Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Characterization of a Novel Human Organotypic Retinal Culture Technique
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This study aims to develop a novel human organotypic retinal culture (HORC) model that prevents compromising retinal integrity during explant handling. This is achieved by culturing the retina with the overlying vitreous and the underlying retinal pigment epithelium-choroid (RPE-choroid) and sclera.
Streszczenie
Previous human organotypic retinal culture (HORC) models have utilized detached retinas; however, without the structural support conferred by retinal pigment epithelium-choroid (RPE-choroid) and sclera, the integrity of the fragile retina can easily be compromised. The aim of this study was to develop a novel HORC model that contains the retina, RPE-choroid and sclera to maintain retinal integrity when culturing retinal explants.
After cutting circumferentially along the limbus to remove iris and lens, four deep incisions were made to flatten the eyecup. In contrast to previous HORC protocols, a trephine was used to cut through not only the retina but also the RPE-choroid and sclera. The resultant triple-layered explants were cultured for 72 h. Hematoxylin and Eosin staining (H&E) was used to assess anatomical structures and retinal explants were further characterized by immunohistochemistry (IHC) for apoptosis, Müller cell integrity and retinal inflammation. To confirm the possibility of disease induction, explants were exposed to high glucose (HG) and pro-inflammatory cytokines (Cyt), to mimic diabetic retinopathy (DR). The Luminex magnetic bead assay was used to measure DR-related cytokines released into the culture medium.
H&E staining revealed distinct retinal lamellae and compact nuclei in retinal explants with the underlying RPE-choroid and sclera, while retinas without the underlying structures exhibited reduced thickness and severe nuclei loss. IHC results indicated absence of apoptosis and retinal inflammation as well as preserved Müller cell integrity. The Luminex assays showed significantly increased secretion of DR-associated pro-inflammatory cytokines in retinal explants exposed to HG + Cyt relative to baseline levels at 24 h.
We successfully developed and characterized a novel HORC protocol in which retinal integrity was preserved without apoptosis or retinal inflammation. Moreover, the induced secretion of DR-associated pro-inflammatory biomarkers when exposing retinal explants to HG + Cyt suggests that this model could be used for clinically translatable retinal disease studies.
Wprowadzenie
The retina is a highly specialized ocular structure responsible for transforming incoming light energy to electric signals, which are then processed by the brain for visual perception. The human retina contains a dynamic range of cell types, highly organized in a unique lamellar structure consisting of two synaptic and three nuclei layers1 (Figure 1). Retinal homeostasis is sustained by the intricate connections between neuroretinal cells, blood vessels, nerves, connective tissues and the RPE1. Due to the sophisticated retinal anatomy and physiology, mechanisms of many retinal diseases still remain poorly understood2,3,4,5. To better study retinal diseases, HORC models have been developed6,7,8,9. Compared to animal studies and in vitro cultures, HORC models are advantageous because they retain the dynamic cellular environment and complex neurovascular interactions in situ, providing a good model for clinical translation.
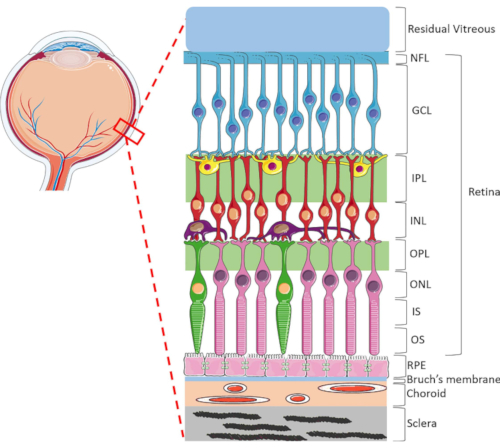
Figure 1: Posterior ocular structures of the human eye. Anterior to posterior, the retinal layers are: nerve fiber layer (NFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor inner segment (IS), and photoreceptor outer layer (OS). Cells within the retina include ganglion cells (blue), amacrine cells (yellow), bipolar cells (red), horizontal cells (purple), rod photoreceptors (pink) and cone photoreceptors (green). The vitreous is located anterior to the retina. The RPE, Bruch's membrane, choroid and sclera are located posterior to the retina. Note that the image shown is only a schematic representation of the retina and the ratio of cells/retinal connectivity within each layer may not be indicative of the in vivo setting. Please click here to view a larger version of this figure.
Previously characterized HORC protocols6,7,8,9 have involved separating the retina from the underlying RPE-choroid and sclera using a surgical trephine. However, without the support provided by these underlying structures, the translucent retina becomes flimsy, difficult to handle and tools such as forceps can easily disrupt its integrity. Furthermore, isolating retina in culture without the RPE has been shown to cause ganglion cell apoptosis and photoreceptor degeneration10,11,12. Thus, an alternative HORC protocol that minimizes the loss of retinal integrity and better mimics the in vivo environment would be useful. This is particularly important when studying retinal disease mechanisms, as physical injury during explant handling could introduce artifacts. Therefore, the aim of this study was to develop a novel HORC model that includes the RPE-choroid and sclera in order to protect retinal integrity during explant handling and culture.
In order to achieve this aim, retinal explants "sandwiched" between the residual vitreous and the underlying RPE-choroid and sclera were extracted. In the sandwich explants, the vitreous weighs down the retina to prevent retinal detachment and folding, whereas the tough, fibrous sclera acts as both a scaffold for structural support and a contact point for forceps. Moreover, animal models have shown that retaining the RPE in culture can prevent retinal degeneration and glial proliferation, a response of Müller cells to danger signals such as hypoxia and inflammation10,11,12.
To characterize the model, sandwich retinal explants were stained with Hematoxylin and Eosin (H&E) to assess anatomical structures and immunohistochemistry (IHC) was performed, labeling explants with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL, an apoptotic cell marker), glial fibrillary acidic protein (GFAP, a retinal inflammation and Müller cell activation marker), and vimentin, a marker of Müller cell integrity. To determine whether this model can be induced to develop molecular disease signs, the explants were exposed to high glucose (HG) with pro-inflammatory cytokines (Cyt), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), a culturing environment that has been shown to mimic diabetic retinopathy (DR) in both cell and animal disease models13,14,15. Luminex assays were used in the DR model to measure cytokines released into the culture medium.
Protokół
Human donor eye cups were obtained from the New Zealand National Eye Bank following corneal excision for transplantation and as approved by the Northern B Health and Disability Ethics Committee (NTX/06/19/CPD/AM07).
NOTE: The culture should be done in a Class II biosafety cabinet to ensure sterile tissue culture conditions. The tissues must be cultured within 24 h post-mortem to avoid significant retinal integrity loss.
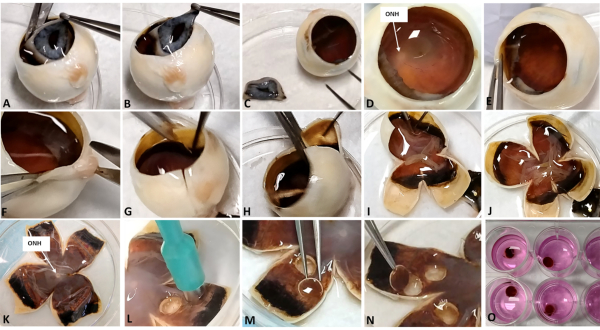
Figure 2: Images showing the procedure for collecting the sandwich retinal explants. For explant preparation, use dissecting scissors with one sharp tip and one blunt end, with the blunt end facing the inside of the globe to reduce tissue damage during incision. Also use forceps with blunt ends to avoid scratching the intraocular tissues during handling. Using surgical scissors, with the blunt end facing the inside, cut along the limbus to remove the iris and lens (A-C). The ONH can be located if looking straight into the opened eyecup (D). Using forceps with blunt tips, hold the sclera to stabilize the globe (E). Divide the globe vertically into two halves by making two deep incisions towards the ONH but do not cut through the ONH. Repeat this along the horizontal meridian (G). Apply blunt forceps at the sclera and gently open the globe to a clover shape (H-K). Use forceps to carefully manipulate the vitreous to smoothen the folded retina, as the vitreous tugs onto the retina, but do not touch the retina directly (I). Remove the vitreous if it is causing retinal detachment or folding (not shown in Figure 2 as the transparent vitreous is not well captured on photos). Locate areas where the retina is flat and extract retinal explants using a surgical trephine (L). Applying forceps at the sclera, carefully transfer the retinal explants into the pre-prepared culture medium (M,N). The entire sandwich explant should sink to the bottom of the well due to its weight, therefore each explant is submersed in the medium (O). Please click here to view a larger version of this figure.
1. Extraction of the sandwich retinal explants from the eyecup
- Remove the iris and lens.
- Place the eye cup inside a Petri dish, with the iris and lens facing upwards and the optic nerve head (ONH) contacting the Petri dish (Figure 2A).
- Hold the eye cup steady at the limbus using forceps (Figure 2B).
- Detach the iris and lens by making small cuts circumferentially along the outer edge of the limbus (Figure 2A).
- Remove the iris and lens carefully. Avoid disturbing the retina during manipulation (Figure 2C).
NOTE: The lens will not fall into the eye cup as it is attached, via zonule fibers, to the ciliary body.
- Flatten the eye cup.
- With the eye cup still sitting upright, identify the ONH. This is easier with a bright white light source (Figure 2D).
- Incise at the four quadrants towards the ONH (Figure 2E). The Petri dish can be rotated for easier handling. Do NOT cut the ONH.
- Spread and flatten the eye cup carefully (Figure 2E).
- Apply the forceps on the sclera instead of the retina to avoid disrupting retinal integrity.
NOTE: Peripheral retinal detachment and folding is unavoidable, as the weight of the overflowing vitreous will pull on the retina. In these areas, remove the vitreous to prevent further retinal folding. Remember to leave residual vitreous to stabilize the retina on top of the RPE-choroid and sclera.
- Collect sandwich retinal explants.
- Place a surgical trephine on the retina in a region without retinal folds (Figure 2F).
- Press hard to penetrate the sclera, which should generate a cracking sound.
- Twist the trephine by 180° to ensure the sclera has been penetrated fully such that the retinal explant is now separated from the remaining tissue.
- Apply the forceps at the sclera and transfer the sandwich retinal explant to the culture medium (Figure 2G-I).
- Obtain 2-3 sandwich retinal explants from the peripheral retina of each quadrant.
NOTE: The sandwich retinal explant can sometimes be trapped in the opening of the trephine. Using forceps, gently tease out a small section of the base of the sclera. This occurs more often when the blade is blunt and can cause loss of the retina (Figure 3A-C). - Use a new trephine after cutting out 1-2 retinal explants as the blade becomes blunt easily (Figure 3C
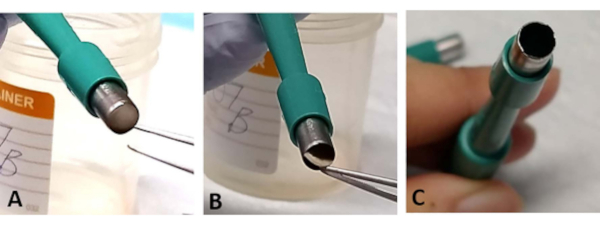
Figure 3: Troubleshooting. During the extraction process, the retinal explant may be trapped at the opening of the surgical trephine (A). Gently tease out the tissue from the base of the sclera without touching the retina (B). This can happen more often if the surgical trephine is used for extracting more than two retinal explants, as the blade can easily become blunt (C). Please click here to view a larger version of this figure.
- Culture the retinal explants.
- Prepare the culture medium containing Dulbecco's Modified Eagle Medium nutrient mixture F-12 (DMEM-F12) and a 1x antibiotics and antimycotics mixture (AA, 100x stock).
- Prior to explant extraction, place 500 µL of medium in the wells of a 24-well plate and equilibrate in the incubator. This is important as adding the medium afterwards can dislodge the retina.
- Culture the sandwich retinal explants at 37 °C for up to 72 h in a humidified 5% CO2 incubator in medium prepared in step 1.4.2.
- To induce DR-like changes, culture retinal explants in medium containing DMEM-F12 with a combination of 32.5 mM HG with Cyt, TNF-α (10 ng/mL) and IL-1β (10 ng/mL).
2. Paraffin-embedding of the sandwich retinal explants
- Fix the sandwich retinal explants.
- Immerse the sandwich retinal explants in 10% formalin for at least 24 h.
- Remove the formalin solution and transfer the sandwich retinal explants gently into tissue pads and cassettes.
- Soak the sandwich retinal explants in 70% ethanol for at least 24 h.
- Paraffin-embed retinal explants into blocks.
- Cut paraffin-embedded retinal tissues into 5 µm thick sections using a microtome and mount onto glass slides. Store at room temperature until imaging.
- Deparaffinize sections.
- Immerse the sections in 70% xylene for 5 min.
- Immerse the sections in 100% xylene for 5 min.
- Rehydrate the sections in 70% ethanol for 5 min.
- Rehydrate the sections in 100% ethanol for 5 min.
- Wash the sections under running tap water for 10 min.
3. Characterization using H&E, IHC and a Magnetic Luminex assay
- H&E staining protocol
- Deparaffinize the sections using step 2.2.
- Hydrate the sections in tap water for 5 min.
- Stain the sections in Gill's 2 hematoxylin solution for 5 min.
- Wash the sections thoroughly under running tap water to remove excess stain.
- Differentiate by dipping the sections twice in 1% acid alcohol.
- Wash the sections quickly under running tap water.
- Stain the sections blue by dipping six times in 1% lithium carbonate (10 mg/mL).
- Wash the sections thoroughly under running tap water for 5 min to remove excess blue stain.
- Dip the sections in 1% eosin 10 times.
- Wash the sections quickly under tap water to remove excess stain.
- Dehydrate the sections by dipping them 10 times in 100% ethanol. Do this twice.
- Dip the sections in 70% xylene 10 times.
- Dip the sections in 100% xylene 10 times.
- Mount with a coverslip using dibutylphthalate polystyrene xylene (DPX) mounting medium.
- Take images using a light microscope.
- IHC labeling protocol'
- Deparaffinize the sections using step 2.2.
- Place the slides into a solution containing 10 mM sodium citrate buffer with 0.05% Tween 20 at pH 6.0 and run an antigen retrieval in a pressure cooker automated at 121 °C for 2 min.
- Wash sections in phosphate buffered saline (PBS) for 5 min. Do this 3 times.
- Block the sections with PBS containing 0.1% Triton X-100 and 10% normal goat serum for 1 h at room temperature.
- Incubate sections overnight at 4 °C with primary antibodies conjugated to secondary antibodies (Table of Materials).
- Wash sections in PBS for 5 min. Do this 3 times.
- Stain nuclei using 4′,6-diamidino-2-phenylindole (DAPI) for 2 min.
- Wash and mount sections using an anti-fade reagent.
- Seal coverslips with nail polish.
- Take images using a confocal laser scanning microscope.
- Magnetic Luminex assay
- Transfer 75 µL of media from each well to a 96-well-u-bottom plate at 24 and 72 h.
- Analyze the cell supernatant for IL-18, IL-6, IL-8 and vascular endothelial growth factor (VEGF) after 24 and 72 h using Luminex cytokine assay. Follow the manufacturer's instructions to conduct the assay16.
Wyniki
Retinal integrity was preserved in this HORC model. Retinal integrity was preserved in the cultured sandwich retinal explants but was lost in the retina cultured without adjacent structures. H&E was conducted to examine the structural integrity of sectioned sandwich retinal explants after 72 h in culture. The sandwich retinal explants showed preserved integrity and a distinct lamellae structure from GCL to ONL with compact nuclei in INL and ONL (F...
Dyskusje
HORC is currently the most clinically translatable model in preclinical retinal research. Compared to in vitro cell culture models, HORC can better represent the anatomy of the human retina in situ, through retaining the dynamic retinal cell types and their connections with neurons, vasculatures and the extracellular environment19. Compared to animal models, HORC are more advantageous in studying the pathophysiology of and designing pharmaceutical treatments for human retinal diseases due to inter...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors would like to thank the generous donors of eye tissues and the team from the New Zealand National Eye Bank for their support. This work was financially supported by project grants from the Maurice and Phyllis Paykel Trust and the Auckland Medical Research Foundation (1117015). IDR's directorship is supported by the Buchanan Charitable Foundation. CK's scholarship is provided by the New Zealand Association of Optometrists Education and Research Fund (CC36812) and HHL's scholarship is provided by the Buchanan Charitable Foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Disposable Biopsy Punches (5 mm) | Integra York PA Inc., USA | 21909-142 | Referred as surgical trephines in this article |
| Human Recombinant IL-1β | Peprotech, USA | 200-01B | Working concentration: 10 ng/mL |
| Human Recombinant TNF-a | Peprotech, USA | 300-01A | Working concentration: 10 ng/mL |
| DAPI (1 µg/mL) | Sigma Aldrich, Germany | #D9542 | Nuclear stain. Working dilution 1:1000 |
| DMEM/F-12, GlutaMAX supplement | Gibco, Thermofisher, Scientific Inc., USA | 10565018 | Dulbecco’s Modified Eagle Medium nutrient mixture F-12 containing a 1× antibiotics and antimycotics mixture (AA, 100× stock) |
| Fine Scissors - Sharp-Blunt | Fine Science Tools (F.S.T) | 14028-10 | Tips: Sharp-Blunt, Cutting Edge: 27mm, Length: 10cm, Alloy/Material: Stainless Steel, Serrated: No, Tip Shape: Straight |
| Graefe Forceps | Fine Science Tools (F.S.T) | 11050-10 | Length:10cm, Tip shape: Straight, Tips: Serrated, Tip Width: 0.8mm, Tip Dimensions:0.8 x 0.7mm, Alloy/Materials: Stainless Steel |
| Mouse monoclonal GFAP-Cy3 | Sigma Aldrich, Germany | #C9205 | Primary antibody conjugated to Cy3. Working dilution 1:1000. |
| Mouse monoclonal Vimentin-Cy3 | Sigma Aldrich, Germany | #C9080 | Primary antibody conjugated to Cy3. Working dilution 1:50. |
| Rabbit polyclonal TUNEL (In Situ Cell Death Detection Kit, Fluorescein) | Sigma Aldrich, Germany | #11684795910 | Primary antibody conjugated to Fluorescein-dUTP. Working dilution 1:10 with enzyme-buffer solution. |
Odniesienia
- Kolb, H., Fernandez, E., Nelson, R. Facts and Figures Concerning the Human Retina. Webvision-The Organization of the Retina and Visual System. , (2005).
- Gemenetzi, M., De Salvo, G., Lotery, A. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye. 24 (12), 1743-1756 (2010).
- Buschini, E., Piras, A., Nuzzi, R., Vercelli, A. Age related macular degeneration and drusen: neuroinflammation in the retina. Progress in Neurobiology. 95 (1), 14-25 (2011).
- Chen, S. -. Y., et al. Current concepts regarding developmental mechanisms in diabetic retinopathy in Taiwan. Biomedicine. 6 (2), (2016).
- Kaaja, R., Loukovaara, S. Progression of retinopathy in type 1 diabetic women during pregnancy. Current Diabetes Reviews. 3 (2), 85-93 (2007).
- Azizzadeh Pormehr, L., et al. Human organotypic retinal flat-mount culture (HORFC) as a model for retinitis pigmentosa11. Journal of Cellular Biochemistry. 119 (8), 6775-6783 (2018).
- Fernandez-Bueno, I., et al. Time course modifications in organotypic culture of human neuroretina. Experimental Eye Research. 104, 26-38 (2012).
- Niyadurupola, N., Sidaway, P., Osborne, A., Broadway, D. C., Sanderson, J. The development of human organotypic retinal cultures (HORCs) to study retinal neurodegeneration. British Journal of Ophthalmology. 95 (5), 720-726 (2011).
- Osborne, A., Hopes, M., Wright, P., Broadway, D. C., Sanderson, J. Human organotypic retinal cultures (HORCs) as a chronic experimental model for investigation of retinal ganglion cell degeneration. Experimental Eye Research. 143, 28-38 (2016).
- Caffe, A., Visser, H., Jansen, H., Sanyal, S. Histotypic differentiation of neonatal mouse retina in organ culture. Current Eye Research. 8 (10), 1083-1092 (1989).
- Kaempf, S., Walter, P., Salz, A. K., Thumann, G. Novel organotypic culture model of adult mammalian neurosensory retina in co-culture with retinal pigment epithelium. Journal of Neuroscience Methods. 173 (1), 47-58 (2008).
- Liu, L., Cheng, S. -. H., Jiang, L. -. Z., Hansmann, G., Layer, P. G. The pigmented epithelium sustains cell growth and tissue differentiation of chicken retinal explants in vitro. Experimental Eye Research. 46 (5), 801-812 (1988).
- Kuo, C., Green, C. R., Rupenthal, I. D., Mugisho, O. O. Connexin43 hemichannel block protects against retinal pigment epithelial cell barrier breakdown. Acta Diabetologica. 57 (1), 13-22 (2020).
- Mugisho, O. O., et al. The inflammasome pathway is amplified and perpetuated in an autocrine manner through connexin43 hemichannel mediated ATP release. Biochimica et Biophysica Acta (BBA)-General Subjects. 1862 (3), 385-393 (2018).
- Mugisho, O. O., et al. Intravitreal pro-inflammatory cytokines in non-obese diabetic mice: Modelling signs of diabetic retinopathy. PLoS ONE. 13 (8), 0202156 (2018).
- . R&D Systems, I Available from: https://www.rndsystems.com/protocol-types/luminex (2020)
- Nakazawa, T., et al. Attenuated glial reactions and photoreceptor degeneration after retinal detachment in mice deficient in glial fibrillary acidic protein and vimentin. Investigative Ophthalmology, Visual Science. 48 (6), 2760-2768 (2007).
- Okada, M., Matsumura, M., Ogino, N., Honda, Y. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefe's Archive for Clinical and Experimental Ophthalmology. 228 (5), 467-474 (1990).
- Murali, A., Ramlogan-Steel, C. A., Andrzejewski, S., Steel, J. C., Layton, C. J. Retinal explant culture: A platform to investigate human neuro-retina. Clinical & Experimental Ophthalmology. 47 (2), 274-285 (2019).
- Koleva-Georgieva, D. N., Sivkova, N. P., Terzieva, D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Medica (Plovdiv). 53 (2), 44-50 (2011).
- Lee, J. -. H., et al. Cytokine profile of peripheral blood in type 2 diabetes mellitus patients with diabetic retinopathy. Annals of Clinical & Laboratory Science. 38 (4), 361-367 (2008).
- Chorostowska-Wynimko, J., et al. In vitro angiomodulatory activity of sera from type 2 diabetic patients with background retinopathy. Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society. 56, 65-70 (2005).
- Khalifa, R. A., Khalef, N., Moemen, L. A., Labib, H. M. The role interleukin 12 (IL-12), interferon-inducible protein 10 (IP-10) and Interleukin 18 (IL-18) in the angiogenic activity of diabetic retinopathy. Research Journal of Medicine and Medical Sciences. 4, 510-514 (2009).
- Zhou, J., Wang, S., Xia, X. Role of intravitreal inflammatory cytokines and angiogenic factors in proliferative diabetic retinopathy. Current Eye Research. 37 (5), 416-420 (2012).
- Song, Z., et al. Increased intravitreous interleukin-18 correlated to vascular endothelial growth factor in patients with active proliferative diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology. 252 (8), 1229-1234 (2014).
- Louie, H. H., Shome, A., Kuo, C. Y. J., Rupenthal, I. D., Green, C. R., Mugisho, O. O. Connexin43 hemichannel block inhibits NLRP3 inflammasome activation in a human retinal explant model of diabetic retinopathy. Experimental Eye Research. , (2020).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone