Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Identification and Characterization of Immunogenic RNA Species in HDM Allergens that Modulate Eosinophilic Lung Inflammation
W tym Artykule
Podsumowanie
Environmental allergens such as house dust mites (HDM) often contain microbial substances that activate innate immune responses to regulate allergic inflammation. The protocol presented here demonstrates the identification of dsRNA species in HDM allergens and characterization of their immunogenic activities in modulating eosinophilic lung inflammation.
Streszczenie
Environmental allergens such as house dust mites (HDM) are often in complex forms containing both allergic proteins that drive aberrant type 2 responses and microbial substances that induce innate immune responses. These allergen-associated microbial components play an important role in regulating the development of type 2 inflammatory conditions such as allergic asthma. However, the underlying mechanisms remain largely undefined. The protocol presented here determines the structural characteristics and in vivo activity of allergen-associated immunostimulatory RNA. Specifically, common allergens are examined for the presence of double-stranded RNA (dsRNA) species that can stimulate IFN responses in lungs and restrain the development of severe lung eosinophilia in a mouse model of HDM-induced allergic asthma. Here, we have included the following three assays: Dot blot to show the dsRNA structures in total RNA isolated from allergens including HDM species, RT-qPCR to measure the activities of HDM RNA in interferon stimulating genes (ISGs) expression in mouse lungs and FACS analysis to determine the effects of HDM RNA on the number of eosinophils in BAL and lung, respectively.
Wprowadzenie
Based on the hygiene hypothesis originally proposed by Strachan1, early childhood exposure to environmental microbial factors such as endotoxin can protect against the development of allergic disorders2,3. During microbial infections, e.g., viral infections, the innate immune detection of foreign nucleic acids (RNA/DNA) triggers host defense responses4,5,6. However, the existence and prevalence of immunogenic nucleic acids such as long double-stranded RNA (dsRNA) species in house dust mites (HDM) or other insect allergens remain unknown. This protocol was designed to determine whether HDM or insect and non-insect allergens contain long dsRNA species that can activate a protective immune response to counteract the development of severe eosinophilic lung inflammation in a mouse model of allergic asthma. Here, we provide three simple and fast methods to evaluate the structural determinants in HDM total RNA that are required for regulating allergen-induced eosinophilic lung inflammation.
The mucosal immune system is the largest immune organ in the body and serves as the first line of host defense against both microbial infections and allergic insults7,8. The long dsRNA, the replication intermediate of many viruses, is known to function as a pathogen-associated molecular pattern (PAMP) to potently stimulate innate responses via Toll like receptor 3 (TLR3) to induce the expression of interferon stimulated genes (ISGs)9,10,11,12,13,14. We have recently shown that HDM total RNA contained dsRNA structures, which upregulated the expression of ISGs and reduced severe eosinophilic lung inflammation when administered via the intratracheal instillation in a murine model of allergic asthma induced by HDM extracts15. The severity of lung inflammations is determined by analyzing the immune cell types in bronchoalveolar lavage (BAL) and lung tissue via flow cytometry16,17,18,19,20.
This protocol includes three assays: 1) rapid detection of dsRNA structures with RNA dot blot using a mouse monoclonal antibody J2 which specifically binds to the dsRNA (≥40bp) in a sequence-independent manner; 2) quick evaluation for in vivo effects of immunostimulatory RNA in mouse lungs by measuring the induction of ISGs using RT-qPCR; 3) accurate quantification of eosinophils in BAL and lung in the context of HDM-induced lung inflammation using flow cytometry analysis.
The above assays can be used to study not only allergic lung diseases, but also respiratory bacterial and viral infections. For example, the dsRNA specific J2 antibody can also be used in other applications such as immunoaffinity chromatography, immunohistochemistry, enzyme-linked immunosorbent assay (ELISA) and immunostaining21,22,23. In addition, several applications downstream of BAL fluid collection can be utilized for quantifying soluble contents such as cytokines and chemokines using ELISA, and transcriptional profiling of cells in the airways (e.g., alveolar macrophages). Although there are a variety of protocols available in the literature to evaluate lung conditions, most of these protocols often focus on the target validation. The procedures described here can be applied to identify components in environmental allergens that are important for regulating the development of allergic diseases.
Protokół
Experimental procedures described here were approved by the Institutional Animal Care and Use Committee of University of Texas Health San Antonio.
1. Dot blot to show the presence of dsRNA structures in HDM total RNA
- Total RNA isolation from allergens, insects, and non-insect allergens
- Put HDM, insects, or non-insect animals collected alive or obtained commercially into 50 mL tubes, and quickly freeze with liquid-N2. Then store at -70 °C for subsequent total RNA isolation.
NOTE: In this experiment, HDM, insect, and non-insect animals were selected because they are known to be common sources of allergens. Further, an immunostimulatory function of their RNAs remain unclear. - Transfer a proper amount (equivalent to 100 μL in volume or less) of HDM, insects or non-insect animals stored at -70 °C into a 2 mL tube containing beads (1.4 mm ceramic spheres), then freeze tubes in a liquid-N2 container for ~10 min.
- For the total RNA isolation, add 1 mL of guanidinium thiocyanate-based RNA isolation reagent24 to each tube, then break the insect and non-insect small animals with a high-energy cell disrupter at the maximum speed for 45 s and chill on ice. Repeat this step twice.
- Transfer the solution from step 1.1.3 into a new 1.5 mL tube and add 200 μL of chloroform to each tube and vortex. Centrifuge tubes at 14,000 x g for 14 min at 4 °C.
- Once centrifugation is completed, transfer the upper aqueous phase (200 μL) into a new 1.5 mL tube containing 500 μL of isopropanol to precipitate RNA pellet. Do not disturb the interphase. The recommended volume ratio of the upper phase versus isopropanol is 1:2.5 ratio.
- Mix by gentle vortexing, then centrifuge tubes at 14,000 x g for 14 min at 4 °C.
- Aspirate the supernatant with caution then wash the RNA pellet with 500 μL of 75% ethanol and centrifuge at 7,500 x g for 10 min at 4 °C. Remove all liquid with caution, air-dry the pellet and dissolve the RNA pellet with 20-50 μL of RNase-free H2O.
- Measure the RNA concentration with a spectrophotometer using the following parameters:
- Open the associated software and select the type of nucleic acids to measure. Change the sample type to RNA.
- Perform the blank measurement with 1-2 μL of RNase-free H2O. Wipe off the RNase-free H2O. Now, the instrument is ready for the measurement.
- Load 1-2 μL of the RNA sample and measure the RNA concentration (μg/μL).
NOTE: The ratio of the absorbance at 260 and 280 nm (A260/280) at ~2.0 (1.9-2.2) is generally accepted as “pure” for RNA. If not processed immediately, store RNA samples at -70 ˚C and avoid the freeze-thaw cycles to keep the RNA intact.
- Put HDM, insects, or non-insect animals collected alive or obtained commercially into 50 mL tubes, and quickly freeze with liquid-N2. Then store at -70 °C for subsequent total RNA isolation.
- Detection of dsRNA structure in the total RNA using dsRNA specific J2 antibody
- Prepare two 20 μL of RNA samples (200 ng/μL). One with RNase-III treatment (1 μL for 1 μg RNA, incubate at 37 °C for 60 min), and the other without RNase-III treatment.
NOTE: RNase III is used here to specifically degrade dsRNA, but not single-stranded RNA25. - Use a pencil to draw grids where RNA samples will be blotted on the membrane.
- Spot 2 μL of the 200 ng/μL of the RNA sample onto the positively charged nylon membrane.
- Crosslink the samples to the membrane at 1,200 microjoules x 100 in a UV crosslinker. Repeat steps 1.2.3 and 1.2.4 two more times in the sample spot place. This will result in total 0.8 μg per blot.
NOTE: Do not spot more than 2 μL of RNA sample on the membrane at a time. - Block non-specific binding with 5% milk in TBS-T for 1 h with shaking at room temperature. Remove the blocking solution from step 1.2.5 and add the anti-dsRNA J2 antibody at the 1:1,000 dilution in 1% milk in TBS-T and incubate overnight with shaking at 4 °C.
- Wash the membrane with TBS-T for 5 min and repeat this step for 3 times. Add the secondary antibody (Alkaline phosphatase-conjugated Anti-Mouse IgG diluted in 1% milk 1:5,000) and incubate for 1 h on a shaker at room temperature. Wash the membrane with TBS-T for 5 min and repeat this step for 3x.
- Add the substrate (BCIP/NBT) and incubate for 5-15 min until a desired signal is visible.
- Stop the reaction by rinsing the membrane with ddH2O.
- Dry the membrane on tissue papers and take a photograph using a smartphone (a representative result is shown in Figure 1).
- Prepare two 20 μL of RNA samples (200 ng/μL). One with RNase-III treatment (1 μL for 1 μg RNA, incubate at 37 °C for 60 min), and the other without RNase-III treatment.
2. RT-qPCR to measure the ability of HDM total RNA in stimulating lung ISGs expression
- RNA isolation from mice lung tissues
NOTE: Mice (female, 8-12 weeks old, C57BL/6J) were maintained under specific pathogen-free conditions.- Briefly anesthetize the animal with isoflurane and administer via the intratracheal instillation with 5 μg (diluted in 80 μL PBS) of HDM RNAs treated with or without RNase III.
- After 16-18 h post HDM RNA treatment, sacrifice the mouse by CO2 inhalation for a few minutes. Then, place the mouse on a platform and pin limbs with needles.
- Disinfect the mouse with 70% ethanol then cut the skin starting from abdomen to the neck with a sterilized scissor.
- Fix the skin with needles and cut the ribs to expose the lungs. Remove the whole lungs and wash them with cold PBS. Place the lungs on tissue papers and excise one small piece of each lung-lobe into a 2 mL tube containing beads (200-300 μL in volume, 1.4 mm ceramic spheres).
NOTE: The purpose of using ceramic beads is to grind whole lung tissues - Freeze the lung samples by placing tubes into a liquid-N2 container for ~10 min.
- Add 500 μL of guanidinium thiocyanate-based RNA isolation reagent to each tube and break the lung tissues with a homogenizer for 45 s. Chill on ice between each step. Repeat this step twice.
- Follow the steps 1.1.4- 1.1.7 for lung RNA isolation.
- Air-dry the pellet and dissolve the RNA pellet with proper amount of RNase-free H2O (~20-30 μL).
- Measure the RNA concentration as described in step 1.1.8.
- RT-qPCR to determine the ability of HDM RNA in stimulating lung gene expression.
- Using 100 ng/μL of RNA extracted from lung tissues as the template, perform the cDNA synthesis according to the referenced protocol26.
- Set up an RT-qPCR reaction at 10 μL/well for a 384-well plate using cDNA generated above and the gene-specific primer pairs (Table 1 and Table 2).
- Seal the wells tightly with a transparent adhesive film and vortex the plate for 30 s. Spin the plate at 1,000 x g for 30 s to collect samples at the bottom of the wells.
- Load the plate onto a RT-qPCR machine and start to run the RT-qPCR reaction using the thermal cycler protocol (Table 3).
- Export the results into a spreadsheet file or analyze the data using the software provided by the manufacture after the program is completed (a representative result is shown in Figure 2).
3. FACS analysis to determine the effects of HDM RNA on the infiltration of eosinophils in BAL and lung
- BAL fluid collection for FACS analysis
- Euthanize mice (female, 8-12 weeks old, C57BL/6J) that were treated with HDM allergen extracts (according to the experimental design shown in Figure 3B) by CO2 inhalation.
- Place the mouse on a platform and pin limbs with needles.
- Disinfect the mouse with 70% ethanol. Use scissors to cut the skin from the upper area of the abdomen to the neck.
- Gently, pull the salivary glands and the sternohyoid muscle carefully apart using the forceps to expose the trachea. Place a nylon string (~10 cm) under the trachea using forceps.
- Make an incision in the trachea (~2 mm under the larynx) just enough to insert a cannula. Do not cut through the trachea. Knot the string around trachea and cannula.
- Load the syringe with 1 mL of PBS+EDTA and attach it to the end of the cannula. Inject 1 mL of PBS+EDTA into the lung and completely aspirate the solution. Detach the syringe from the cannula carefully and transfer the solution into a 15 mL tube on ice.
- Reload the syringe with the fresh PBS+EDTA and repeat this step 2x.
- Centrifuge the tube containing the pooled BAL obtained in step 3.1.7 to pellet the cells at 500 x g for 7 min at 4 °C. Record the volume of BAL fluid then transfer the supernatant to two 1.5 mL tubes without disturbing the pellet.
NOTE: The supernatant of BAL can be stored at -70 °C for future analysis e.g., ELISA. - In case there are RBCs present in the pellet due to severe lung inflammation, after removing the supernatant, add 500 μL of RBC lysis buffer and mix well by resuspension. Transfer the solution into a new 1.5 mL tube and centrifuge for 7 min at the speed of 500 x g at 4 °C.
- Remove the supernatant and resuspend the pellet in 150 μL of FACS buffer.
- Transfer the 150 μL of the resuspended sample into 96-well plate and centrifuge the plate for 7 min at the speed of 500 x g at 4 °C.
- Quickly, invert the plate on tissue papers to collect the cells residing at the bottom of the wells.
- Stain the cells with antibodies in FACS buffer in the presence of 2.4G2 blocking antibody (2.5 μg / 100 μL). Incubate the plate at room temperature for 30 min in a dark place.
- After staining, centrifuge the plate to pellet the cells at 500 x g for 7 min at 4 °C.
- Remove the staining solution by inverting the plate on tissue paper then wash by resuspending with 100 μL of FACS buffer. Next, centrifuge the plate again at 500 x g for 7 min at 4 °C and remove the FACS buffer by inverting the plate on tissue paper.
- Resuspend samples into 150 μL of FACS buffer and transfer samples to the FACS tubes containing 350 μL of FACS buffer. Add 25 μL of counting beads to each sample. Samples are now ready for flow cytometry analysis.
NOTE: Various cell types in BAL fluid were labeled with antibodies as indicated. Counting beads were added before the FACS run. Flow cytometry data were analyzed using a commercially available software. Refer to Figure 3 and Table 4 for gating strategy.
- Lung tissue digestion for the FACS analysis
- Follow steps 3.1.1 - 3.1.3.
- Cut the skin starting from abdomen to the neck with a sterilized scissor. Fix the skin with needles and cut the ribs to expose the lungs.
- Remove the whole lungs and wash them with cold PBS. Place samples in the 1.5 mL tube containing 50 μL of lung digestion solution.
- Mince the lung tissues into small pieces with a curved scissor. Transfer the lung tissues into a 6-well plate, then add 8 mL of lung digestion solution. Place the plate on a shaker in 37 °C incubator for 45 min.
- After incubation, use the top of 1.5 mL tube to grind the lung tissues. Place a 70 µm strainers on a new 6-well plate and apply the sample through 0.22 µm filter.
- Transfer the filtered solution into a 15 mL tube, then centrifuge the tubes at 500 x g for 7 min at 4 °C. Aspirate the supernatant and resuspend the pellet in 1 mL of RBC lysis buffer and leave it on ice for 3 min.
- Transfer the sample into 1.5 mL tube and centrifuge at 500 x g for 7 min at 4 °C. Repeat 2x
- Wash the lung cells 2x with 1 mL FACS buffer. Aspirate the supernatant and resuspend the pellet in 1 mL of FACS buffer, and then transfer 100 μL of the sample into 96 well plate.
- Centrifuge the plate for 7 min at the speed of 500 x g at 4 °C. Follow the steps described in BAL fluid collection for FACS analysis (3.1.13 to 3.1.16) to stain the cells of digested lung tissue samples.
NOTE: Eosinophils in the lungs were labeled with antibodies as indicated, then mixed with counting beads for further FACS analysis. Flow cytometry data were analyzed using associated software. Refer to Figure 3 for evaluating HDM RNA-induced immune responses.
4. Statistical analysis
- Perform statistical analysis using a commercially available software.
- Determine the p values by unpaired two-tailed Student t test for the comparison of two groups.
- Calculate the absolute numbers of eosinophils based on reference beads (top panel) using the formula
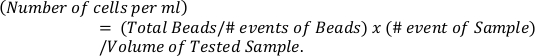
- Determine the p values by two-way ANOVA and Sidak’s multiple comparisons test for the comparison of more than two groups.
- Consider a p value smaller than 0.05 as statistically significant. The p values are indicated on plots as *p <0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
NOTE: All buffer recipes are provided in Table 5.
Wyniki
The presence of long dsRNA structures in HDM, insects and non-insect small animals was examined by dot blot using a dsRNA-specific mouse monoclonal antibody J2 (≥ 40bp). RNase III was used to digest dsRNA into 12–15 bp dsRNA fragments, which were undetectable by J2 (Figure 1).
The ability of HDM total RNA to stimulate an innate immune response in mouse lungs in a dose-dependent manner was analyzed by RT-qPCR (Figure 2...
Dyskusje
The current protocol describes how to evaluate the immunostimulatory properties of allergen-associated microbial RNA and their impacts on the development of eosinophilic lung inflammation in a mouse model of allergic asthma. Although long dsRNAs are known as the replication intermediates of many viruses that can potently activate interferon responses in mammalian cells, their presences in HDM allergens have been unknown until our recent work15. The combination of RNA dot blot, RT-qPCR and FACS ana...
Ujawnienia
We have nothing to disclose.
Podziękowania
We thank Ms. Karla Gorena for technical assistance in flow cytometry. L.S. is supported by the China Scholarship Council and Hunan Provincial Innovation Foundation for Postgraduate (CX201713068). H.H.A. is supported by the Department of Clinical Laboratory Sciences, College of Applied Medical Sciences, Jouf University, Sakaka, Saudi Arabia. X.D.L. is supported by the UT Health San Antonio School of Medicine Startup Fund and the Max and Minnei Voelcker Fund.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.40 µm Falcon Cell Strainer | Thermo Fisher Scientific | 08-771-1 | |
| 1 mL syringes | Henke Sass Wolf | 5010.200V0 | |
| 15 mL Tube | TH.Geyer | 7696702 | |
| 50 mL Tube | TH.Geyer | 7696705 | |
| 70% ethanol | Decon Labs | 2701 | |
| Absolute Counting Beads | Life Technologies Europe B.V. | C36950 | |
| ACK-RBC lysing buffer | Lonza | 10-548E | |
| Amersham Hybond-N+ Membrane | GE Healthcare | RPN203B | |
| Ant | San Antonio | Note: Locally collected | |
| Antibody dilution buffer | (see Table 5 for recipe) | ||
| Anti-Mouse CD11b V450 Rat (clone M1/70) | BD Bioscience | 560456 | 1 to 200 dilution |
| Anti-Mouse CD11c PE-Cy7 (clone N418) | BioLegend | 117317 | 1 to 200 dilution |
| Anti-Mouse CD19 Alexa Flour 647 (clone 1D3) | eBioscience | 15-0193-81 | 1 to 200 dilution |
| Anti-Mouse CD3e APC (clone 145-2C11) | Invitrogen | 15-0031-81 | 1 to 200 dilution |
| Anti-Mouse CD45 APC-Cy7 (clone: 30-F11) | BioLegend | 103130 | 1 to 200 dilution |
| Anti-Mouse Fixable Viabillity Dye eFluor 506 | Invitrogen | 65-0866-14 | 1 to 200 dilution |
| Anti-Mouse IgG (H+L), AP Conjugate | Promega | S3721 | |
| Anti-Mouse Ly-6G FITC (clone RB6-8C5) | Invitrogen | 11-5931-82 | 1 to 200 dilution |
| Anti-Mouse MHC II APC-eFluor 780 (clone M5/114.15.2) | eBioscience | 47-5321-80 | 1 to 200 dilution |
| Anti-Mouse Siglec-F PE (clone E50-2440) | BD Pharmingen | 552126 | 1 to 200 dilution |
| BCIP/NBT substrate | Thermo Fisher Scientific | PI34042 | |
| Blocking Buffer | (see Table 5 for recipe) | ||
| Cannual, 20G X 1.5” | CADENCE SCIENCE | 9920 | |
| Centrifuge | Thermo Fisher Scientific | 75004030 | |
| CFX384 Touch Real-Time PCR Detection System | Bio-Rad Laboratories | 1855485 | |
| Chloroform | Thermo Fisher Scientific | C298-500 | |
| Cockroach | Greer Laboratories | B26 | |
| Counting beads | Thermo Fisher Scientific | 01-1234-42 | |
| D. farinae | Greer Laboratories | B81 | |
| D. pteronyssinus | Greer Laboratories | B82 | |
| Denville Cell Culture Plates with lid, 96 well cell culture plate | Thomas Scientific | 1156F03 | |
| Digital Dry Bath - Four Blocks | Universal Medical, Inc. | BSH1004 | |
| Earthworm | San Antonio | Note: Locally collected | |
| Ethylenediaminetetraacetic acid (EDTA) | Sigma-Aldrich | E6511 | |
| FACS buffer | (see recipe in Table 5) | ||
| Falcon Round-Bottom Polypropylene Tubes, 5 mL | STEMCELLTM TECHNOLOGIES | 38056 | |
| Flow cytometer (BD FACS Celesta) | BD Biosciences | ||
| Fly | Greer Laboratories | B8 | |
| Forceps | Roboz Surgical Instrument | RS-5135 | |
| Hemocytometer | Hausser Scientific | 3110 | |
| HT-DNA | Sigma | D6898 | |
| In Vivo MAb anti-mouse CD16/CD32 (clone: 2.4G2) | Bio X Cell | BE0307 | |
| iScript cDNA Synthesis Kit | Bio-Rad Laboratories | 1708891 | |
| Isoflurane | Abbott Labs | sc-363629Rx | |
| Isopropanol | Thermo Fisher Scientific | BP2618500 | |
| J2 anti-dsRNA monoclonal antibody | SCICONS | 10010200 | |
| Lung digestion solution | (see recipe in Table 5) | ||
| Lysing Matrix D | MP Biomedicals | 116913050-CF | |
| Lysing Matrix D, 2 mL tube | MP Biomedicals | SKU:116913100 | |
| Mice (female, 8-12 weeks old, C57BL/6J) | Jackson Laboratory | #000664 | |
| Microcentrifuge tube 1.5 mL | Sigma-Aldrich | 30120.094 | |
| Microscope | Olympus | CK30 | |
| Mini-BeadBeater | Homogenizers | SKU:BS:607 | |
| Mini-Beadbeater-16 | Biospec | 607 | |
| Mosquito | Greer Laboratories | B55 | |
| NanoDrop 2000C | Thermo Scientific Spectophotometer Medex Supply | TSCND2000C | |
| Needle, 21 G x 1 1/2 in | BD Biosciences | 305167 | |
| Non-fat milk | Bio-Rad Laboratories | 1706404 | |
| Nylon string | Dynarex | 3243 | |
| Phosphate-buffered Saline (PBS) | Lonza | BE17-516F | |
| RNase III | Thermo Fisher Scientific | AM2290 | |
| RNase T1 | Thermo Fisher Scientific | AM2283 | |
| Scissors | Roboz Surgical Instrument | RS-6802 | |
| Shaker or Small laboratory mixer | Boekel Scientific | 201100 | |
| SPHERO AccuCount Fluorescent | Spherotech | ACFP-70-5 | 1 to 10 dilution |
| Spider | San Antonio | Note: Locally collected | |
| TBS | (see recipe in Table 5) | ||
| TBS-T | (see recipe in Table 5) | ||
| Total cell medium | (see recipe in Table 5) | ||
| TRIzol Reagent | Thermo Fisher Scientific | 15596018 | |
| Tween 20 | Sigma-Aldrich | P9416 | |
| UV Stratalinker 2400 UV | LabX | 20447 | |
| Wasp | San Antonio | Note: Locally collected |
Odniesienia
- Strachan, D. P. Hay fever, hygiene, and household size. BMJ. 299, 1259-1260 (1989).
- Schuijs, M. J., et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 349, 1106-1110 (2015).
- Stein, M. M., et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. New England Journal of Medicine. 375, 411-421 (2016).
- Roers, A., Hiller, B., Hornung, V. Recognition of Endogenous Nucleic Acids by the Innate Immune System. Immunity. 44, 739-754 (2016).
- Schlee, M., Hartmann, G. Discriminating self from non-self in nucleic acid sensing. Nature Reviews Immunology. 16, 566-580 (2016).
- Wu, J., Chen, Z. J. Innate immune sensing and signaling of cytosolic nucleic acids. Annual Reviews Immunology. 32, 461-488 (2014).
- O'Hara, A. M., Shanahan, F. The gut flora as a forgotten organ. EMBO Reports. 7 (7), 688-693 (2006).
- . Focused Meeting 2018: Microbes and Mucosal Surfaces Available from: https://microbiologysociety.org/event/society-events-and-meetings/focused-meeting-2018-microbes-and-mucosal-surfaces.html (2018)
- Weber, F., et al. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. Journal of Virology. 80, 5059-5064 (2006).
- Barral, P. M., et al. Functions of the cytoplasmic RNA sensors RIG-I and MDA-5: Key regulators of innate immunity. Pharmacology and Therapeutics. 124, 219-234 (2009).
- Netea, M. G., et al. From the Th1/Th2 paradigm towards a Toll-like receptor/T-helper bias. Antimicrobial Agents and Chemotherapy. 49, 3991-3996 (2005).
- McNally, B., et al. Intranasal administration of dsRNA analog poly(I:C) induces interferon-alpha receptor-dependent accumulation of antigen experienced T cells in the airways. PLoS One. 7, 51351 (2012).
- Seya, T., Takeda, Y., Matsumoto, M. Tumor vaccines with dsRNA adjuvant ARNAX induces antigen-specific tumor shrinkage without cytokinemia. Oncoimmunology. 5, 1043506 (2016).
- Toussi, D. N., Massari, P. Immune Adjuvant Effect of molecularly defined Toll-Like Receptor Ligands. Vaccines (Basel). 2, 323-353 (2014).
- She, L., et al. Immune Sensing of Aeroallergen-Associated Double-Stranded RNA Triggers an IFN Response and Modulates Type 2 Lung Inflammation. Journal of Immunology. 203, 2520-2531 (2019).
- Fujimoto, Y., et al. Pulmonary inflammation and cytokine dynamics of bronchoalveolar lavage fluid from a mouse model of bronchial asthma during A(H1N1)pdm09 influenza infection. Science Reports. 7, 9128 (2017).
- Yao, Y., et al. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 175, 1634-1650 (2018).
- Dua, K., Shukla, S. D., Hansbro, P. M. Aspiration techniques for bronchoalveolar lavage in translational respiratory research: Paving the way to develop novel therapeutic moieties. Journal of Biological Methods. 4, 73 (2017).
- Van Hoecke, L., et al. Bronchoalveolar Lavage of Murine Lungs to Analyze Inflammatory Cell Infiltration. Journal of Visualized Experiments. (123), e55398 (2017).
- Salahuddin, S., et al. Processing of Bronchoalveolar Lavage Fluid and Matched Blood for Alveolar Macrophage and CD4+ T-cell Immunophenotyping and HIV Reservoir Assessment. Journal of Visualized Experiments. (148), e59427 (2019).
- Son, K. N., Liang, Z., Lipton, H. L. Double-Stranded RNA Is Detected by Immunofluorescence Analysis in RNA and DNA Virus Infections, Including Those by Negative-Stranded RNA Viruses. Journal of Virology. 89, 9383-9392 (2015).
- Monsion, B., et al. Efficient Detection of Long dsRNA in Vitro and in Vivo Using the dsRNA Binding Domain from FHV B2 Protein. Front Plant Sci. 9, 70 (2018).
- Redente, E. F., et al. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 301, 510-518 (2011).
- Card, J. W., et al. Gender differences in murine airway responsiveness and lipopolysaccharide-induced inflammation. Journal of Immunology. 177, 621-630 (2006).
- Gueders, M. M., et al. Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflammation Research. 58, 845-854 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone