Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Assembly of Cell Mimicking Supported and Suspended Lipid Bilayer Models for the Study of Molecular Interactions
W tym Artykule
Podsumowanie
This protocol describes the formation of cell mimicking uni-lipid and multi-lipid vesicles, supported lipid bilayers, and suspended lipid bilayers. These in vitro models can be adapted to incorporate a variety of lipid types and can be used to investigate various molecule and macromolecule interactions.
Streszczenie
Model cell membranes are a useful screening tool with applications ranging from early drug discovery to toxicity studies. The cell membrane is a crucial protective barrier for all cell types, separating the internal cellular components from the extracellular environment. These membranes are composed largely of a lipid bilayer, which contains outer hydrophilic head groups and inner hydrophobic tail groups, along with various proteins and cholesterol. The composition and structure of the lipids themselves play a crucial role in regulating biological function, including interactions between cells and the cellular microenvironment, which may contain pharmaceuticals, biological toxins, and environmental toxicants. In this study, methods to formulate uni-lipid and multi-lipid supported and suspended cell mimicking lipid bilayers are described. Previously, uni-lipid phosphatidylcholine (PC) lipid bilayers as well as multi-lipid placental trophoblast-inspired lipid bilayers were developed for use in understanding molecular interactions. Here, methods for achieving both types of bilayer models will be presented. For cell mimicking multi-lipid bilayers, the desired lipid composition is first determined via lipid extraction from primary cells or cell lines followed by liquid chromatography-mass spectrometry (LC-MS). Using this composition, lipid vesicles are fabricated using a thin-film hydration and extrusion method and their hydrodynamic diameter and zeta potential are characterized. Supported and suspended lipid bilayers can then be formed using quartz crystal microbalance with dissipation monitoring (QCM-D) and on a porous membrane for use in a parallel artificial membrane permeability assay (PAMPA), respectively. The representative results highlight the reproducibility and versatility of in vitro cell membrane lipid bilayer models. The methods presented can aid in rapid, facile assessment of the interaction mechanisms, such as permeation, adsorption, and embedment, of various molecules and macromolecules with a cell membrane, helping in the screening of drug candidates and prediction of potential cellular toxicity.
Wprowadzenie
The cell membrane, composed primarily of phospholipids, cholesterol, and proteins, is a crucial component of all living cells1. With organization driven by lipid amphiphilicity, the cell membrane functions as a protective barrier and regulates how the cell interacts with its surrounding environment2. Several cellular processes are dependent on the lipid and protein composition of the membrane1,2. For example, cell membrane interactions are important for effective drug delivery3. Pharmaceuticals, biologics, nanomaterials, biological toxins, and environmental toxicants can impact the integrity of a cell membrane, thereby affecting cellular function4. The construction of in vitro cell mimicking membrane models based on the lipid composition of cell membranes has the potential to provide facile tools to greatly enhance the study of the potential impact of these materials on cells.
Model lipid bilayers include lipid vesicles, supported lipid bilayers, and suspended lipid bilayers. Supported lipid bilayers are a model of the phospholipid cell membrane commonly used in biotechnology applications where lipid vesicles are ruptured on a supported substrate material5,6,7,8,9. One common technique used to monitor bilayer formation is quartz crystal microbalance with dissipation monitoring (QCM-D), which examines the adsorption of vesicles in comparison to the bulk liquid properties in situ8,10,11,12,13,14. Previously, QCM-D has been used to demonstrate that under flow conditions, once a critical vesicle coverage of phosphatidylcholine (PC) lipid vesicles is achieved on the surface, they spontaneously rupture into rigid lipid bilayers15. Previous work has also investigated supported lipid bilayer formation with varying lipid compositions16, incorporation of lipid proteins17,18,19, and utilizing polymer cushions20, yielding supported lipid bilayers capable of mimicking various aspects of cell membrane function.
Lipid bilayers have been used to mimic various biological barriers from sub-cellular to organ levels including mitochondrion, red blood cell, and liver cell membranes by altering the phospholipid, cholesterol, and glycolipid components21. These more complex multi-lipid vesicles may require additional methods to achieve vesicle rupture, depending on the lipid composition. For example, previous studies have utilized an α-helical (AH) peptide derived from the hepatitis C virus's nonstructural protein 5A to induce bilayer formation by destabilizing the adsorbed lipid vesicles22,23. Using this AH peptide, supported lipid bilayers mimicking placental cells have previously been formed24. The great potential of supported lipid bilayers for biomedical applications has been demonstrated with investigations spanning molecular and nanoparticle transport25, 26, environmental toxicant interactions27, protein assembly and function17,18,19, peptide arrangement and insertion28, 29, drug screening30, and microfluidic platforms31.
Suspended lipid bilayers have been used for pharmaceutical screening studies via a parallel artificial membrane permeability assay (PAMPA) where a lipid bilayer is suspended across a porous hydrophobic insert32,33,34,35. PAMPA lipid models have been developed for different biological interfaces including the blood-brain, buccal, intestinal, and transdermal interfaces36. By combining both the supported lipid bilayer and PAMPA techniques, adsorption, permeability, and embedment of compounds within lipid components of a desired tissue or cell type can be thoroughly studied.
This protocol describes the fabrication and application of in vitro cell membrane lipid bilayer models to investigate several molecular interactions. Preparation of both uni-lipid and multi-lipid supported and suspended lipid bilayers is detailed. To form a supported lipid bilayer, lipid vesicles are first developed using thin-film hydration and extrusion methods followed by physicochemical characterization. Formation of a supported lipid bilayer using QCM-D monitoring and fabrication of suspended lipid membranes for use in PAMPA is discussed. Finally, multi-lipid vesicles for the development of more complex cell mimicking membranes are examined. Using both types of fabricated lipid membranes, this protocol demonstrates how this tool can be used to study molecular interactions. Overall, this technique constructs cell mimicking lipid bilayers with high reproducibility and versatility.
Protokół
1. Developing uni-lipid vesicles
- Thin-film hydration method
- Preparation and storage of lipid stock solutions
NOTE: All steps using chloroform need to be performed in a chemical fume hood. Chloroform should always be pipetted using solvent safe carbon fiber pipette tips. Solutions containing chloroform should always be stored in glass vials.- Prepare a 10 mg/mL lipid stock solution by adding the appropriate volume of chloroform into the vial containing the lipid powder and mix well. For example, add 20 mL of chloroform to 200 mg of L-α-phosphatidylcholine (egg, chicken) (eggPC). The stock solution may be made at a different concentration if needed.
NOTE: If the powder lipid was stored in an ampule, after adding chloroform transfer to a glass vial with a polytetrafluoroethylene (PTFE) lined cap. - Seal the vial cap with Parafilm and store at -20 °C for up to 6 months.
- Prepare a 10 mg/mL lipid stock solution by adding the appropriate volume of chloroform into the vial containing the lipid powder and mix well. For example, add 20 mL of chloroform to 200 mg of L-α-phosphatidylcholine (egg, chicken) (eggPC). The stock solution may be made at a different concentration if needed.
- Formation of a dry-lipid film
- Add the appropriate volume of lipid stock solution into a clean glass vial needed for a final vesicle concentration of 2.5 mg/mL. For example, to form 1 mL of egg PC vesicles at 2.5 mg/mL, pipette 250 µL of egg PC stock solution into the vial.
NOTE: The prepared volume may depend on the extruder process being used (see step 1.3). The mini extruder maximum recommended volume is 1 mL while the large extruder volume range is 5-50 mL. - Remove chloroform from lipid stock solution using a stream of N2 gas (ultrapure 5.0 Grade).
- To ensure full removal of chloroform, connect the dried lipid film to vacuum and leave for at least 4 h.
NOTE: The process can be stopped here. If the lipid film will not be used immediately after vacuum drying, store in a desiccator until used. We have observed that these lipid films yield similar quality vesicles after 1 week of storage at these conditions; the vesicle quality following lengthier storage durations, if necessary, should be further explored.
- Add the appropriate volume of lipid stock solution into a clean glass vial needed for a final vesicle concentration of 2.5 mg/mL. For example, to form 1 mL of egg PC vesicles at 2.5 mg/mL, pipette 250 µL of egg PC stock solution into the vial.
- Performing freeze-thaw-vortex cycles
- Prepare a Tris sodium chloride (NaCl) buffer solution containing 10 mM of Tris base and 100 mM of NaCl. Rehydrate the dried lipid film with the required volume of Tris NaCl buffer to yield a final vesicle concentration of 2.5 mg/mL and vortex for approximately 15-30 s.
- Transfer the vesicle suspension into a container with dry ice until frozen, approximately 30 min. After the sample is completely frozen, thaw the suspension in a 30-40 °C water bath. Vortex the thawed vesicle suspension.
NOTE: Liquid N2 may be used in place of dry ice. Transfer the vesicle suspension into liquid nitrogen for 30 s, and then immediately thaw in an 80°C water bath. - Repeat step 1.1.3.2 an additional 4 times, for a total of 5 freeze-thaw-vortex cycles.
- Preparation and storage of lipid stock solutions
- Extrusion
NOTE: After freeze-thaw-vortex cycles are completed, multilamellar vesicles are formed. Extrusion aids in reducing size and developing large unilamellar vesicles.- Mini (1 mL) extruder process
- Thoroughly clean all components of the extruder using a mild detergent in ultrapure water and rinse at least three times with ultrapure water ensuring all detergent is removed. Dry with N2 gas.
- Assemble the two internal membrane supports and O-rings (inner diameter of 12.7 mm; outer diameter of 15.2 mm). Position each membrane support so the O-ring is facing up.
- Pre-wet a filter support with ultrapure water. Place it on the membrane support surface inside the O-ring. Repeat for the second internal membrane support.
- Position one internal membrane support into the extruder outer casing. Place one 100 nm polycarbonate membrane onto the internal membrane support, directly over the filter support.
NOTE: The polycarbonate membranes are stored separately between pieces of blue colored paper. Remove the separating paper before inserting onto the membrane support. - Position the second internal membrane support into the extruder outer casing with the O-ring and filter support side facing the polycarbonate membrane. Attach the PTFE bearing into the retainer nut and screw closed with the extruder outer casing. Clip the extruder into the heating block.
- Load the lipid vesicle suspension into one of the syringes and position the syringe into the extruder heat block, inserting the needle fully into one end of the extruder. Insert the second, empty syringe into the opposite side and lock in both syringes using the arm clips on the heat block.
NOTE: If needed, place the extruder heat block on a hot plate and set the temperature to a value above the transition temperature of the lipid. Insert a thermometer into the holder built into the heat block for accurate temperature readings and wait until required temperature is reached (~15 min). Egg PC lipid vesicles do not require heat during extrusion. - Slowly push the vesicle suspension into the empty syringe, and then back into the original syringe. Monitor for pressure-changes throughout the extrusion that indicate a leak. Repeat 20 more times for a total of 21 passes through the polycarbonate membrane. Transfer the lipid vesicles into to a clean glass vial for storage.
NOTE: The number of extrusions can be optimized depending on the lipid composition. - If heat was used, allow for extruded vesicle suspension to reach room temperature. Store the extruded lipid vesicles at 4 °C until further use.
NOTE: The recommended vesicle storage duration is highly dependent on the lipid composition, and the vesicle physicochemical properties (e.g., hydrodynamic diameter, zeta potential) should be monitored over time. For example, egg PC vesicles have been stored for at least two weeks with no change in vesicle size or bilayer formation capacity.
- Large (5-50 mL) extruder process
NOTE: Follow steps 1.2.2.1-1.2.2.5 if heat is required for chosen lipid. Skip to step 1.2.2.5 if heat is not needed. Steps 1.2.2.1-1.2.2.4 are not required for egg PC.- Fill a 1 L flask with reverse osmosis (RO) water.
NOTE: Do not use ultrapure water to circulate through the 50 mL system as it can cause metal ions to leach from the extruder cylinder. - Place the 1 L flask in a water bath on a hot plate and set the hot plate to a temperature above the transition temperature of the lipid.
- Attach the sample cylinder to the flask with flexible tubing via the inlet on the sample cylinder. Attach tubing on the outlet of the cylinder to the top of the 1 L flask. Secure tubing at both the inlet and outlet, as needed. This will create a uni-directional flow of the water through the sample cylinder.
- Turn on the pump to start water circulation. If heat is needed, allow for approximately 30-45 minutes for sample cylinder to reach desired temperature.
- Connect the cap of the sample cylinder to a nitrogen tank via the flexible connector attached to the pressure relief valve unit.
- Clean all parts of the 50 mL extruder with 70% (v/v) ethanol.
- Assemble the extruder by placing the large hole screen support, sintered disk, drain disks, and polycarbonate membrane into the space in the extruder lower support. Connect the extruder upper and lower supports using the four screws and tighten.
- Attach the extruder unit to the sample cylinder by screwing to the bottom and tightening with a wrench to secure.
NOTE: If heat is used, place a thermometer into the cylinder and wait until water has reached desired temperature before continuing. This will ensure that the sample temperature is maintained throughout the entire extrusion process. - Fill the sample cylinder with ultrapure water. Extrude the water through the extruder unit prior to adding the sample into the sample cylinder. This is done to pre-wet the membranes, similar to the mini extruder.
NOTE: Ensure the cap is fully screwed on and the pressure relief valve is closed completely before turning on the nitrogen. Minimal pressure is required for this step (~5-10 psi). - Add the lipid vesicle suspension into the sample cylinder and screw the top closed. Slowly increase the pressure until the sample begins to drip from the extruder unit at a rate of approximately 2-3 drops/s into a clean glass vial.
NOTE: Do not increase the pressure quickly at this step, as too much pressure may negatively impact the membranes and lead to unsuccessful extrusion. - Once all sample has been extruded, turn off the N2 supply and release the pressure in the sample cylinder by opening the pressure relief valve slowly. Pour the lipid vesicles back into the sample cylinder and repeat step 1.2.2.11, 9 more times for a total of 10 extrusions.
NOTE: The required pressure for extrusion may decrease with increasing number of extrusions, as the sample becomes more homogeneous and closer in size to the polycarbonate membrane pore size. - Store extruded lipid vesicle suspension at 4 °C until further use.
- Fill a 1 L flask with reverse osmosis (RO) water.
- Mini (1 mL) extruder process
2. Characterizing lipid vesicles
- Hydrodynamic diameter measurement using dynamic light scattering (DLS)
- Vortex lipid vesicles and pipette 50 µL of the lipid vesicle suspension into a disposable low-volume cuvette. Cover to prevent contamination with dust and debris.
- Load the vesicle suspension into the DLS instrument, input the sample details, and perform the measurement using the associated software.
- Zeta potential
- Prepare a folded capillary zeta cell by washing with ultrapure water, 70% ethanol, and ultrapure water using syringes that connect to the inputs of the cell. Gently push the liquid through the cell 3-4 times and empty the cell completely before switching to the next solution.
- Vortex the lipid vesicles and prepare a 1:10 (v/v) dilution of lipid vesicles in ultrapure water.
- Load the diluted lipid vesicle suspension. Remove air bubbles by pushing the suspension back and forth between the syringes. Attach the stoppers to each inlet.
NOTE: It is crucial to remove all bubbles, as this will impact the measurement. - Place the zeta cell in the sample chamber, ensuring that the electrodes are in contact. Close the sample chamber top. In the associated software, input the sample details and collect measurement.
3. Forming a uni-lipid supported lipid bilayer using QCM-D
- Solution preparations
- Prepare a 2% (w/v) sodium dodecyl sulfate (SDS) solution in ultrapure water. Mix on a stir plate until completely dissolved. Aliquot working solutions of at least 10 mL of ultrapure water, 2% SDS, and Tris NaCl.
- Prepare a dilution of lipid vesicles in Tris NaCl buffer. The concentration of vesicles is dependent on the application. For egg PC, concentrations in the range of 0.01-0.5 mg/mL have been shown to result in successful supported lipid bilayer formation.
- Cleaning silica-coated quartz crystal sensors
NOTE: Cleaning QCM-D crystals is dependent on the surface material of the sensor being used. To form supported lipid bilayers, silica-coated quartz crystals are used in this protocol and detailed below as adapted from the manufacturer's standard operating procedure.- Insert the silica-coated quartz crystal sensor into the flow module ensuring that the "t" on the crystal aligns with the "t" on the module. Screw the flow module closed.
NOTE: If the QCM-D utilized allows for multiple flow modules to be connected and run simultaneously, repeat the following procedures for the additional modules as needed. - Insert the flow module into the base of the instrument with the electrodes from the flow module connecting with the analyzer system. Lock the module into place.
- Connect the inlet and outlet tubing to the flow module and pump. Place the tubing into the holding guards and close the lid of the analyzer system. Place a waste container at the outlet of the pump to collect spent solutions.
- To perform the cleaning, first turn on the pump. Set the flow speed to 400 μL/min. Insert the inlet tubing into ultrapure water and flow 5-10 mL through the module.
- Switch the inlet tubing into 2% SDS and flow 5-10 mL through the module. Switch the inlet tubing back into ultrapure water and flow 10-20 mL through the module. Remove the inlet tubing from the solution and flow air through the tubing until all liquid is ejected.
NOTE: The cleaning protocol above is used daily before and after every measurement. A thorough cleaning can be performed as needed. Briefly, to perform a thorough cleaning, disassemble the flow modules. All components except for the electrode side of the flow module should be immersed in 2% (w/v) SDS and bath sonicated, followed by thorough rinsing with ultrapure water and drying with a stream of N2 gas. The component of the flow module containing the electrode pins should never be in contact with liquid. - Remove the sensor from the flow module and rinse the sensor with ultrapure water. Dry the sensor with an N2 gas stream. Dry the flow module with an N2 gas stream. Ensure the electrode always remain free of any liquid.
- In a chemical fume hood, insert the silica-coated quartz crystal sensor into an ultra-violet (UV)/ozone cleaning instrument. Turn on the instrument and allow treatment for at least 2 min. Remove the sensors carefully and return into the flow module.
- Insert the silica-coated quartz crystal sensor into the flow module ensuring that the "t" on the crystal aligns with the "t" on the module. Screw the flow module closed.
- Forming a Tris NaCl baseline
- Turn the analyzer instrument on to connect to the associated software and set temperature to the desired value for the supported lipid bilayer. Allow the temperature to stabilize to the desired input.
NOTE: If the set temperature is above room temperature, all solutions should be heated to the same temperature using a heat block. - Configure the measurement and find all sensor resonance frequencies and dissipations for overtones 3, 5, 7, 9, 11, and 13 before starting the measurement.
NOTE: The 1st overtone can be disregarded as this harmonic is overly sensitive and produces noisy data. - Turn on the pump and set the flow rate to 175 μL/min or the desired experimental flow rate.
- Wipe the inlet tubing with ethanol prior to inserting into Tris NaCl. Start the measurement and begin flowing Tris NaCl.
NOTE: Data is collected and monitored in real-time. The change from air to liquid in the flow module will be observed in the data collection software by a rapid dissipation change (ΔD) increase and frequency change (ΔF) decrease. - Allow Tris NaCl to flow through the module for 5-10 min, ensuring that the baseline ΔF and ΔD values in liquid remain stable.
- Turn the analyzer instrument on to connect to the associated software and set temperature to the desired value for the supported lipid bilayer. Allow the temperature to stabilize to the desired input.
- Forming a uni-lipid supported lipid bilayer
- Stop the pump and remove the inlet tubing from the Tris NaCl solution and carefully insert into the lipid vesicle solution. Back flow for 5 s to remove any air bubbles from the inlet tubing, and then continue forward flow. Restart the measurement in the software to zero the baseline.
NOTE: Be careful to avoid air bubbles in the tubing, which can flow through the module and disrupt bilayer formation and the data recording. - Flow lipid vesicles until bilayer formation is observed in real-time in the data acquisition software (at least 8 min for egg PC vesicles).
- Repeat step 3.4.1 to change the inlet tubing from lipid vesicles back into Tris NaCl buffer.
NOTE: If the desired application is to study molecular interactions, continue directly to step 6.1 without stopping the solution flow or data acquisition. If bilayer formation is the endpoint, proceed to step 3.4.4. - In the software, stop the measurement and save the file. Stop the pump.
- Clean the flow module and silica-coated quartz crystal sensor following the protocol steps 3.2.4 and 3.2.5.
- Stop the pump and remove the inlet tubing from the Tris NaCl solution and carefully insert into the lipid vesicle solution. Back flow for 5 s to remove any air bubbles from the inlet tubing, and then continue forward flow. Restart the measurement in the software to zero the baseline.
4. Forming a suspended lipid bilayer
NOTE: The protocol for forming a suspended lipid bilayer is adapted from the parallel artificial membrane permeability assay (PAMPA) protocol provided by the filter plate manufacturer37.
- Solubilize desired lipid in dodecane at 20 mg/mL (e.g., 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC)).
- Add 5 μL of the lipid solution to the donor compartment, which is a porous polyvinylidene difluoride (PVDF) 96-well multiscreen filter plate (0.45 μm pore size).
- Immediately submerge the filter plate into the acceptor compartment, which is a transport receiver plate containing 300 μL of 1× phosphate buffered saline (PBS). Add 200 μL of 1× PBS to the donor compartment.
NOTE: Controls of filters with lipid only and untreated filters exposed to 1× PBS may be included. - Continue directly to section 6.2 to investigate molecular interactions with the suspended lipid bilayer. It is recommended to complete the study within 16 h of forming the suspended bilayer.
5. Developing multi-lipid cell mimicking vesicles and bilayers
- Lipid extraction from mammalian cells
NOTE: Lipid extraction follows the Bligh-Dyer approach38.- Culture the desired cell line as appropriate. After achieving 70-80% confluence (T75 flask), detach cells using trypsin-ethylenediaminetretaacetic acid at 37 °C for 5 min.
- Centrifuge cells at 200 × g for 5 min. Remove the supernatant and resuspend the cell pellet in 1 mL of ultrapure water.
- Add 3.75 mL of a 1:2 (v/v) mixture of chloroform:methanol to the cell suspension and vortex for 15 min. Then, add 1.25 mL of chloroform and vortex for 1 min. Finally, add 1.25 mL of water and vortex for 1 min.
- Centrifuge cell mixture at 1000 x g for 10 min. Collect the bottom layer of liquid, which contains lipids in the organic phase. Dry under a stream of N2 gas.
- Quantify the lipid content using liquid chromatography-mass spectrometry (LC-MS) using a C18 reverse phase, 3.5 μm × 50 mm column.
- For the mobile phase, prepare two solutions, the first with 60:40 (v/v) acetonitrile:water and the second with 90:10 (v/v) isopropanol:acetonitrile. Ammonium formate should be added to both solutions at a final concentration of 10 mM. Over 60 min, increase the mobile phase gradient from 35% (v/v) of the second solution to 95% (v/v).
- Detect the effluent in negative ionization mode, with consecutive full-scan MS and tandem MS/MS. Identify the individual phospholipid species from their mass-to-charge (m/z) ratios. Analyze the mass spectra from collision-induced dissociation fragmentation, using LIPID MAPS mass spectrometry analysis tools. Obtain extracted ion chromatograms to integrate the area under the curve, determining the abundance of each lipid species.
- Perform steps 5.1.5-5.1.7 for a lipid standard containing the major lipid classes to determine the relative sensitives of detection for each different phospholipid class.
- Developing multi-lipid vesicles
- Follow steps in 1.1.1 to prepare lipid stock solutions for lipids representing each desired bilayer component, as identified in step 5.1.
- Based on the lipid compositions obtained from step 5.1, add the appropriate volume of lipid/chloroform stock into a clean glass vial needed for a final vesicle concentration of 2.5 mg/mL. Remove bulk chloroform drying solution under a stream of N2 gas.
- Follow steps 1.1.2, 1.1.3, and 1.2 to form multi-lipid vesicles. Follow step 2 for vesicle characterization.
- Forming a multi-lipid supported lipid bilayer using QCM-D
NOTE: Some multi-lipid vesicles can result in spontaneous lipid vesicle rupture and bilayer formation similar to uni-lipid PC vesicles presented in step 3. However, more complex multi-lipid vesicles may require external input to aid in vesicle rupture. Here, the AH peptide is used to destabilize the outer leaflet of the vesicle resulting in bilayer formation. Other methods to achieve destabilization and vesicle rupture may be considered if desired.- Follow step 3 to form the multi-lipid supported lipid bilayer utilizing the multi-lipid vesicles formed in step 5.2.
- If spontaneous rupture of the vesicles into a bilayer is not observed, attempt vesicle destabilization using the AH peptide. Prepare the AH peptide (peptide sequence: H-Ser−Gly−Ser−Trp−Leu−Arg−Asp−Val−Trp−Asp−Trp−Ile−Cys−Thr−Val−Thr−Asp−Phe−Lys−Thr−Trp−Leu−Gln−Ser−Lys−Leu−Asp−Tyr−Lys−Asp-NH2) solution at 13 μM in Tris NaCl with 1% (v/v) dimethylsulfoxide, DMSO.
- Follow steps 3.4.1-3.4.3. After step 3.4.3, change the inlet tubing into the AH peptide solution. Introduce the solution into the flow module until ΔF and ΔD are observed from the new solution addition. Stop the pump and allow the AH peptide to incubate with the vesicles for 10 min.
- Switch the inlet tubing into Tris NaCl and start the flow to remove the AH peptide from the ruptured vesicles leading to successful formation of a lipid bilayer.
NOTE: If the desired application is to study molecular interactions, continue direction to step 6.1 without stopping the solution flow or data acquisition. - In the software, stop the measurement and save the file. Stop the pump.
- Clean the flow module and silica-coated quartz crystal sensor following the protocol steps 3.2.4-3.2.6.
- Suspended multi-lipid bilayers
- Solubilize mixture of desired lipids in dodecane at 20 mg/mL.
- Make up a 5 μL lipid mix solution using the desired cell mimicking composition.
- Follow steps 4.2 and 4.3.
NOTE: Continue directly to step 6.2 to investigate molecular interactions with the suspended lipid bilayer.
6. Molecule interaction studies with uni-lipid and multi-lipid bilayers
- Studying molecular interactions with a supported lipid bilayer using QCM-D
- Prepare a solution of the desired molecule to investigate adsorption with a supported lipid bilayer. For example, prepare a solution of 200 μM di(2-ethylhexyl) phthalate (DEHP) in Tris NaCl with 1% (v/v) DMSO.
- If the molecule solution is prepared in Tris NaCl, it can be flowed directly following step 3.4.3 for a uni-lipid bilayer or 5.3.4 for a multi-lipid bilayer. If the molecule must be prepared in a different solvent, instead insert the inlet tubing into the desired solvent alone for at least 5 min (e.g., Tris NaCl with 1% (v/v) DMSO for DEHP).
NOTE: Viscosity changes due to the solvent can be monitored and considered by flowing it prior to and after introduction of the molecule of interest. - Switch the inlet tubing into the solution containing the molecule of interest and flow for at least 5 min. The flow may also be stopped and liquid containing the desired molecule allowed to incubate with the bilayer if desired.
- Change the inlet tubing back to the molecule solvent alone if something other than Tris NaCl. Flow for at least 5 min. Then, switch the inlet tubing into Tris NaCl and flow for at least 5 min.
- In the software, stop the measurement and save the file. Stop the pump.
- Clean the flow module and silica-coated quartz crystal sensor following the protocol steps 3.2.4-3.2.6.
- Studying molecular interactions with suspended lipid bilayers using PAMPA
- Prepare a solution of the desired molecule. For example, prepare a 200 μM DEHP in 1× PBS with 1% (v/v) DMSO.
- Prepare a new transport receiver plate with 300 μL of fresh 1x PBS per well.
- Immediately following step 3.3 for a uni-lipid suspended bilayer or 4.4.3 for a multi-lipid suspended bilayer, remove the 1× PBS from the donor compartment of the multiscreen filter plate and replace with 200 μL of the test solution. Immediately submerge in the transport receiver plate prepared in step 6.2.2.
- Incubate with gentle rocking for a desired amount of time (e.g., 2 h) at 25 °C.
- After incubation, collect 150 μL of the solution from the donor and acceptor compartments. Measure the molecule concentration in both samples using an appropriate method based on properties of this molecule.
- For example, use a microplate spectrophotometer with the appropriate absorbance wavelength, such as 280 nm for DEHP, and compare with a standard curve of the molecule of interest.
- Calculate the apparent permeability (Papp) of the molecule of interest using the following equations:
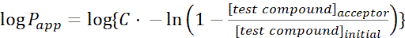 (1)
(1)
Where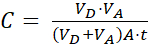 (2)
(2)
NOTE: [test compound]acceptor is the concentration of the molecule of interest (e.g., DEHP) at time, t, in the acceptor compartment; and [test compound]initial is the initial concentration of the molecule. A is the membrane area, t is the time, VD is the donor compartment volume, and VA is the acceptor compartment volume.
Wyniki
This protocol details methods for forming supported and suspended lipid bilayers (Figure 1). The first step to forming a supported lipid bilayer is to develop lipid vesicles. The mini extruder allows for small volumes of lipid vesicles to be prepared (1 mL or less), while the large extruder allows for 5-50 mL of lipid vesicles to be prepared in one batch. Size distributions of uni-lipid vesicles formed by either the mini or large extruder are shown in Figure 2A....
Dyskusje
This protocol allows for the formation of lipid vesicles, supported lipid bilayers, and suspended lipid bilayers. Here, critical steps are presented to form each of these structures. When forming lipid vesicles, it is important to extrude above the transition temperature of the lipid39. When below the transition temperature, the lipid is physically present in its ordered gel phase39. In this ordered phase the hydrocarbon lipid tails are fully extended allowing for close pac...
Ujawnienia
The authors declare that they have no conflict of interest or competing financial interests.
Podziękowania
This material is based upon work supported by the National Science Foundation under Grant No. 1942418 awarded to A.S., and a National Science Foundation Graduate Research Fellowship awarded to C.M.B.H., under Grant No. 1644760. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors thank Dr. Noel Vera-González for lipid vesicle characterization data acquisition. The authors thank Professor Robert Hurt (Brown University) for the use of his Zetasizer. The authors thank the Brown University Mass Spectrometry Facility, in particular, Dr. Tun-Li Shen for assistance with quantifying lipid composition.
Materiały
| Name | Company | Catalog Number | Comments |
| 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC, 16:0-18:1 PC) | Avanti Polar Lipids | 850457 | |
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (sodium salt) (POPS, 16:0-18:1 PS) | Avanti Polar Lipids | 840034 | |
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (16:0-18:1 PE) | Avanti Polar Lipids | 850757 | |
| 1,2-dioleoyl-sn-glycero-2-phospho-L-serine (DOPS, 18:1 PS) | Avanti Polar Lipids | 840035 | |
| 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC, 18:1 (Δ9-Cis) PC) | Avanti Polar Lipids | 850375 | |
| 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, 18:1 (Δ9-Cis) PE) | Avanti Polar Lipids | 850725 | |
| 1,2-distearoyl-sn-glycero-3-ethylphosphocholine (chloride salt) (18:0 EPC (Cl Salt)) | Avanti Polar Lipids | 890703 | |
| 3 mL Luer-Loc syringes | BD | 309657 | |
| 40 mL sample vial, amber with polytetrafluoroethylene (PTFE)/rubber liner | Duran Wheaton Kimble | W224605 | |
| Acetonitrile | Sigma-Aldrich | 271004 | |
| Alconox | Fisher Scientific | 50-821-781 | |
| Ammonium formate | Millipore Sigma | LSAC70221 | |
| C18, 3.5 um x 50 mm column, SunFire | Waters | 186002551 | |
| Chloroform | Millipore Sigma | LSAC288306 | |
| Cuvette UV Micro LCH 8.5 mm, 50 um, RPK | Sarstedt | 67.758.001 | |
| Di(2-ethylhexyl) phthalate (DEHP) | Millipore Sigma | 36735 | |
| Dimethyl sulfoxide (DMSO) | Millipore Sigma | LSAC472301 | |
| Ethanol | Pharmco | 111000200 | |
| Filter supports, 10 mm | Avanti Polar Lipids | 610014 | Size for mini extruder |
| Folded capillary zeta cell | Malvern Panalytical | DTS1070 | |
| Isopropanol | Sigma-Aldrich | 190764-4L | |
| Kimwipes | Kimberly Clark | 34256 | |
| L-α-phosphatidylinositol (soy) (Soy PI) | Avanti Polar Lipids | 840044 | |
| L-α-phosphitidylcholine (Egg, Chicken) | Avanti Polar Lipids | 840051 | |
| LiposoFast ® LF-50 | Avestin, Inc. | ||
| Methanol | Sigma-Aldrich | 179337 - 4L | |
| Mini-extruder set with holder/heating block | Avanti Polar Lipids | 610000 | |
| MultiScreen-IP Filter Plate, 0.45 µm, clear, sterile | Millipore Sigma | MAIPS4510 | for PAMPA studies |
| Nitrogen gas, ultrapure | TechAir | NI T5.0 | |
| Nuclepore hydrophilic membranes, polycarbonate, 19 mm, 0.1 um | Whatman | 800309 | Size for mini extruder |
| Nuclepore hydrophilic membranes, polycarbonate, 25 mm, 0.1 um | Whatman | 110605 | Size for large extruder |
| Parafilm | Bemis | PM999 | |
| Phosphate buffer saline (PBS), 10x | Genesee Scienfitic | 25-507X | Dilute to 1x |
| Qsoft 401 software | Biolin Scientific | ||
| Quartz Crystal Microbalance with Dissipation Q-Sense Analyzer | Biolin Scientific | ||
| Scintillation vials, borosilicate glass vials, 20 mL | Duran Wheaton Kimble | 986561 | |
| Silicon Dioxide, thin QSensors | Biolin Scientific | QSX 303 | |
| Sodium chloride (NaCl) | Millipore Sigma | LSACS5886 | |
| Sodium dodecyl sulfate (SDS) | Fisher Scientific | BP166-100 | |
| Solvent Safe pipette tips | Sigma-Aldrich | S8064 | |
| Sphingomyelin (Egg, Chicken) | Avanti Polar Lipids | 860061 | |
| Trizma base | Millipore Sigma | LSACT1503 | |
| Trypsin-ethylenediaminetretaacetic acid | Caisson Labs | TRL01-6X100ML | |
| Whatman drain disc, 25 mm | Whatman | 230600 | Size for large extruder |
| Zetasizer ZS90 | Malvern Panalytical | ||
| Zetasizer 7.01 software | Malvern Panalytical |
Odniesienia
- Lucio, M., Lima, J. L. F. C., Reis, S. Drug-Membrane Interactions: Significance for Medicinal Chemistry. Current Medicinal Chemistry. 17 (17), 1795-1809 (2010).
- Mayne, C. G., et al. The cellular membrane as a mediator for small molecule interaction with membrane proteins. Biochimica et Biophysica Acta - Biomembranes. 1858 (10), 2290-2304 (2016).
- Bunea, A. I., Harloff-Helleberg, S., Taboryski, R., Nielsen, H. M. Membrane interactions in drug delivery: Model cell membranes and orthogonal techniques. Advances in Colloid and Interface Science. 281, 102177 (2020).
- Peetla, C., Stine, A., Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Molecular Pharmaceutics. 6 (5), 1264-1276 (2009).
- Richter, R., Mukhopadhyay, A., Brisson, A. Pathways of Lipid Vesicle Deposition on Solid Surfaces: A Combined QCM-D and AFM Study. Biophysical Journal. 85 (5), 3035-3047 (2003).
- Lind, T. K., Cárdenas, M., Wacklin, H. P. Formation of supported lipid bilayers by vesicle fusion: Effect of deposition temperature. Langmuir. 30 (25), 7259-7263 (2014).
- Mingeot-Leclercq, M. -. P., Deleu, M., Brasseur, R., Dufrêne, Y. F. Atomic force microscopy of supported lipid bilayers. Nature protocols. 3 (10), 1654-1659 (2008).
- Richter, R. P., Bérat, R., Brisson, A. R. Formation of solid-supported lipid bilayers: an integrated view. Langmuir the ACS journal of surfaces and colloids. 22 (8), 3497-3505 (2006).
- Chan, Y. -. H. M., Boxer, S. G. Model membrane systems and their applications. Current Opinion in Chemical Biology. 11 (6), 581-587 (2007).
- Edvardsson, M., Svedhem, S., Wang, G., Richter, R., Rodahl, M., Kasemo, B. QCM-D and reflectometry instrument: applications to supported lipid structures and their biomolecular interactions. Analytical chemistry. 81 (1), 349-361 (2009).
- Rodahl, M., et al. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discussions. 107, 229-246 (1997).
- Keller, C. A., Glasmästar, K., Zhdanov, V. P., Kasemo, B. Formation of Supported Membranes from Vesicles. Physical Review Letters. 84 (23), 5443-5446 (2000).
- Keller, C. A., Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophysical journal. 75 (3), 1397-1402 (1998).
- Cho, N. -. J., Frank, C. W., Kasemo, B., Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nature protocols. 5 (6), 1096-1106 (2010).
- Bailey, C. M., Tripathi, A., Shukla, A. Effects of Flow and Bulk Vesicle Concentration on Supported Lipid Bilayer Formation. Langmuir. 33 (43), 11986-11997 (2017).
- van Meer, G., Voelker, D. R., Feigenson, G. W. Membrane lipids: where they are and how they behave. Nature reviews. Molecular cell biology. 9 (2), 112-124 (2008).
- Rossi, C., Chopineau, J. Biomimetic tethered lipid membranes designed for membrane-protein interaction studies. European Biophysics Journal. 36 (8), 955-965 (2007).
- Hatty, C. R., et al. Investigating the interactions of the 18 kDa translocator protein and its ligand PK11195 in planar lipid bilayers. Biochimica et Biophysica Acta - Biomembranes. 1838 (3), 1019-1030 (2014).
- Min, Y., Kristiansen, K., Boggs, J. M., Husted, C., Zasadzinski, J. a., Israelachvili, J. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proceedings of the National Academy of Sciences of the United States of America. 106 (9), 3154-3159 (2009).
- Heath, G. R., et al. Layer-by-layer assembly of supported lipid bilayer poly-l-lysine multilayers. Biomacromolecules. 17 (1), 324-335 (2016).
- Alberts, B., Lewis, J. The Lipid Bilayer. Molecular Biology of the Cell. , 6-11 (2013).
- Cho, N. J., Wang, G., Edvardsson, M., Glenn, J. S., Hook, F., Frank, C. W. Alpha-helical peptide-induced vesicle rupture revealing new insight into the vesicle fusion process as monitored in situ by quartz crystal microbalance-dissipation and reflectometry. Analytical Chemistry. 81 (12), 4752-4761 (2009).
- Hardy, G. J., Nayak, R., Munir Alam, S., Shapter, J. G., Heinrich, F., Zauscher, S. Biomimetic supported lipid bilayers with high cholesterol content formed by α-helical peptide-induced vesicle fusion. Journal of Materials Chemistry. 22 (37), 19506-19513 (2012).
- Bailey-Hytholt, C. M., Shen, T. L., Nie, B., Tripathi, A., Shukla, A. Placental Trophoblast-Inspired Lipid Bilayers for Cell-Free Investigation of Molecular Interactions. ACS Applied Materials and Interfaces. 12 (28), 31099-31111 (2020).
- Domenech, O., Francius, G., Tulkens, P. M., Van Bambeke, F., Dufrêne, Y., Mingeot-Leclercq, M. -. P. Interactions of oritavancin, a new lipoglycopeptide derived from vancomycin, with phospholipid bilayers: Effect on membrane permeability and nanoscale lipid membrane organization. Biochimica et biophysica acta. 1788 (9), 1832-1840 (2009).
- Bailey, C. M., Kamaloo, E., Waterman, K. L., Wang, K. F., Nagarajan, R., Camesano, T. a. Size dependence of gold nanoparticle interactions with a supported lipid bilayer: A QCM-D study. Biophysical Chemistry. 203-204, 51-61 (2015).
- Bailey-Hytholt, C. M., Puranik, T., Tripathi, A., Shukla, A. Investigating interactions of phthalate environmental toxicants with lipid structures. Colloids and Surfaces B: Biointerfaces. 190, 110923 (2020).
- Wang, K. F., Nagarajan, R., Camesano, T. A. Antimicrobial peptide alamethicin insertion into lipid bilayer: a QCM-D exploration. Colloids and surfaces. B, Biointerfaces. 116, 472-481 (2014).
- Lozeau, L. D., Rolle, M. W., Camesano, T. A. A QCM-D study of the concentration- and time-dependent interactions of human LL37 with model mammalian lipid bilayers. Colloids and Surfaces B: Biointerfaces. 167 (1), 229-238 (2018).
- Kongsuphol, P., Fang, K. B., Ding, Z. Lipid bilayer technologies in ion channel recordings and their potential in drug screening assay. Sensors and Actuators B: Chemical. 185, 530-542 (2013).
- Ren, X., et al. Design, fabrication, and characterization of archaeal tetraether free-standing planar membranes in a PDMS-and PCB-based fluidic platform. ACS Applied Materials & Interfaces. 6 (15), 12618-12628 (2014).
- Seo, P. R., Teksin, Z. S., Kao, J. P. Y., Polli, J. E. Lipid composition effect on permeability across PAMPA. European Journal of Pharmaceutical Sciences. 29 (3-4), 259-268 (2006).
- Avdeef, A. The rise of PAMPA. Expert Opinion on Drug Metabolism & Toxicology. 1 (2), 325-342 (2005).
- Avdeef, A., Artursson, P., Neuhoff, S., Lazorova, L., Gråsjö, J., Tavelin, S. Caco-2 permeability of weakly basic drugs predicted with the Double-Sink PAMPA method. European Journal of Pharmaceutical Sciences. 24 (4), 333-349 (2005).
- Campbell, S. D., Regina, K. J., Kharasch, E. D. Significance of Lipid Composition in a Blood-Brain Barrier-Mimetic PAMPA Assay. Journal of Biomolecular Screening. 19 (3), 437-444 (2014).
- Berben, P., et al. Drug permeability profiling using cell-free permeation tools: Overview and applications. European Journal of Pharmaceutical Sciences. 119, 219-233 (2018).
- Schmidt, D., Lynch, J. Evaluation of the reproducibility of Parallel Artificial Membrane Permation Assays (PAMPA). EMD Millipore Corporation. , (2020).
- Bligh, E. G., Dyer, W. J. A Rapid Method of Total Lipid Extraction and Purification. Canadian Journal of Biochemistry and Physiology. 37 (8), 911-917 (1959).
- Nayar, R., Hope, M. J., Cullis, P. R. Generation of large unilamellar vesicles from long-chain saturated phosphatidylcholines by extrusion technique. BBA - Biomembranes. 986 (2), 200-206 (1989).
- Lind, T. K., Skida, M. W. A., Cárdenas, M. Formation and Characterization of Supported Lipid Bilayers Composed of Phosphatidylethanolamine and Phosphatidylglycerol by Vesicle Fusion, a Simple but Relevant Model for Bacterial Membranes. ACS Omega. 4 (6), 10687-10694 (2019).
- Berben, P., et al. Drug permeability profiling using cell-free permeation tools: Overview and applications. European Journal of Pharmaceutical Sciences. 119, 219-233 (2018).
- Bermejo, M., et al. PAMPA-a drug absorption in vitro model: 7. Comparing rat in situ, Caco-2, and PAMPA permeability of fluoroquinolones. European Journal of Pharmaceutical Sciences. 21 (4), 429-441 (2004).
- Kerns, E. H., Di, L., Petusky, S., Farris, M., Ley, R., Jupp, P. Application of parallel artificial membrane permeability assay and Caco-2 permeability. Journal of Pharmaceutical Sciences. 93 (6), 1440-1453 (2004).
- Masungi, C., et al. Parallel artificial membrane permeability assay (PAMPA) combined with a 10-day multiscreen Caco-2 cell culture as a tool for assessing new drug candidates. Pharmazie. 63 (3), 194-199 (2008).
- Vera-González, N., et al. Anidulafungin liposome nanoparticles exhibit antifungal activity against planktonic and biofilm Candida albicans. Journal of Biomedical Materials Research - Part A. 108 (11), 2263-2276 (2020).
- Barenholz, Y., Gibbes, D., Litman, B. J., Goll, J., Thompson, T. E., Carlson, F. D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 16 (1), 2806-2810 (1977).
- El Kirat, K., Morandat, S., Dufrêne, Y. F. Nanoscale analysis of supported lipid bilayers using atomic force microscopy. Biochimica et Biophysica Acta - Biomembranes. 1798 (4), 750-765 (2010).
- Tawa, K., Morigaki, K. Substrate-supported phospholipid membranes studied by surface plasmon resonance and surface plasmon fluorescence spectroscopy. Biophysical Journal. 89 (4), 2750-2758 (2005).
- Koenig, B. W., et al. Neutron Reflectivity and Atomic Force Microscopy Studies of a Lipid Bilayer in Water Adsorbed to the Surface of a Silicon Single Crystal. Langmuir. 12 (5), 1343-1350 (1996).
- Lind, T. K., Cárdenas, M. Understanding the formation of supported lipid bilayers via vesicle fusion-A case that exemplifies the need for the complementary method approach (Review). Biointerphases. 11 (2), 020801 (2016).
- Castellana, E. T., Cremer, P. S. Solid supported lipid bilayers: From biophysical studies to sensor design. Surface Science Reports. 61 (10), 429-444 (2006).
- Isaksson, S., et al. Protein-Containing Lipid Bilayers Intercalated with Size-Matched Mesoporous Silica Thin Films. Nano Letters. 17 (1), 476-485 (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone