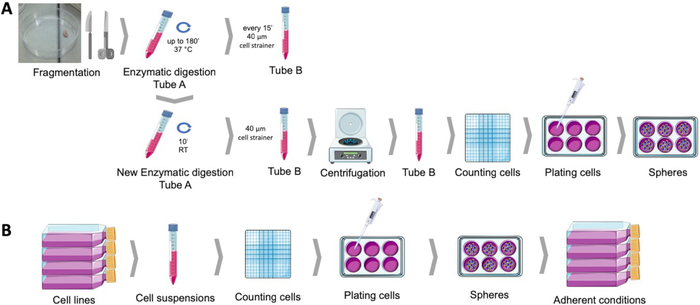
Figure 1: Obtaining cancer stem cells from human endometrial tumor samples (A) and breast and gynecological cancer cell lines (B). Human tumor samples are fragmented, enzymatically digested and plated in sphere culturing medium into poly-HEMA coated dishes. Cancer cell lines are detached, cell suspensions are counted, and single cells are distributed at low density into poly-HEMA coated plates under appropriate conditions. The spheres obtained, when placed under adherent culture conditions, produce derived adherent populations. Please click here to view a larger version of this figure.
3. Sphere-forming Capacity, Self-renewal, and Sphere Projection Area
NOTE: Sphere-forming capacity is the ability of a tumor cell population to produce spheres. Self-renewal is the ability of sphere cells to produce new colonies of spherical cells in suspension. The sphere projection area is representative of the area occupied by the sphere and therefore expressive of their size and the number of cell divisions undergone in a certain time period.
- Determining the sphere-forming capacity
- After completion of the sphere-forming protocol, collect the spheres in a centrifuge tube and centrifuge at 125 x g for 5 min.
- Discard the SCM and gently suspend the pellet in a known volume of fresh media. With the aim of concentrating the spheres to facilitate counting, suspend the spheres in a small media volume. Be careful not to disturb the spheres.
- Use a hemocytometer to count the spheres with more than 40 μm in diameter. Alternatively, spheres can be counted directly on the plate by using a microscope equipped with a graticule25 or using an automated system26,27.
- Calculate the percentage ratio of spheres obtained vs. the number of cells initially plated.
- Determining self-renewal
- After completion of the sphere-forming protocol, collect the spheres in a centrifuge tube and centrifuge at 125 x g for 5 min.
- Discard the sphere culturing media and gently suspend the pellet in trypsin-EDTA.
- Incubate up to 5 min at 37 °C.
- Add enzyme inactivation media and pipette up and down to ensure a single cell suspension.
- Using a hemocytometer and the trypan blue exclusion method, count the viable cells in the suspension.
- Initiate the sphere-forming protocol as described in section 1.
- After 8 days, use a hemocytometer to count the spheres with more than 40 μm in diameter.
- Calculate the percentage ratio of spheres obtained vs. the number of cells initially plated.
- Determining the sphere projection area
- To evaluate the area occupied by the spheres, obtain images of at least 10 random fields per condition, in an inverted microscope equipped with an image acquisition module. A magnification of 100X to 400X is recommended.
- Analyze images using imaging software, such as ImageJ software28, by drawing areas of interest corresponding to the spheres and measuring its area in pixels.
- Calculate sphere projection area as the mean area of pixels measured.
4. Cancer Stem Cell Marker Assessment with Flow Cytometry
NOTE: CD44+/CD24-/low phenotype was consistently associated with breast and gynecological cancer stem cells. The procedure described may be used to evaluate this and other cell surface markers.
- After completion of the sphere-forming protocol, collect the spheres in a centrifuge tube and centrifuge at 125 x g for 5 min.
- Discard the SCM and gently suspend the pellet in trypsin-EDTA.
- Incubate up to 5 min at 37 °C.
- Add enzyme inactivation media and pipette up and down to ensure a single cell suspension.
- Centrifuge at 125 x g for 5 min, discard the supernatant and gently suspend the cells in PBS.
- Allow the cells to rest in suspension for 30 min to ensure recovery of the membrane conformation.
- Using a hemocytometer and the trypan blue exclusion method, count the cells in the suspension.
- Adjust the cell suspension volume to 106 cells/500 µL.
- Incubate with the monoclonal antibodies according to the instructions of the suppliers (concentration, time, temperature, and light/dark) and considering the experiment set represented in Table 2 or the markers given in Table 1.
- Immediately after staining, perform the flow cytometric analysis using a flow cytometer with appropriate detection modules.
- Standardize cytometer setup, following protocols established by the EuroFlow Consortium29.
- Set up primary gates based on the forward and side scatter excluding debris and dead cells. This can be improved by concomitant labelling with annexin V and gating negative cells.
- Set fluorescence gates based on the unstained samples and compensation for a spectral overlap using single stained controls.
5. Cancer Stem Cell Marker Assessment with Western Blot
NOTE: In addition to ALDH1 activity, high expression of this marker was consistently associated with breast and gynecological cancer stem cells13,14. The procedure described may be used to evaluate this and other cell markers.
- After completion of the sphere-forming protocol, collect the spheres in a centrifuge tube and centrifuge at 125 x g for 5 min.
- Preparation of the whole cell lysates
- Place the centrifuge tubes on ice and discard the supernatant without disrupting the pellet.
- Wash the pellet with 1 mL of cold PBS and discard by centrifugation.
- Suspend the pellet in a small volume (200-500 µL) of RIPA lysis buffer30 (NaCl 150 mM, Tris-HCl 1.50 mM pH 7.4, Triton-X100 1% vol./vol., sodium deoxycholic acid 0.5% wt./vol., sodium dodecyl sulfate 0.5% wt./vol.) supplemented with cOmplete Mini and dithiothreitol 1 mM.
- Maintaining the samples cold (on ice), submit them to vortex and sonication with a 30% amplitude.
- Centrifuge the samples for 15 min at 14,000 x g in a refrigerated centrifuge set to 4 °C.
- Transfer the supernatants to new, properly identified microtubes.
- Determine the protein concentrations using the BCA or Bradford assays31.
- If necessary, store the samples at -80 °C until further western blot analysis.
- Perform sample denaturation, electrophoresis, electron transfer and protein detection according to standard western blotting protocols, as described32,33,34.
Wyniki
The sphere-forming protocol allows spherical colonies to be obtained in suspension from several endometrial and breast cancer cell lines (Figure 2A) or after gentle enzymatic digestion of tissue from human tumor samples (Figure 2E). In both cases, a few days after plating, monoclonal spherical colonies in suspension are obtained. Both endometrial and breast cancer spheres give rise to a cell monolayer with similar morphology to ...
Dyskusje
This protocol details an approach to obtain tumorspheres from cancer cell lines and primary human samples. Tumorspheres are enriched in a sub-population with stem cell-like properties36. This enrichment in CSC is dependent on viability in an anchorage-free environment while differentiated cells are reliant on adhesion to a substrate37. As primary plating of tumor cells in a low adherence environment that imposes suspension does not ensure enrichment in CSC per se, we provid...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This study was funded by the Portuguese Society of Gynecology through the 2016 Research Prize and by CIMAGO. CNC.IBILI is supported through the Foundation for Science and Technology, Portugal (UID/NEU/04539/2013), and co-funded by FEDER-COMPETE (POCI-01-0145-FEDER-007440). The Coimbra Hospital and Universitary Center (CHUC) Tumor Bank, approved by CHUC's Ethics Committee for Health and by the Portuguese National Data Protection Commission, was the source of endometrial samples of patients followed at the institution's Gynecology Service. Figure 1 was produced using Servier Medical Art, available from www.servier.com.
Materiały
| Name | Company | Catalog Number | Comments |
| Absolute ethanol | Merck Millipore | 100983 | |
| Accutase | Gibco | A1110501 | StemPro Accutas Cell Dissociation Reagent |
| ALDH antibody | Santa Cruz Biotechnology | SC166362 | |
| Annexin V FITC | BD Biosciences | 556547 | |
| Antibiotic antimycotic solution | Sigma | A5955 | |
| BCA assay | Thermo Scientific | 23225 | Pierce BCA Protein Assay Kit |
| Bovine serum albumin | Sigma | A9418 | |
| CD133 antibody | Miteny Biotec | 293C3-APC | Allophycocyanin (APC) |
| CD24 antibody | BD Biosciences | 658331 | Allophycocyanin-H7 (APC-H7) |
| CD44 antibody | Biolegend | 103020 | Pacific Blue (PB) |
| Cell strainer | BD Falcon | 352340 | 40 µM |
| Collagenase, type IV | Gibco | 17104-019 | |
| cOmplete Mini | Roche | 118 361 700 0 | |
| Dithiothreitol | Sigma | 43815 | |
| DMEM-F12 | Sigma | D8900 | |
| DNAse I | Roche | 11284932001 | |
| ECC-1 | ATCC | CRL-2923 | Human endometrium adenocarcinoma cell line |
| Epidermal growth factor | Sigma | E9644 | |
| Fibroblast growth factor basic | Sigma | F0291 | |
| Haemocytometer | VWR | HERE1080339 | |
| HCC1806 | ATCC | CRL-2335 | Human mammary squamous cell carcinoma cell line |
| Insulin, transferrin, selenium Solution | Gibco | 41400045 | |
| MCF7 | ATCC | HTB-22 | Human mammary adenocarcinoma cell line |
| Methylcellulose | AlfaAesar | 45490 | |
| NaCl | JMGS | 37040005002212 | |
| Poly(2-hydroxyethyl-methacrylate | Sigma | P3932 | |
| Putrescine | Sigma | P7505 | |
| RL95-2 | ATCC | CRL-1671 | Human endometrium carcinoma cell line |
| Sodium deoxycholic acid | JMS | EINECS 206-132-7 | |
| Sodium dodecyl sulfate | Sigma | 436143 | |
| Tris | JMGS | 20360000BP152112 | |
| Triton-X 100 | Merck | 108603 | |
| Trypan blue | Sigma | T8154 | |
| Trypsin-EDTA | Sigma | T4049 | |
| ß-actin antibody | Sigma | A5316 |
Odniesienia
- Hardin, H., Zhang, R., Helein, H., Buehler, D., Guo, Z., Lloyd, R. V. The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors. Laboratory Investigation. 97 (10), 1142 (2017).
- Plaks, V., Kong, N., Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells?. Cell Stem Cell. 16 (3), 225-238 (2015).
- Visvader, J. E., Lindeman, G. J. Cancer stem cells in solid tumours accumulating evidence and unresolved questions. Nature reviews. Cancer. 8, 755-768 (2008).
- Allegra, A., et al. The Cancer Stem Cell Hypothesis: A Guide to Potential Molecular Targets. Cancer Investigation. 32 (9), 470-495 (2014).
- Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences. 100 (7), 3983-3988 (2003).
- Friel, A. M., et al. Functional analyses of the cancer stem cell-like properties of human endometrial tumor initiating cells. Cell Cycle. 7 (2), 242-249 (2008).
- Zhang, S., et al. Identification and Characterization of Ovarian Cancer-Initiating Cells from Primary Human Tumors. Cancer Research. 68 (11), 4311-4320 (2008).
- Bapat, S. A., Mali, A. M., Koppikar, C. B., Kurrey, N. K. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer research. 65 (8), 3025-3029 (2005).
- Carvalho, M. J., Laranjo, M., Abrantes, A. M., Torgal, I., Botelho, M. F., Oliveira, C. F. Clinical translation for endometrial cancer stem cells hypothesis. Cancer and Metastasis Reviews. 34 (3), 401-416 (2015).
- Morel, A. P., Lièvre, M., Thomas, C., Hinkal, G., Ansieau, S., Puisieux, A. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. PLoS ONE. 3 (8), e2888 (2008).
- Tirino, V., et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. The FASEB Journal. 27 (1), 13 (2013).
- Ajani, J. A., et al. ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Molecular Oncology. 8 (1), 142-149 (2014).
- Carvalho, M. J., et al. Endometrial Cancer Spheres Show Cancer Stem Cells Phenotype and Preference for Oxidative Metabolism. Pathology and Oncology Research. , (2018).
- Laranjo, M., et al. Mammospheres of hormonal receptor positive breast cancer diverge to triple-negative phenotype. The Breast. 38, 22-29 (2018).
- Cui, M., et al. Non-Coding RNA Pvt1 Promotes Cancer Stem Cell–Like Traits in Nasopharyngeal Cancer via Inhibiting miR-1207. Pathology & Oncology Research. , (2018).
- Deng, S., et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 ALDH1), in human epithelial cancers. PloS one. 5 (4), e10277 (2010).
- Weiswald, L. B., Guinebretière, J. M., Richon, S., Bellet, D., Saubaméa, B., Dangles-Marie, V. In situ protein expression in tumour spheres: development of an immunostaining protocol for confocal microscopy. BMC Cancer. 10 (1), 106 (2010).
- Weiswald, L. B., Bellet, D., Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia. 17 (1), 1-15 (2015).
- Picon-Ruiz, M., et al. Low adherent cancer cell subpopulations are enriched in tumorigenic and metastatic epithelial-to-mesenchymal transition-induced cancer stem-like cells. Scientific Reports. 6 (1), 1-13 (2016).
- Dontu, G., et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes & development. 17 (10), 1253-1270 (2003).
- Ballout, F., et al. Sphere-Formation Assay: Three-Dimensional in vitro Culturing of Prostate Cancer Stem/Progenitor Sphere-Forming Cells. Frontiers in Oncology. 8 (August), 1-14 (2018).
- Ishiguro, T., Ohata, H., Sato, A., Yamawaki, K., Enomoto, T., Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Science. 108 (3), 283-289 (2017).
- Noseda, M., Nasatto, P., Silveira, J., Pignon, F., Rinaudo, M., Duarte, M. Methylcellulose, a Cellulose Derivative with Original Physical Properties and Extended Applications. Polymers. 7 (5), 777-803 (2015).
- Shaw, F. L., et al. A detailed mammosphere assay protocol for the quantification of breast stem cell activity. Journal of Mammary Gland Biology and Neoplasia. 17 (2), 111-117 (2012).
- Zhou, M., et al. LncRNA-Hh Strengthen Cancer Stem Cells Generation in Twist-Positive Breast Cancer via Activation of Hedgehog Signaling Pathway. Stem cells (Dayton, Ohio). 34 (1), 55-66 (2016).
- Ha, J. R., et al. Integration of Distinct ShcA Signaling Complexes Promotes Breast Tumor Growth and Tyrosine Kinase Inhibitor Resistance. Molecular cancer research MCR. 16 (5), 894-908 (2018).
- Jurmeister, S., et al. Identification of potential therapeutic targets in prostate cancer through a cross-species approach. EMBO molecular medicine. 10 (3), (2018).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 9 (7), 671-675 (2012).
- Kalina, T., et al. EuroFlow standardization of flow cytometer instrument settings and immunophenotyping protocols. Leukemia. 26 (9), 1986-2010 (2012).
- Peach, M., Marsh, N., Miskiewicz, E. I., MacPhee, D. J. . Solubilization of Proteins: The Importance of Lysis Buffer Choice. , 49-60 (2015).
- Olson, B. J. S. C. Assays for Determination of Protein Concentration. Current Protocols in Pharmacology. , A.3A.1-A.3A.32 (2016).
- Eslami, A., Lujan, J. Western Blotting: Sample Preparation to Detection. Journal of Visualized Experiments. (44), 1-2 (2010).
- Silva, J. M., McMahon, M. The Fastest Western in Town: A Contemporary Twist on the Classic Western Blot Analysis. Journal of Visualized Experiments. 84 (84), 1-8 (2014).
- Oldknow, K. J., et al. A Guide to Modern Quantitative Fluorescent Western Blotting with Troubleshooting Strategies. Journal of Visualized Experiments. 8 (93), 1-10 (2014).
- Serambeque, B. . Células estaminais do cancro do endométrio - a chave para o tratamento personalizado? [Stem Cells of Endometrial Cancer: The Key to Personalized Treatment?]. , (2018).
- Lee, C. H., Yu, C. C., Wang, B. Y., Chang, W. W. Tumorsphere as an effective in vitro platform for screening anti-cancer stem cell drugs. Oncotarget. 7 (2), (2015).
- De Luca, A., et al. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 6 (17), (2015).
- Masuda, A., et al. An improved method for isolation of epithelial and stromal cells from the human endometrium. Journal of Reproduction and Development. 62 (2), 213-218 (2016).
- Del Rio-Tsonis, K., et al. Facile isolation and the characterization of human retinal stem cells. Proceedings of the National Academy of Sciences. 101 (44), 15772-15777 (2004).
- Wang, L., Guo, H., Lin, C., Yang, L., Wang, X. I. Enrichment and characterization of cancer stem-like cells from a cervical cancer cell line. Molecular Medicine Reports. 9 (6), 2117-2123 (2014).
- Chen, Y. C., et al. High-throughput single-cell derived sphere formation for cancer stem-like cell identification and analysis. Scientific Reports. 6 (April), 1-12 (2016).
- Kim, J., Jung, J., Lee, S. J., Lee, J. S., Park, M. J. Cancer stem-like cells persist in established cell lines through autocrine activation of EGFR signaling. Oncology Letters. 3 (3), 607-612 (2012).
- Hwang-Verslues, W. W., et al. Multiple Lineages of Human Breast Cancer Stem/Progenitor Cells Identified by Profiling with Stem Cell Markers. PloS one. 4 (12), e8377 (2009).
- Feng, Y., et al. Metformin reverses stem cell-like HepG2 sphere formation and resistance to sorafenib by attenuating epithelial-mesenchymal transformation. Molecular Medicine Reports. 18 (4), 3866-3872 (2018).
- Wang, H., Paczulla, A., Lengerke, C. Evaluation of Stem Cell Properties in Human Ovarian Carcinoma Cells Using Multi and Single Cell-based Spheres Assays. Journal of Visualized Experiments. (95), 1-11 (2015).
- Stebbing, J., Lombardo, Y., Coombes, C. R., de Giorgio, A., Castellano, L. Mammosphere Formation Assay from Human Breast Cancer Tissues and Cell Lines. Journal of Visualized Experiments. (97), 1-5 (2015).
- Zhao, H., et al. Sphere-forming assay vs. organoid culture: Determining long-term stemness and the chemoresistant capacity of primary colorectal cancer cells. International Journal of Oncology. 54 (3), 893-904 (2019).
- Bagheri, V., et al. Isolation and identification of chemotherapy-enriched sphere-forming cells from a patient with gastric cancer. Journal of Cellular Physiology. 233 (10), 7036-7046 (2018).
- Kaowinn, S., Kaewpiboon, C., Koh, S., Kramer, O., Chung, Y. STAT1-HDAC4 signaling induces epithelial-mesenchymal transition and sphere formation of cancer cells overexpressing the oncogene, CUG2. Oncology Reports. , 2619-2627 (2018).
- Lonardo, E., Cioffi, M., Sancho, P., Crusz, S., Heeschen, C. Studying Pancreatic Cancer Stem Cell Characteristics for Developing New Treatment Strategies. Journal of Visualized Experiments. (100), 1-9 (2015).
- Lu, H., et al. Targeting cancer stem cell signature gene SMOC-2 Overcomes chemoresistance and inhibits cell proliferation of endometrial carcinoma. EBioMedicine. 40, 276-289 (2019).
- Bu, P., Chen, K. Y., Lipkin, S. M., Shen, X. Asymmetric division: a marker for cancer stem cells. Oncotarget. 4 (7), (2013).
- Islam, F., Qiao, B., Smith, R. A., Gopalan, V., Lam, A. K. Y. Cancer stem cell: fundamental experimental pathological concepts and updates. Experimental and molecular pathology. 98 (2), 184-191 (2015).
- Liu, W., et al. Comparative characterization of stem cell marker expression, metabolic activity and resistance to doxorubicin in adherent and spheroid cells derived from the canine prostate adenocarcinoma cell line CT1258. Anticancer research. 35 (4), 1917-1927 (2015).
- Broadley, K. W. R., et al. Side Population is Not Necessary or Sufficient for a Cancer Stem Cell Phenotype in Glioblastoma Multiforme. STEM CELLS. 29 (3), 452-461 (2011).
- Cojoc, M., Mäbert, K., Muders, M. H., Dubrovska, A. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Seminars in Cancer Biology. 31, 16-27 (2015).
- Batlle, E., Clevers, H. Cancer stem cells revisited. Nature Medicine. 23 (10), 1124-1134 (2017).
- Zhang, X. L., Jia, Q., Lv, L., Deng, T., Gao, J. Tumorspheres Derived from HCC Cells are Enriched with Cancer Stem Cell-like Cells and Present High Chemoresistance Dependent on the Akt Pathway. Anti-cancer agents in medicinal chemistry. 15 (6), 755-763 (2015).
- Fukamachi, H., et al. CD49fhigh Cells Retain Sphere-Forming and Tumor-Initiating Activities in Human Gastric Tumors. PLoS ONE. 8 (8), e72438 (2013).
- Gao, M. Q., Choi, Y. P., Kang, S., Youn, J. H., Cho, N. H. CD24+ cells from hierarchically organized ovarian cancer are enriched in cancer stem cells. Oncogene. 29 (18), 2672-2680 (2010).
- Cariati, M., et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. International Journal of Cancer. 122 (2), 298-304 (2008).
- López, J., Valdez-Morales, F. J., Benítez-Bribiesca, L., Cerbón, M., Carrancá, A. Normal and cancer stem cells of the human female reproductive system. Reproductive Biology and Endocrinology. 11 (1), 53 (2013).
- Alvero, A. B., et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 8 (1), 158-166 (2009).
- Charafe-Jauffret, E., Ginestier, C., Birnbaum, D. Breast cancer stem cells: tools and models to rely on. BMC Cancer. 9 (1), 202 (2009).
- Leccia, F., et al. ABCG2, a novel antigen to sort luminal progenitors of BRCA1- breast cancer cells. Molecular Cancer. 13 (1), 213 (2014).
- Croker, A. K., Allan, A. L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44+ human breast cancer cells. Breast Cancer Research and Treatment. 133 (1), 75-87 (2012).
- Sun, M., et al. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomaterialia. 28, 171-182 (2015).
- Shao, J., Fan, W., Ma, B., Wu, Y. Breast cancer stem cells expressing different stem cell markers exhibit distinct biological characteristics. Molecular Medicine Reports. 14 (6), 4991-4998 (2016).
- Croker, A. K., et al. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. Journal of Cellular and Molecular Medicine. 13 (8b), 2236-2252 (2009).
- Cheung, S. K. C., et al. Stage-specific embryonic antigen-3 (SSEA-3) and β3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proceedings of the National Academy of Sciences. 113 (4), 960-965 (2016).
- Meyer, M. J., Fleming, J. M., Lin, A. F., Hussnain, S. A., Ginsburg, E., Vonderhaar, B. K. CD44 pos CD49f hi CD133/2 hi Defines Xenograft-Initiating Cells in Estrogen Receptor–Negative Breast Cancer. Cancer Research. 70 (11), 4624-4633 (2010).
- Ahn, S. M., Goode, R. J. A., Simpson, R. J. Stem cell markers: Insights from membrane proteomics?. PROTEOMICS. 8 (23-24), 4946-4957 (2008).
- Chefetz, I., et al. TLR2 enhances ovarian cancer stem cell self-renewal and promotes tumor repair and recurrence. Cell Cycle. 12 (3), 511-521 (2013).
- Alvero, A. B., et al. Stem-Like Ovarian Cancer Cells Can Serve as Tumor Vascular Progenitors. Stem Cells. 27 (10), 2405-2413 (2009).
- Yin, G., et al. Constitutive proteasomal degradation of TWIST-1 in epithelial–ovarian cancer stem cells impacts differentiation and metastatic potential. Oncogene. 32 (1), 39-49 (2013).
- Wei, X., et al. Mullerian inhibiting substance preferentially inhibits stem/progenitors in human ovarian cancer cell lines compared with chemotherapeutics. Proceedings of the National Academy of Sciences. 107 (44), 18874-18879 (2010).
- Meirelles, K., et al. Human ovarian cancer stem/progenitor cells are stimulated by doxorubicin but inhibited by Mullerian inhibiting substance. Proceedings of the National Academy of Sciences. 109 (7), 2358-2363 (2012).
- Shi, M. F., et al. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cellular and Molecular Life Sciences. 67 (22), 3915-3925 (2010).
- Meng, E., et al. CD44+/CD24− ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clinical & Experimental Metastasis. 29 (8), 939-948 (2012).
- Witt, A. E., et al. Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian. Oncogene. 36 (12), 1707-1720 (2017).
- Wu, H., Zhang, J., Shi, H. Expression of cancer stem markers could be influenced by silencing of p16 gene in HeLa cervical carcinoma cells. European journal of gynaecological oncology. 37 (2), 221-225 (2016).
- Huang, R., Rofstad, E. K. Cancer stem cells (CSCs), cervical CSCs and targeted therapies. Oncotarget. 8 (21), 35351-35367 (2017).
- Zhang, X., et al. Imatinib sensitizes endometrial cancer cells to cisplatin by targeting CD117-positive growth-competent cells. Cancer Letters. 345 (1), 106-114 (2014).
- Luo, L., et al. Ovarian cancer cells with the CD117 phenotype are highly tumorigenic and are related to chemotherapy outcome. Experimental and Molecular Pathology. 91 (2), 596-602 (2011).
- Zhao, P., Lu, Y., Jiang, X., Li, X. Clinicopathological significance and prognostic value of CD133 expression in triple-negative breast carcinoma. Cancer Science. 102 (5), 1107-1111 (2011).
- Ferrandina, G., et al. Expression of CD133-1 and CD133-2 in ovarian cancer. International Journal of Gynecologic Cancer. 18 (3), 506-514 (2008).
- Rutella, S., et al. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clinical cancer research an official journal of the American Association for Cancer Research. 15 (13), 4299-4311 (2009).
- Friel, A. M., et al. Epigenetic regulation of CD133 and tumorigenicity of CD133 positive and negative endometrial cancer cells. Reproductive Biology and Endocrinology. 8 (1), 147 (2010).
- Nakamura, M., et al. Prognostic impact of CD133 expression as a tumor-initiating cell marker in endometrial cancer. Human Pathology. 41 (11), 1516-1529 (2010).
- Saha, S. K., et al. KRT19 directly interacts with β-catenin/RAC1 complex to regulate NUMB-dependent NOTCH signaling pathway and breast cancer properties. Oncogene. 36 (3), 332-349 (2017).
- LV, X., Wang, Y., Song, Y., Pang, X., Li, H. Association between ALDH1+/CD133+ stem-like cells and tumor angiogenesis in invasive ductal breast carcinoma. Oncology Letters. 11 (3), 1750-1756 (2016).
- Ruscito, I., et al. Exploring the clonal evolution of CD133/aldehyde-dehydrogenase-1 (ALDH1)-positive cancer stem-like cells from primary to recurrent high-grade serous ovarian cancer (HGSOC). A study of the Ovarian Cancer Therapy–Innovative Models Prolong Survival (OCTIPS). European Journal of Cancer. 79, 214-225 (2017).
- Sun, Y., et al. Isolation of Stem-Like Cancer Cells in Primary Endometrial Cancer Using Cell Surface Markers CD133 and CXCR4. Translational Oncology. 10 (6), 976-987 (2017).
- Rahadiani, N., et al. Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Science. 102 (4), 903-908 (2011).
- Mamat, S., et al. Transcriptional Regulation of Aldehyde Dehydrogenase 1A1 Gene by Alternative Spliced Forms of Nuclear Factor Y in Tumorigenic Population of Endometrial Adenocarcinoma. Genes & Cancer. 2 (10), 979-984 (2011).
- Mukherjee, S. A., et al. Non-migratory tumorigenic intrinsic cancer stem cells ensure breast cancer metastasis by generation of CXCR4+ migrating cancer stem cells. Oncogene. 35 (37), 4937-4948 (2016).
- Lim, E., et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nature Medicine. 15 (8), 907-913 (2009).
- Liang, Y. J., et al. Interaction of glycosphingolipids GD3 and GD2 with growth factor receptors maintains breast cancer stem cell phenotype. Oncotarget. 8 (29), 47454-47473 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone