Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Measurement of Fatty Acid β-Oxidation in a Suspension of Freshly Isolated Mouse Hepatocytes
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Fatty acid β-oxidation is an essential metabolic pathway responsible for generating energy in many different cell types, including hepatocytes. Here, we describe a method to measure fatty acid β-oxidation in freshly isolated primary hepatocytes using 14C-labeled palmitic acid.
Streszczenie
Fatty acid β-oxidation is a key metabolic pathway to meet the energy demands of the liver and provide substrates and cofactors for additional processes, such as ketogenesis and gluconeogenesis, which are essential to maintain whole-body glucose homeostasis and support extra-hepatic organ function in the fasted state. Fatty acid β-oxidation occurs within the mitochondria and peroxisomes and is regulated through multiple mechanisms, including the uptake and activation of fatty acids, enzyme expression levels, and availability of cofactors such as coenzyme A and NAD+. In assays that measure fatty acid β-oxidation in liver homogenates, cell lysis and the common addition of supraphysiological levels of cofactors mask the effects of these regulatory mechanisms. Furthermore, the integrity of the organelles in the homogenates is hard to control and can vary significantly between preparations. The measurement of fatty acid β-oxidation in intact primary hepatocytes overcomes the above pitfalls. This protocol describes a method for the measurement of fatty acid β-oxidation in a suspension of freshly isolated primary mouse hepatocytes incubated with 14C-labeled palmitic acid. By avoiding hours to days of culture, this method has the advantage of better preserving the protein expression levels and metabolic pathway activity of the original liver, including the activation of fatty acid β-oxidation observed in hepatocytes isolated from fasted mice compared to fed mice.
Wprowadzenie
Fatty acid β-oxidation is an essential process in lipid metabolism, providing a catabolic pathway to balance fatty acid synthesis and intake from the diet. This process generates energy for multiple organs, including the cardiac muscle, kidney cortex, and fasted liver, and utilizes fatty acids obtained from the diet, adipose tissue lipolysis, and internal triglyceride stores1,2.
Oxidation of fatty acid through the β-oxidation pathway results in the sequential shortening of the fatty acyl chain by two carbons at a time, released as acetyl-CoA, and this process occurs both in the mitochondria and the peroxisomes. While most fatty acids undergo only β-oxidation, some are oxidized at different carbons before entering this pathway. For example, 3-methyl-substituted fatty acids, such as phytanic acid, undergo removal of one carbon by α-oxidation in the peroxisomes before entering the β-oxidation pathway. Similarly, some fatty acids are first converted to dicarboxylic fatty acids by oxidation of the terminal methyl group (ω-oxidation) in the endoplasmic reticulum before being preferentially oxidized in the peroxisomes by β-oxidation3.
Regardless of the specific organelle, a fatty acid must first be converted to a coenzyme A (CoA) thioester, or acyl-CoA, to be oxidized through the β-oxidation pathway. β-Oxidation of long-chain acyl-CoAs in the mitochondrial matrix requires the carnitine shuttle for their translocation, where carnitine palmitoyltransferase 1 (CPT1) catalyzes the conversion of acyl-CoAs to acylcarnitines and is the rate-limiting enzyme in this process4. Once translocated to the mitochondrial matrix, the acyl-CoAs are re-formed and serve as substrates for the mitochondrial β-oxidation machinery. In the fasted state, the acetyl-CoA produced through β-oxidation in hepatic mitochondria is primarily channeled to ketogenesis. Peroxisomes serve as the primary site for the β-oxidation of very long-chain, branched-chain, and dicarboxylic fatty acids. Peroxisomes do not require the carnitine shuttle to import fatty acid substrates, instead importing the correspondent acyl-CoAs through the activity of the ATP-binding cassette (ABC) transporters ABCD1-35. Within the peroxisomes, acyl-CoAs are then oxidized by a dedicated set of enzymes, distinct from the mitochondrial fatty acid β-oxidation machinery. Both mitochondria and peroxisomes also require a supply of NAD+ and free CoA to oxidize fatty acyl chains. CoA levels in the liver have been shown to increase in response to fasting, supporting the increased rate of fatty acid oxidation which occurs in this state6. Furthermore, increased CoA degradation in the peroxisomes results in a selective decrease in peroxisomal fatty acid oxidation7. Therefore, the process of fatty acid oxidation within the cell is regulated by the expression levels and activities of enzymes involved in the activation, transport, and oxidation of fatty acids, as well as the concentrations of cofactors and other metabolites throughout multiple subcellular compartments.
Procedures using tissue homogenates to measure fatty acid oxidation destroy the cellular architecture regulating and supporting this process, leading to a collection of data that does not accurately reflect the in vivo metabolism. While techniques using plated primary hepatocytes preserve this system, culturing isolated cells for extended periods of time results in a loss of the in vivo gene expression profile that was present in the cells when they were still living within the animal8,9. The following protocol describes a method to isolate primary hepatocytes and assay their capacity for fatty acid β-oxidation immediately after isolation and in suspension, using [1-14C]palmitic acid. The assay is based on the measurement of the radioactivity associated with the acid-soluble metabolites (ASM) or products, like acetyl-CoA, produced by the β-oxidation of [1-14C]palmitic acid10,11.
Protokół
All experimental procedures on mice (C57BL/6J, males, 9-11 weeks of age) were approved by the Institutional Animal Care and Use Committees (IACUC) of West Virginia University.
1. Hepatocyte isolation
- Preparation
- In the days before the hepatocyte isolation, prepare the buffers and cell culture media listed in Table 1. Set up a water bath with the temperature set to 37 °C close to where the surgery will be performed.
- On the day of the hepatocyte isolation, under a laminar flow hood, transfer 35 mL of Buffer 1 to a sterile 50 mL centrifuge tube and 70 mL of Buffer 2 to a 100 mL sterile beaker or bottle.
- Add antibiotics gentamicin (50 µg/mL) and penicillin/streptomycin (1x) to both buffers.
- Transfer 20 mL of Buffer 2 as prepared in step 1.1.3 to a 100 mm cell culture dish and place on ice.
- Transfer the remaining 50 mL of Buffer 2 to a sterile 50 mL centrifuge tube. Place the 50 mL tubes containing the antibiotic-supplemented Buffers 1 and 2 in a water bath set at 37 °C and let them warm up for at least 15 min before starting the perfusion.
NOTE: If conducting multiple hepatocytes isolations in a session, scale up the number of antibiotic-supplemented aliquots of Buffers 1 and 2 to prepare accordingly. - Thaw an aliquot of collagenase solution and keep on ice.
NOTE: If properly stored, there is no significant loss of enzyme activity in collagenase solutions frozen and thawed up to 3 times and used within 3 weeks from preparation. - Prepare the surgical instruments and peristaltic pump. Sterilize the lines of the peristaltic pump by circulating 15 mL of 70% ethanol, followed by 15 mL of sterile water.
- Connect a 22 G needle to the exit line (Figure 1A). The hollowed filter of a catheter works well as a connector. Fill the lines with Buffer 1 and inspect the lines, connector, and needle to ensure no air bubbles are trapped.
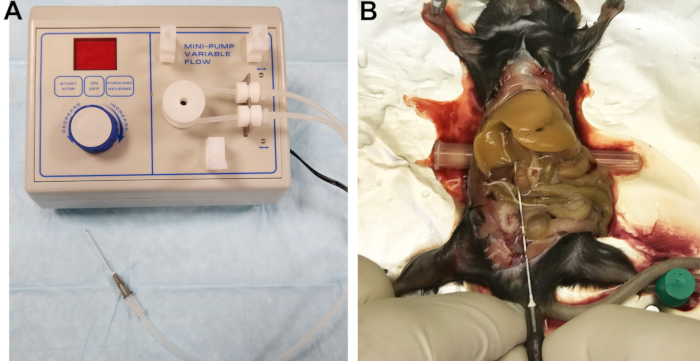
Figure 1: Perfusion apparatus and perfused liver. (A) Peristaltic pump with outlet line connected to the needle used to cannulate and perfuse the liver. (B) Successful cannulation is indicated by immediate and homogeneous blanching of the liver. Please click here to view a larger version of this figure.
- Liver perfusion and dissociation
- Anesthetize a mouse via isoflurane inhalation with medical grade air as carrier gas, using 4% isoflurane for induction and 1.5% isoflurane to maintain anesthesia. Verify the depth of anesthesia by assessing the loss of pedal reflex.
- When there is no response to toe pinching, place the mouse in a supine position on a surgery board, outstretch the limbs, and secure them to the board with pins.
- Liberally spray the abdomen and chest of the mouse with 70% ethanol.
- Using forceps, pull up the skin and abdominal wall near the base of the abdomen and cut laterally, on either side of the midline and up to the diaphragm, to expose the organs.
- Expose the inferior vena cava (IVC) by moving the intestines to the right side and gently flipping the lobes of the liver up. Insert a small cylindrical object, such as a needle cap, under the back of the mouse to slightly tilt the IVC and facilitate its cannulation (Figure 1B).
- Start the pump at the lowest speed and, with Buffer 1 flowing, insert the needle into the IVC.
- Cut the portal vein to relieve the pressure and allow for drainage of blood and perfusion buffers, then immediately increase the flow rate to 7 mL/min. If done correctly, the liver will uniformly blanch within a few seconds (Figure 1B).
- For more consistent results, hold the needle in position by hand for the entire duration of the perfusion.
- Perfuse the liver with warm Buffer 1. To avoid introducing air bubbles, ensure that the line inserted in the tube containing Buffer 1 remains continuously submerged.
- While perfusion occurs, add 130 µL of collagenase solution to Buffer 2 and mix by pipetting up and down or stirring with a 5 mL or 10 mL serological pipette.
- As the volume in the tube containing Buffer 1 decreases to about 5 mL, slowly add 5 mL of Buffer 2 to Buffer 1 by pipetting on the side of the tube. The goal is to avoid introducing air bubbles in the line while changing from Buffer 1 to Buffer 2.
- Wait until the volume decreases again to 5 mL and slowly add another 5 mL of Buffer 2. Repeat one more time. As Buffer 2 replaces Buffer 1 and the dissociation starts, the liver will swell.
- Add the remaining Buffer 2 to the tube originally containing Buffer 1. Stop the perfusion when there is about 5-10 mL of Buffer 2 left in the tube.
NOTE: While Buffer 2 is perfusing the liver, the portal vein can be intermittently clamped with forceps for 5 s. This step is optional, but the resultant increase in pressure throughout the liver can improve its dissociation and, thus, the final hepatocyte yield. - Carefully excise the liver and transfer it to the 100 mm culture dish containing the 20 mL of ice-cold Buffer 2 set aside at step 1.1.4.
- Under the laminar flow hood, gently break the liver apart using surgical scissors and tweezers.
- Add about 20 mL of ice-cold M199 to the hepatocyte suspension and filter it through a 100 µm cell strainer using the plunger of a syringe to gently promote the release of additional hepatocytes from larger liver pieces.
- Wash the 100 mm culture dish and the cell strainer with additional M199 until the collection tube is full.
- Centrifuge the suspension at 50 x g for 2 min at 4 °C. Carefully aspirate the supernatant and gently resuspend the hepatocyte pellet in 30 mL of cold M199 by swirling.
- Pellet the hepatocytes as mentioned in step 1.2.18. Repeat the wash one more time.
- Resuspend the hepatocytes in 10 mL of warm M199 and determine the viability and yield using the trypan blue exclusion method and a hemocytometer12.
- Dilute the cells in M199 warmed to 37 ºC to a final concentration of 1.0 x 106 viable cells/mL and immediately start the assay.
2. Fatty acid β-oxidation assay
NOTE: The assay is conducted in triplicate, and each reaction mixture contains 750,000 cells, 1.35 mg/mL bovine serum albumin (BSA), 100 µM palmitic acid, and 0.4 µCi [1-14C]palmitic acid in a final volume of 2 mL.
CAUTION: Radioactive compounds are hazardous. Purchase, handle, store, and dispose of radioactive material in accordance with Institutional, State, and Federal regulations.
- Preparation
- In the days before the assay, prepare the palmitic acid and BSA solutions (Table 1) and store them at -20 °C.
- On the day of the assay, complete steps 2.1.3-2.1.9 before starting the liver perfusion.
- Thaw the palmitic acid and BSA solutions. Prepare the substrate mixture for multiple reactions plus a 20%-30% excess, with a typical assay setup shown in Table 2.
- Aliquot 13.5 µL of BSA solution per reaction in a microcentrifuge tube and warm to 41 °C, then add 1 µL of the 200 mM palmitic acid solution (BSA: palmitic acid molar ratio = 1:5) per reaction.
NOTE: It is preferable to dispense solutions prepared using organic solvents, such the radioactive and non-radioactive palmitic acid solutions, with a positive displacement pipette and appropriate tips. - Vortex vigorously and incubate at 41 °C to facilitate the formation of the soluble palmitic acid: BSA complex. Vortex occasionally during the incubation period.
- The mixture will initially appear cloudy but will clarify completely after 20-30 min of incubation at 41 °C. Keep it at 41 °C until ready to start the reactions.
- Aliquot 133 µL of 1 M perchloric acid in 1.5 mL microcentrifuge tubes to stop the reactions.
CAUTION: Perchloric acid is a strong acid and a strong oxidant. Appropriate protective gear is required for handling this compound. - Aliquot 485.5 µL of M199 per reaction into a tube and keep it at 37 °C to dilute the radioactive BSA: palmitic acid complex prepared at steps 2.1.4-2.1.6 before starting the reactions.
- Dispense 750 µL of M199 in as many 14 mL round-bottom tubes as the samples. If desired, add inhibitors of fatty acid β-oxidation, such as etomoxir, rotenone, and antimycin, including a vehicle control (Table 2).
- During the hepatocyte wash steps, 10-15 min before starting the reactions, transfer the tubes prepared at step 2.1.9 to a shaking water bath set to 37 °C and shaking at 180-200 rpm.
- Starting, stopping, and analyzing the fatty acid β-oxidation reactions
- If the viability of the hepatocytes is acceptable (typically ≥ 75%, Figure 2), for each reaction, transfer 0.8 µL of [1-14C]palmitic acid (0.5 mCi/mL) to the microcentrifuge tube containing the clarified BSA: palmitic acid solution (steps 2.1.4-2.1.6). Vortex and return to the water bath at 41 °C.
- To equilibrate the hepatocytes to 37 °C and to pre-incubate them with inhibitors (if present), immediately after the final hepatocyte resuspension (step 1.2.21), transfer 750 µL of the hepatocyte suspension with a 1 mL pipette to each of the 14 mL round-bottom tubes in the shaking water bath (steps 2.1.9-2.1.10) and vortex briefly at low speed to mix.
- Stagger each addition by 30 s and incubate for 15 min. To save a sample for protein determination, transfer another aliquot of hepatocytes to a 1.5 mL microcentrifuge tube and spin at 3,000 x g for 5 min.
NOTE: While dispensing, the hepatocyte suspension needs to be continuously swirled or gently stirred with the dispensing 1 mL pipette to prevent settling and large variability in cell number across samples. - Remove the supernatant and store the pellet at -80 °C until ready to measure the total amount of protein in the sample to normalize the results (Figure 3).
- While the hepatocytes are under pre-incubation at 37 °C, add the radioactive BSA: palmitic acid complex to the warm medium in 2.1.8, and keep at 37 °C until ready to start the reactions. This is the final substrate mix.
- To start the reactions, remove the hepatocytes from the water bath and add 500 µL of substrate mix.
- Vortex at low speed for 5 s to completely resuspend the cells and return to the water bath. Repeat with all the samples, staggering by 30 s.
- Incubate for 15 min. Start a set of reactions and immediately stop (see steps 2.2.10-2.2.11) to determine the background radioactivity (Table 2).
- Transfer duplicate aliquots (200-250 µL) of the leftover substrate mix to 6 mL scintillation vials and set aside to count. Use these counts to calculate the radioactivity corresponding to the total nmoles of palmitic acid available for oxidation in 500 µL of substrate mix.
- To stop the reactions, remove the hepatocytes from the water bath, resuspend the hepatocytes by vortexing at moderate speed, and then transfer 400 µL of the hepatocyte suspension to the microcentrifuge tubes containing perchloric acid.
- Immediately cap the tubes. Repeat this sequence for all the samples, staggering by 30 s.
- Vigorously vortex the 1.5 mL microcentrifuge tubes and spin them down the 1.5 mL microcentrifuge tubes at 13,000 x g for 10 min.
- Transfer 300 µL of the supernatant to a 6 mL scintillation vial, add 4 mL of scintillation fluid, and count the radioactivity in the samples and the substrate mix aliquots (step 2.2.9) in a scintillation counter.
CAUTION: After centrifuging, open the tubes under a fume hood to avoid breathing the 14C-CO2 produced by the complete oxidation of 14C-acetyl-CoA generated by fatty acid β-oxidation and released by the acidic conditions.
| Buffers/Media Components | Amount | Final Concentration | Instructions |
| Solution C | |||
| KCl | 1.79 g | 480 mM | Add water to 50 mL. Store at 4 °C |
| MgSO4 heptahydrate | 1.48 g | 120 mM | |
| KH2PO4 | 0.81 g | 119 mM | |
| Krebs-Henseleit Buffer (KHB), calcium-free | |||
| NaCl | 7.0 g | 120 mM | Add water to 900 mL, adjust the pH to 7.4, and bring the final volume to 1 L. Store at 4 °C |
| NaHCO3 | 2.0 g | 24 mM | |
| 1 M HEPES pH 7.45 | 5 mL | 5 mM | |
| Glucose | 1 or 2 g | 5.6 or 11 mM | |
| Solution C | 10 mL | ||
| Buffer 1 | |||
| KHB | 500 mL | Mix components and filter sterilize. Store at 4 °C | |
| 50 mM EGTA | 1.0 mL | 0.1 mM | |
| Buffer 2 | |||
| KHB | 500 mL | Mix components and filter sterilize. Store at 4 °C | |
| 1 M CaCl2 dihydrate | 686 µL | 1.4 mM | |
| Gentamicin solution | |||
| Gentamicin sulphate | 0.5 g | 50 mg/mL | Add water to 10 mL and filter sterilize. Aliquot and store at -20 °C |
| Collagenase solution | |||
| Collagenase I and II blend | 10 mg | 7 mg/mL | Dissolve the entire content of the vial in 1.43 mL of water. Aliquot and store at -20 °C |
| M199 | |||
| M199 | 1 pouch | Add water to 900 mL and adjust the pH to 7.2-7.4. Bring the final volume to 1 L and filter sterilize. Store at 4 °C | |
| NaHCO3 | 2.2 g | 26 mM | |
| 1 M HEPES (cell culture grade) | 25 mL | 25 mM | |
| Extra glucose (only for fed mice) | 1 g | 11 mM | |
| BSA solution | |||
| Fatty acid-free BSA | 400 mg | 20% (w/v) | Dissolve in 2 mL of water. Aliquot and store at -20 °C |
| Non-radioactive palmitic acid solution | |||
| Palmitic acid | 103 mg | 200 mM | Dissolve in 2 mL of ethanol, store at -20 °C |
| 1 M Perchloric acid | |||
| 70% Perchloric acid | 3.5 mL | 1 M | Dilute to 40 mL with water. Store at room temperature |
Table 1: Buffers, media, and other solutions required for the hepatocyte isolation and the fatty acid β-oxidation assay
| Reaction number | M199 ± Inhibitors | Hepatocyte suspension (µL) | Substrate mix (µL) | ||||
| Volume (µL) | Etomoxir | ||||||
| 1 | 750 | - | Pre-warm at 37 °C | 750 | Pre-incubate at 37 °C for 15 min | 500 | Incubate at 37 °C for 15 min |
| 2 | |||||||
| 3 | |||||||
| 4 | + | ||||||
| 5 | |||||||
| 6 | |||||||
| 7 | + | Stop immediately | |||||
| 8 | |||||||
| 9 | |||||||
Table 2: Example of the experimental setup for a hepatocyte suspension assayed in triplicate in the presence and absence of etomoxir.
Wyniki
The liver perfusion described here typically yields 30-40 million cells/liver with average viability of 80%, as estimated by trypan blue exclusion (Figure 2). The typical concentration of glucose in the Krebs-Henseleit buffer (KHB), which is used to prepare the perfusion Buffers 1 and 2, is 11 mM. When measuring fatty acid β-oxidation in hepatocytes isolated from fasted mice, the concentration of glucose in the KHB can be lowered to better represent the fasted state. As shown in
Dyskusje
During the liver perfusion, it is critical to avoid the introduction of air bubbles, as they block the microcapillaries in the liver, preventing or restricting the buffer circulation and overall decreasing the hepatocyte yield and viability20,21. Precautions, such as closely inspecting the buffer-filled inlet line before cannulation of the IVC and avoiding lifting the inlet line off the tube containing Buffer 1 to switch to Buffer 2, as described herein, can succ...
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
This work was supported by the National Institutes of Health grant R35GM119528 to Roberta Leonardi.
Materiały
| Name | Company | Catalog Number | Comments |
| (R)-(+)-Etomoxir sodium salt | Tocris Bioscience | 4539/10 | |
| [1-14C]-Palmitic acid, 50–60 mCi/mmol, 0.5 mCi/mL | American Radiolabeled Chemicals | ARC 0172A | |
| 1 M HEPES, sterile | Corning | 25060CI | |
| 10 µL disposable capillaries/pistons for positive displacement pipette | Mettler Toledo | 17008604 | |
| 1000 µL, 200 µL, and 10 µL pipettes and tips | |||
| 5 mL, 10 mL, and 25 mL serological pipettes | |||
| 50 mL sterile centrifuge tubes | CellTreat | 229421 | |
| 70% Perchloric acid | Fisher Scientific | A2296-1LB | |
| BSA, fatty acid-free | Fisher Scientific | BP9704100 | |
| CaCl2 dihydrate | MilliporeSigma | 223506 | |
| D-(+)-Glucose | MilliporeSigma | G7021 | |
| EGTA | Gold Biotechnology | E-217 | |
| Ethanol | Pharmco | 111000200CSPP | |
| Filter System, 0.22 μm PES Filter, 500 mL, Sterile | CellTreat | 229707 | |
| Gentamicin sulphate | Gold Biotechnology | G-400-25 | |
| HDPE, 6.5 mL scintillation vials | Fisher Scientific | 03-342-3 | |
| Hemocytometer | |||
| Hypodermic needles 22 G, 1.5 in | BD Biosciences | 305156 | |
| Isoflurane | VetOne | 502017 | |
| KCl | Fisher Scientific | BP366-1 | |
| KH2PO4 | MilliporeSigma | P5655 | |
| Liberase TM Research Grade | MilliporeSigma | 5401119001 | Defined blend of purified collagenase I and II with a medium concentration of thermolysin |
| M199 medium | MilliporeSigma | M5017 | |
| MgSO4 heptahydrate | MilliporeSigma | M1880 | |
| Microcentrifuge | Fisher Scientific | accuSpin Micro 17 | |
| Microdissecting Scissors | Roboz Surgical Instrument Co | RS-5980 | |
| NaCl | Chem-Impex International | 30070 | |
| NaHCO3 | Acros Organics | 424270010 | |
| Palmitic acid | MilliporeSigma | P0500 | |
| Penicillin/streptomycin (100x) | Gibco | 15140122 | |
| Phosphate buffered saline (PBS) | Cytiva Life Sciences | SH30256.01 | |
| Positive displacement pipette MR-10, 10 µL | Mettler Toledo | 17008575 | |
| Refrigerated centrifuge with inserts for 50 mL conical tubes | Eppendorf | 5810 R | |
| Round-bottom, 14 mL, polypropylene culture test tubes | Fisher Scientific | 14-956-9A | |
| Scintillation counter | Perkin Elmer | TriCarb 4810 TR | |
| ScintiVerse BD cocktail | Fisher Scientific | SX18-4 | |
| Shaking water bath, 30 L capacity | New Brunswick Scientific | Model G76 | |
| Sterile cell strainers, 100 µm | Fisher Scientific | 22363549 | |
| Thumb Dressing Forceps | Roboz Surgical Instrument Co | RS-8120 | |
| Trypan Blue | Corning | 25900CI | |
| Variable-flow peristaltic pump | Fisher Scientific | 138762 | |
| Water baths, 2–2.5 L capacity |
Odniesienia
- Alves-Bezerra, M., Cohen, D. E. Triglyceride Metabolism in the Liver. Comprehensive Physiology. 8 (1), 1-8 (2017).
- Lopaschuk, G. D., Ussher, J. R., Folmes, C. D., Jaswal, J. S., Stanley, W. C. Myocardial fatty acid metabolism in health and disease. Physiological Reviews. 90 (1), 207-258 (2010).
- Mannaerts, G. P., van Veldhoven, P. P. Functions and organization of peroxisomal beta-oxidation. Annals of the New York Academy of Sciences. 804, 99-115 (1996).
- Kerner, J., Hoppel, C. Fatty acid import into mitochondria. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1486 (1), 1-17 (2000).
- Baker, A., et al. Peroxisomal ABC transporters: functions and mechanism. Biochemical Society Transactions. 43 (5), 959-965 (2015).
- Leonardi, R., Rehg, J. E., Rock, C. O., Jackowski, S. Pantothenate kinase 1 is required to support the metabolic transition from the fed to the fasted state. PloS One. 5 (6), 11107 (2010).
- Shumar, S. A., Kerr, E. W., Fagone, P., Infante, A. M., Leonardi, R. Overexpression of Nudt7 decreases bile acid levels and peroxisomal fatty acid oxidation in the liver. Journal of Lipid Research. 60 (5), 1005-1019 (2019).
- Richert, L., et al. Gene expression in human hepatocytes in suspension after isolation is similar to the liver of origin, is not affected by hepatocyte cold storage and cryopreservation, but is strongly changed after hepatocyte plating. Drug Metabolism and Disposition: The Biological Fate of Chemicals. 34 (5), 870-879 (2006).
- Colbert, R. A., Amatruda, J. M., Young, D. A. Changes in the expression of hepatocyte protein gene-products associated with adaptation of cells to primary culture. Clinical Chemistry. 30 (12), 2053-2058 (1984).
- Spurway, T. D., Sherratt, H. A., Pogson, C. I., Agius, L. The flux control coefficient of carnitine palmitoyltransferase I on palmitate beta-oxidation in rat hepatocyte cultures. Biochemical Journal. 323, 119-122 (1997).
- Consitt, L. A., et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes. 59 (6), 1407-1415 (2010).
- Lee, S. M., Schelcher, C., Demmel, M., Hauner, M., Thasler, W. E. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. Journal of Visualized Experiments: JoVE. (79), e50615 (2013).
- Lilly, K., Chung, C., Kerner, J., VanRenterghem, R., Bieber, L. L. Effect of etomoxiryl-CoA on different carnitine acyltransferases. Biochemical Pharmacology. 43 (2), 353-361 (1992).
- Yu, X. X., Drackley, J. K., Odle, J. Rates of mitochondrial and peroxisomal beta-oxidation of palmitate change during postnatal development and food deprivation in liver, kidney and heart of pigs. Journal of Nutrition. 127 (9), 1814-1821 (1997).
- Yu, X. X., Drackley, J. K., Odle, J., Lin, X. Response of hepatic mitochondrial and peroxisomal beta-oxidation to increasing palmitate concentrations in piglets. Biology of the Neonate. 72 (5), 284-292 (1997).
- Veerkamp, J. H., van Moerkerk, H. T. Peroxisomal fatty acid oxidation in rat and human tissues. Effect of nutritional state, clofibrate treatment and postnatal development in the rat. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 875 (2), 301-310 (1986).
- Hakvoort, T. B., et al. Interorgan coordination of the murine adaptive response to fasting. Journal of Biological Chemistry. 286 (18), 16332-16343 (2011).
- Sokolovic, M., et al. The transcriptomic signature of fasting murine liver. BMC Genomics. 9, 528 (2008).
- Kersten, S., et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. Journal of Clinical Investigation. 103 (11), 1489-1498 (1999).
- Li, W. C., Ralphs, K. L., Tosh, D. Isolation and culture of adult mouse hepatocytes. Methods in Molecular Biology. 633, 185-196 (2010).
- Ng, I. C., et al. Isolation of Primary Rat Hepatocytes with Multiparameter Perfusion Control. Journal of Visualized Experiments: JoVE. (170), e62289 (2021).
- Shen, L., Hillebrand, A., Wang, D. Q., Liu, M. Isolation and primary culture of rat hepatic cells. Journal of Visualized Experiments: JoVE. (64), e3917 (2012).
- Fulgencio, J. P., Kohl, C., Girard, J., Pegorier, J. P. Effect of metformin on fatty acid and glucose metabolism in freshly isolated hepatocytes and on specific gene expression in cultured hepatocytes. Biochemical Pharmacology. 62 (4), 439-446 (2001).
- Leonardi, R., Rock, C. O., Jackowski, S. Pank1 deletion in leptin-deficient mice reduces hyperglycaemia and hyperinsulinaemia and modifies global metabolism without affecting insulin resistance. Diabetologia. 57 (7), 1466-1475 (2014).
- Bougarne, N., et al. PPARalpha blocks glucocorticoid receptor alpha-mediated transactivation but cooperates with the activated glucocorticoid receptor alpha for transrepression on NF-kappaB. Proceedings of the National Academy of Sciences of the United States of America. 106 (18), 7397-7402 (2009).
- Korelova, K., Jirouskova, M., Sarnova, L., Gregor, M. Isolation and 3D collagen sandwich culture of primary mouse hepatocytes to study the role of cytoskeleton in bile canalicular formation in vitro. Journal of Visualized Experiments: JoVE. (154), e60507 (2019).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone