Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Endotracheal Intubation Using a Flexible Intubation Endoscope as a Standardized Model for Safe Airway Management in Swine
W tym Artykule
Erratum Notice
Podsumowanie
The use of pigs in research has increased in recent years. Nevertheless, pigs are characterized by difficult airway anatomy. By demonstrating how to perform endoscopically guided endotracheal intubation, the present protocol aims to further increase laboratory animals' safety to avoid animal suffering and unnecessary death.
Streszczenie
Endotracheal intubation is often a basic requirement for translational research in porcine models for various interventions that require a secured airway or high ventilation pressures. Endotracheal intubation is a challenging skill, requiring a minimum number of successful endotracheal intubations to achieve a high success rate under optimal conditions, which is often unachievable for non-anaesthesiology researchers. Due to the specific porcine airway anatomy, a difficult airway can usually be assumed. The impossibility of establishing a secure airway can result in injury, adverse events, or death of the laboratory animal. Using a prospective, randomized, controlled evaluation approach, it has been shown that fiberoptic-assisted endotracheal intubation takes longer but has a higher first-pass success rate than conventional intubation without causing clinically relevant drops in oxygen saturation. This model presents a standardized regimen for endoscopically guided endotracheal intubation, providing a secured airway, especially for researchers who are inexperienced in the technique of endotracheal intubation via direct laryngoscopy. This procedure is expected to minimize animal suffering and unnecessary animal losses.
Wprowadzenie
Endotracheal intubation is often a basic requirement for translational research in porcine models for various interventions that require a secured airway or high ventilation pressures (such as ventilation during cardiopulmonary resuscitation1 or acute respiratory distress syndrome2) or require that cerebral blood flow is not compromised through internal compression by supraglottic airway devices3, which are occasionally propagated as alternatives in the context of an anticipated difficult airway in pigs4,5.
While the lung physiology of pigs shows similar features to that of humans6, securing the airway is sometimes significantly more difficult7 due to specific differences in the porcine orotracheal anatomy. The snout of a pig has a narrow opening with a very large tongue, the larynx is extremely mobile, and the epiglottis is relatively large, with a free end that extends to the soft palate. Caudally, the larynx forms an obtuse angle with the trachea. The arytenoid cartilages are large8. The narrowest part of the airway is at the subglottic level9, comparable to the airway anatomy of children10. Since the larynx in pigs is very mobile, there is a risk that the end of the endotracheal tube will pass through the vocal cords but the larynx will only be displaced caudally by up to several centimeters, which may be mistaken for a correct intubation8,11. Additionally, esophageal intubation is a common risk when dealing with porcine airway management12.
The rates of difficult or impossible endotracheal intubations with a corresponding negative impact on the experiment or early mortality have not been systematically recorded, but several case reports have been published13,14. In humans, there is the possibility of using a flexible intubation endoscope in the context of an unexpectedly difficult conventional intubation15. Various false intubations often precede this measure. These repeated intubation attempts are associated with adverse events in humans16,17, especially airway complications18. Such events are deleterious in test animals since, in the simplest case, they represent a confounder variable in the experiment; in the worst case, they can lead to the unnecessary loss of the animal.
The present study has developed a model based on the guidelines for expected difficult airway management in humans15,19,20,21,22,23,24. Previously, a similar technique has been described for learning fiberoptic intubation in human studies25,26. The protocol presented in this report aims to provide a standardized and easy-to-adapt intubation model that also allows non-airway specialists to perform successful and safe endotracheal intubation in pigs.
Protokół
The experiments in this protocol were approved by the State and Institutional Animal Care Committee (Landesuntersuchungsamt Rheinland-Pfalz, Koblenz, Germany; approval no. G20-1-135). The experiments were conducted following the ARRIVE guidelines. Overall, 10 anesthetized male pigs (Sus scrofa domestica) with a mean weight of 30 kg ± 2 kg and 12-16 weeks in age were used for the present study.
1. Animal preparation
- Maintain a normal environment for the animals to minimize stress. Withhold food 6 h before the scheduled experiment to lower the risk of aspiration, but allow access to water.
- Sedate the pigs with a combined injection of midazolam (0.5 mg/kg) and azaperone (2-3 mg/kg) (see Table of Materials) in the gluteal muscle or neck with a needle (20 G) for intramuscular injection. Leave the animals undisturbed until sedation sets in (15-20 min).
NOTE: Depending on national regulations, the administration of sedating agents may be subject to scrutiny and may or may not require the supervision of a trained veterinarian. Consult with the local authorities before planning the experiments. - Transport the sedated animals from the stables to the laboratory. The transport time must not exceed adequate sedation time (here, 30-60 min). Ensure sufficient heat retention so that the animal does not get hypothermic (i.e., below 38 °C), such as by covering the body with a blanket depending on the outside temperature.
- Using a sensor (see Table of Materials) clipped to the ear or tail, monitor the peripheral oxygen saturation (SpO2).
- Disinfect the skin with a disinfectant (alcoholic) before inserting a peripheral vein cannula (22 G) into an ear vein. Spray the area, wipe once, then spray again, and allow the disinfectant to dry. Secure the ear cannula with a band-aid (See Table of Materials).
2. Anesthesia and mechanical ventilation
- Administer analgesia through an intravenous injection of 4 µg/kg of fentanyl. Induce anesthesia with an intravenous injection of 3 mg/kg of propofol (see Table of Materials).
NOTE: Due to the bolus application, the drug quickly floods into the active compartment, providing a fast onset of deep anesthesia. - Place the pig on a stretcher in a supine position and fix it with bandages. Apply muscle relaxant through an intravenous injection of 0.5 mg/kg of atracurium (see Table of Materials).
- Instantaneously start noninvasive ventilation via a dog ventilation mask (see Table of Materials) or similar models. To ensure a tight fit of the mask, place the thenar eminence and the thumbs of both hands on top of the mask while performing jaw thrust with the remaining fingers.
NOTE: Ventilation parameters: FiO2 (inspiratory oxygen fraction) = 100%, peak inspiratory pressure = <20 cmH20, respiratory rate = 18-20 breaths/min, PEEP (positive end-expiratory pressure) = 5 cmH20. - Maintain anesthesia through continuous infusion of 0.1-0.2 mg/kg/h of fentanyl and 8-12 mg/kg/h of propofol. Start infusing with 5 mL/kg/h of balanced electrolyte solution (see Table of Materials) continuously. Constantly maintain an adequate depth of anesthesia.
NOTE: Surrogate parameters for this are the absence of movement, the lack of own respiratory efforts after intubation, and the absence of a sudden increase in heart rate. If possible, avoid permanent muscle relaxation to enable motor reactions as a sign of insufficient depth of anesthesia.
3. Endotracheal intubation
- Have an assistant stand on the left side of the head. Have the assistant's left hand open the mouth and pinch the tongue outward and left with a compress. Ask the assistant to press down on the right upper lip with the right index finger to provide a better mouth opening.
- Perform direct laryngoscopy. To do this, insert the laryngoscope (see Table of Materials) into the right side of the mouth and push it forward while pushing the tongue to the left. Advance the tip of the laryngoscope until it rests in the epiglottic vallecula.
NOTE: The epiglottis usually obscures the glottis by sticking to the soft palate. - Carefully push the epiglottis aside with a tube guiding wire (see Table of Materials) with a gentle scooping motion from the right piriform recess to the left along the soft palate.
- Pass the handle of the laryngoscope to the assistant to fix it in the current position.
- Now, take the flexible intubation endoscope onto which an endotracheal tube has already been mounted and that is connected to a video monitor. Insert the endoscope orally and advance it over the base of the tongue until the glottis is visualized.
NOTE: To avoid fogging of the camera, the prior application of anti-fogging agents (see Table of Materials) is recommended. - Advance the endoscope between the vocal ligaments into the trachea. Confirm the anatomy of the trachea by visually identifying the cartilaginous rings and the pars membranacea. Advance the endoscope until it rests above the carina. Try not to touch the sensitive mucosa with the tip of the endoscope to avoid swelling and bleeding.
- While maintaining the position of the endoscope, advance the endotracheal tube until it becomes visible in the camera image.
NOTE: If the endotracheal tube cannot be advanced through the glottic plane, there is a possibility that it has become caught on the arytenoid cartilage. In this case, the endotracheal tube must be withdrawn 1 cm and rotated by 90° before gently advancing again. If necessary, this maneuver can be repeated. Similar calibers of flexible intubation endoscope and endotracheal tube can minimize the risk of this issue occurring. If the endotracheal tube cannot be advanced despite this maneuver, it is likely that the subglottic narrowness-the narrowest part of the porcine larynx-cannot be passed. In this case, a smaller endotracheal tube size needs to be selected. Regular commercially available endotracheal tubes in sizes 6.5 cm or 7.0 cm ID should be able to pass the glottis as long as no anatomic abnormalities are present. Endotracheal tube size requirements vary depending on the piglet size and breed. - Withdraw the flexible intubation endoscope while maintaining the position of the endotracheal tube.
- Using a 10 mL syringe, inflate the cuff with 10 mL of air. Control the cuff pressure with a cuff manager (target value: 30 cmH2O, see Table of Materials).
- Confirm the correct placement of the endotracheal tube and adequate ventilation by periodic and regular exhalation of carbon dioxide via capnography24 and double-sided ventilation via auscultation15.
- Start mechanical ventilation after connecting the tube with a ventilator (PEEP = 5 cmH2O, respiratory rate = variable to achieve an end-tidal CO2 of <6 kPa, usually 30-50 min−1, FiO2 = 0.4, I:E (inspiration to expiration ratio) = 1:2, tidal volume = 6-8 mL/kg).
- Expand the monitoring (e.g., the establishment of an intra-arterial blood pressure measurement, the installation of a central venous or pulmonary arterial catheter27) or continue with the intervention.
NOTE: Depending on the question of the further experiments, define limit values for the vital parameters and intervention options and establish the monitoring accordingly in the study protocol.
Wyniki
Endotracheal intubation was performed on 10 male pigs (age 12-16 weeks, weight 30 kg ± 3 kg) in a prospective, randomized, controlled study setting. The pigs were randomized into two groups: one was conventionally laryngoscopically intubated (CI group), and the other group was intubated assisted via a flexible intubation endoscope as described in the protocol (FIE group). The group assignment was done by pulling sealed envelopes. The investigator was assigned randomly on a daily basis.
Dyskusje
In previous studies, our research group has already described specific details regarding the translational benefits of the porcine model2,27,32,33. Generally, reducing the stress level of the animal and unnecessary pain should be an integral part of any study protocol and is paramount for generating reliably reproducible data. Therefore, awake endoscopically guided intubation of the pig with an...
Ujawnienia
The flexible intubation endoscope and its accessories have been provided unconditionally by the manufacturer for research purposes only. The authors declare no further financial or other conflicts of interest.
Podziękowania
The authors want to thank Dagmar Dirvonskis for her excellent technical support.
Materiały
| Name | Company | Catalog Number | Comments |
| Ambu aScope Regular | Ambu GmbH, Medizinprodukte, Bad Nauheim, Germany | Disposable fiber optic outer diameter 5 mm | |
| Ambu aView Monitor | Ambu GmbH, Medizinprodukte, Bad Nauheim, Germany | monitor | |
| Atracurium Hikma 50 mg/5mL | Hikma Pharma GmbH, Martinsried | atracurium | |
| Azaperone (Stresnil) 40mg/mL | Lilly Deutschland GmbH, Bad Homburg, Germany | azaperone | |
| BD Discardit II Spritze 2, 5, 10, 20 mL | Becton Dickinson S.A. Carretera, Mequinenza Fraga, Spain | syringe | |
| BD Luer Connecta | Becton Dickinson Infusion Therapy, AB Helsingborg, Schweden | 3-way-stopcock | |
| BD Microlance 3 20 G | Becton Dickinson S.A. Carretera, Mequinenza Fraga, Spain | cannula | |
| Curafix i.v. classics | Lohmann & Rauscher International GmbH & Co. KG, Rengsdorf, Germany | Cannula retention dressing | |
| Engström Carestation | GE Heathcare, Madison USA | ventilator | |
| Fentanyl-Janssen 0.05 mg/mL | Janssen-Cilag GmbH, Neuss | fentanyl | |
| Führungsstab, Durchmesser 4.3 | Rüsch | endotracheal tube introducer | |
| IBM SPSS Statistics for Windows, Version 20 | IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) | Statistical software | |
| Incetomat-line 150 cm | Fresenius, Kabi Deutschland, GmbH | perfusor line | |
| Intrafix Primeline | B. Braun Melsungen AG, Melsungen, Germany | Infusion line | |
| JOZA Einmal Nitril Untersuchungshandschuhe | JOZA, München, Germany | disposable gloves | |
| Laryngoscope, 45.48.50, KL 2000 | Medicon | Laryngoscope handle | |
| Littmann Classic III Stethoscope | 3M Deutschland GmbH, Neuss, Germany | stethoscope | |
| Luer Lock | B.Braun Melsungen AG, Germany | ||
| Maimed Vlieskompresse | Maimed GmbH, Neuenkirchen, Germany | Fleece compress to fix the tongue | |
| Masimo LNCS Adtx SpO2 sensor | Masimo Corporation Irvine, Ca 92618 USA | saturation clip for the tail | |
| Masimo LNCS TC-I SpO2 ear clip sensor | Masimo Corporation Irvine, Ca 92618 USA | Saturation clip for the ear | |
| Masimo Radical 7 | Masimo Corporation Irvine, Ca 92618 USA | periphereal oxygen saturation | |
| Midazolam 15 mg/3 mL | Hameln Pharma GmbH, Hameln, Germany | midazolam | |
| Midmark Canine Mask Small Plastic with Diaphragm FRSCM-0005 | Midmark Corp., Dayton, Ohio, USA | dog ventilation mask | |
| Octeniderm farblos | Schülke & Mayr GmbH, Nordenstedt, Germany | Alcoholic disinfectant | |
| Original Perfusor syringe 50 mL | B.Braun Melsungen AG, Germany | perfusor syringe | |
| Perfusor FM Braun | B.Braun Melsungen AG, Germany | syringe pump | |
| Propofol 2% 20 mg/mL (50 mL flasks) | Fresenius, Kabi Deutschland, GmbH | propofol | |
| RÜSCH Führungsstab für Endotrachealtubus (ID 5.6 mm) | Teleflex Medical Sdn. Bhd, Malaysia | PVC coated tube guiding wire | |
| Rüschelit Super Safety Clear >ID 6/6.5 /7.0 mm | Teleflex Medical Sdn. Bhd, Malaysia | endotracheal tube | |
| Stainless Macintosh Größe 4 | Welch Allyn69604 | blade for laryngoscope | |
| Sterofundin | B.Braun Melsungen AG, Melsungen, Germany | Balanced electrolyte solution | |
| Ultrastop Antibeschlagmittel bottle with dropper 25 mL | Sigmapharm Arzneimittel GmbH, Wien, Austria | Antifog agent | |
| Vasofix Safety 22 G-16 G | B.Braun Melsungen AG, Germany | venous catheter | |
| VBM Cuff Manometer | VBM Medizintechnik GmbH, Sulz a.N., Germany | cuff pressure gauge | |
| Zelette | Lohmann & Rauscher International GmbH & Co. KG, Rengsdorf, Germany | Tissue swab |
Odniesienia
- Kleinman, M. E., Oh, W., Stonestreet, B. S. Comparison of intravenous and endotracheal epinephrine during cardiopulmonary resuscitation in newborn piglets. Critical Care Medicine. 27 (12), 2748-2754 (1999).
- Rissel, R., et al. Bronchoalveolar lavage and oleic acid-injection in pigs as a double-hit model for acute respiratory distress syndrome (ARDS). Journal of Visualized Experiments. (159), e61358 (2020).
- Segal, N., et al. Impairment of carotid artery blood flow by supraglottic airway use in a swine model of cardiac arrest. Resuscitation. 83 (8), 1025-1030 (2012).
- Goldmann, K., Kalinowski, M., Kraft, S. Airway management under general anaesthesia in pigs using the LMA-ProSeal: A pilot study. Veterinary Anaesthesia and Analgesia. 32 (5), 308-313 (2005).
- Wemyss-Holden, S. A., Porter, K. J., Baxter, P., Rudkin, G. E., Maddern, G. J. The laryngeal mask airway in experimental pig anaesthesia. Lab Animal. 33 (1), 30-34 (1999).
- Kobayashi, E., Hishikawa, S., Teratani, T., Lefor, A. T. The pig as a model for translational research: overview of porcine animal models at Jichi Medical University. Transplantation Research. 1 (1), 8 (2012).
- Judge, E. P., et al. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. American Journal of Respiratory Cell and Molecular Biology. 51 (3), 334-343 (2014).
- Dondelinger, R. F., et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. European Radiology. 8 (7), 1254-1273 (1998).
- Nickel, R., Schummer, A., Seiferle, E. . Lehrbuch der Anatomie der Haustiere, Band I: Bewegungsapparat. , (2003).
- Wani, T. M., Rafiq, M., Akhter, N., AlGhamdi, F. S., Tobias, J. D. Upper airway in infants-A computed tomography-based analysis. Paediatric Anaesthesia. 27 (5), 501-505 (2017).
- Chum, H., Pacharinsak, C. Endotracheal intubation in swine. Lab Animal. 41 (11), 309-311 (2012).
- Ettrup, K. S., et al. Basic surgical techniques in the Göttingen minipig: Intubation, bladder catheterization, femoral vessel catheterization, and transcardial perfusion. Journal of Visualized Experiments. (52), e2652 (2011).
- Steinbacher, R., von Ritgen, S., Moens, Y. P. S. Laryngeal perforation during a standard intubation procedure in a pig. Laboratory Animals. 46 (3), 261-263 (2012).
- Iliff-Sizemore, S. A., Chrisp, C. E., Rush, H. G. Peritracheolaryngeal abscess: An iatrogenic complication of endotracheal intubation in swine. Laboratory Animal Science. 39 (5), 455-458 (1989).
- Piepho, T., et al. S1 guidelines on airway management. Der Anaesthesist. 64 (11), 859-873 (2015).
- Mort, T. C. Emergency tracheal intubation: Complications associated with repeated laryngoscopic attempts. Anesthesia & Analgesia. 99 (2), 607-613 (2004).
- Hasegawa, K., et al. Association between repeated intubation attempts and adverse events in emergency departments: An analysis of a multicenter prospective observational study. Annals of Emergency Medicine. 60 (6), 749-754 (2012).
- Martin, L. D., Mhyre, J. M., Shanks, A. M., Tremper, K. K., Kheterpal, S. 3,423 emergency tracheal intubations at a university hospital: airway outcomes and complications. Anesthesiology. 114 (1), 42-48 (2011).
- Ahmad, I., et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia. 75 (4), 509-528 (2020).
- Frerk, C., et al. Difficult Airway Society 2015 guidelines for management of unanticipated difficult intubation in adults. British Journal of Anaesthesia. 115 (6), 827-848 (2015).
- Cook, T. M., et al. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 75 (6), 785-799 (2020).
- Kornas, R. L., Owyang, C. G., Sakles, J. C., Foley, L. J., Mosier, J. M. Evaluation and management of the physiologically difficult airway: Consensus recommendations from Society for Airway Management. Anesthesia & Analgesia. 132 (2), 395-405 (2021).
- Higgs, A., et al. Guidelines for the management of tracheal intubation in critically ill adults. British Journal of Anaesthesia. 120 (2), 323-352 (2018).
- Apfelbaum, J. L., et al. American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 136 (1), 31-81 (2022).
- Doyle, D. J. GlideScope-assisted fiberoptic intubation: A new airway teaching method. Anesthesiology. 101 (5), 1252 (2004).
- Lenhardt, R., et al. Is video laryngoscope-assisted flexible tracheoscope intubation feasible for patients with predicted difficult airway? A prospective, randomized clinical trial. Anesthesia & Analgesia. 118 (6), 1259-1265 (2014).
- Ruemmler, R., Ziebart, A., Garcia-Bardon, A., Kamuf, J., Hartmann, E. K. Standardized model of ventricular fibrillation and advanced cardiac life support in swine. Journal of Visualized Experiments. (155), e60707 (2020).
- Dodge, Y. Kolmogorov-Smirnov Test. The Concise Encyclopedia of Statistics. , 283-287 (2008).
- Ross, A., Willson, V. L. Independent Samples T-test. Basic and Advanced Statistical Tests: Writing Results Sections and Creating Tables and Figures. , 13-16 (2017).
- Mann, H. B., Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics. 18 (1), 50-60 (1947).
- Spearman, C. The proof and measurement of association between two things. American Journal of Psychology. 100 (3-4), 441-471 (1987).
- Ziebart, A., et al. Standardized hemorrhagic shock induction guided by cerebral oximetry and extended hemodynamic monitoring in pigs. Journal of Visualized Experiments. (147), e59332 (2019).
- Kamuf, J., et al. Oleic acid-injection in pigs as a model for acute respiratory distress syndrome. Journal of Visualized Experiments. (140), e57783 (2018).
- Kurita, T., Kawashima, S., Morita, K., Nakajima, Y. Assessment of the benefits of head-up preoxygenation using near-infrared spectroscopy with pulse oximetry in a swine model. Journal of Clinical Monitoring and Computing. 35 (1), 155-163 (2021).
- Ruemmler, R., Ziebart, A., Ott, T., Dirvonskis, D., Hartmann, E. K. Flexible fibreoptic intubation in swine - Improvement for resident training and animal safety alike. BMC Anesthesiology. 20 (1), 206 (2020).
- Cook, J. A., Ramsay, C. R., Fayers, P. Using the literature to quantify the learning curve: A case study. International Journal of Technology Assessment in Health Care. 23 (2), 255-260 (2007).
- Buis, M. L., Maissan, I. M., Hoeks, S. E., Klimek, M., Stolker, R. J. Defining the learning curve for endotracheal intubation using direct laryngoscopy: A systematic review. Resuscitation. 99, 63-71 (2016).
- Knapp, S., et al. The assessment of four different methods to verify tracheal tube placement in the critical care setting. Anesthesia & Analgesia. 88 (4), 766-770 (1999).
- Schmidt, R. F. . Physiologie des Menschen. 31, (2010).
- Eberlein, C. M., Luther, I. S., Carpenter, T. A., Ramirez, L. D. First-pass success intubations using video laryngoscopy versus direct laryngoscopy: A retrospective prehospital ambulance service study. Air Medical Journal. 38 (5), 356-358 (2019).
- Lohse, J., Noppens, R. Awake video laryngoscopy - An alternative to awake fiberoptic intubation. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie. 51 (11-12), 656-663 (2016).
- Johnson, C., Roberts, J. T. Clinical competence in the performance of fiberoptic laryngoscopy and endotracheal intubation: A study of resident instruction. Journal of Clinical Anesthesia. 1 (5), 344-349 (1989).
- Geovanini, G. R., Pinna, F. R., Prado, F. A., Tamaki, W. T., Marques, E. Standardization of anesthesia in swine for experimental cardiovascular surgeries. Revista Brasileira de Anestesiologia. 58 (4), 363-370 (2008).
Erratum
Formal Correction: Erratum: Endotracheal Intubation Using a Flexible Intubation Endoscope As a Standardized Model for Safe Airway Management in Swine
Posted by JoVE Editors on 4/03/2023. Citeable Link.
An erratum was issued for: Endotracheal Intubation Using a Flexible Intubation Endoscope As a Standardized Model for Safe Airway Management in Swine. The Protocol, Representative Results, and Discussion sections were updated.
In the Protocol, step 1.5 was updated from:
Disinfect the skin with a disinfectant (alcoholic) before inserting a peripheral vein cannula (22 G) into an ear vein. Spray the area, wipe once, then spray again, and allow the disinfectant to dry.
to:
Disinfect the skin with a disinfectant (alcoholic) before inserting a peripheral vein cannula (22 G) into an ear vein. Spray the area, wipe once, then spray again, and allow the disinfectant to dry. Secure the ear cannula with a band-aid (See Table of Materials).
In the Protocol, step 3.7 was updated from:
While maintaining the position of the endoscope, advance the endotracheal tube until it becomes visible in the camera image.
NOTE: If the endotracheal tube cannot be advanced through the glottic plane, there is a possibility that it has become caught on the arytenoid cartilage. In this case, the endotracheal tube must be withdrawn 1 cm and rotated by 90° before gently advancing again. If necessary, this maneuver can be repeated. Similar calibers of flexible intubation endoscope and endotracheal tube can minimize the risk of this issue occurring. If the endotracheal tube cannot be advanced despite this maneuver, it is likely that the subglottic narrowness-the narrowest part of the porcine larynx-cannot be passed. In this case, a smaller endotracheal tube size needs to be selected. Regular commercially available endotracheal tubes in sizes 6.5 cm or 7.0 cm ID should be able to pass the glottis as long as no anatomic abnormalities are present.
to:
While maintaining the position of the endoscope, advance the endotracheal tube until it becomes visible in the camera image.
NOTE: If the endotracheal tube cannot be advanced through the glottic plane, there is a possibility that it has become caught on the arytenoid cartilage. In this case, the endotracheal tube must be withdrawn 1 cm and rotated by 90° before gently advancing again. If necessary, this maneuver can be repeated. Similar calibers of flexible intubation endoscope and endotracheal tube can minimize the risk of this issue occurring. If the endotracheal tube cannot be advanced despite this maneuver, it is likely that the subglottic narrowness-the narrowest part of the porcine larynx-cannot be passed. In this case, a smaller endotracheal tube size needs to be selected. Regular commercially available endotracheal tubes in sizes 6.5 cm or 7.0 cm ID should be able to pass the glottis as long as no anatomic abnormalities are present. Endotracheal tube size requirements vary depending on the piglet size and breed.
In the Representative Results, the sixth paragraph was updated from:
Statistical analyses were performed using commercially available software (see Table of Materials). Normal distribution was examined using the Kolmogorov-Smirnoff test28. If a normal distribution was determined, group differences were analyzed using t-tests of independent samples29 or the Mann-Whitney U test30 for the non-parametric version. Data are presented as mean (± standard deviation). Correlations of ordinal-scale data were examined using Spearman's correlation coefficient31. A significance level of p < 0.05 was assumed.
to:
Statistical analyses were performed using commercially available software (see Table of Materials). Normal distribution was examined using the Kolmogorov-Smirnoff test28. If a normal distribution was determined, group differences were analyzed using t-tests of independent samples29 or the Mann-Whitney U test30 for the non-parametric version. Data are presented as mean (± standard deviation). Correlations of ordinal-scale data were examined using Spearman's correlation coefficient31. A significance level of p < 0.05 was assumed. All tests were performed with exploratory intention; therefore p-values are descriptive. Nevertheless, p < 0.05 was accepted as indicative of statistical significance.
In the Representative Results, the legend for figure 1 was updated from:
Figure 1: Number of intubation attempts in group comparison. For the group that was intubated using a flexible intubation endoscope, every intubation attempt was successful; in the group that was conventionally intubated, it took an average of 1.4 attempts before the endotracheal tube could be placed correctly. Error bars show the standard deviation. Please click here to view a larger version of this figure.
to:
Figure 1: Number of intubation attempts in group comparison. For the group that was intubated using a flexible intubation endoscope, every intubation attempt was successful; in the group that was conventionally intubated, it took an average of 1.4 attempts before the endotracheal tube could be placed correctly. Error bars show the standard deviation. n = 5 (for each group). Please click here to view a larger version of this figure.
In the Representative Results, figure 2 was updated from:
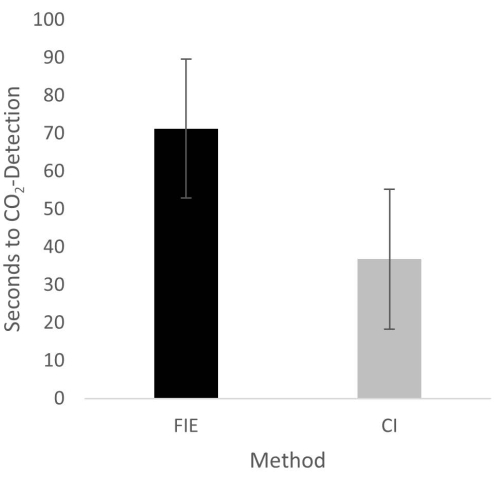
Figure 2: Time until CO2 detection in group comparison. For the group that was intubated using a flexible intubation endoscope, it took significantly longer until end-tidal CO2 could be detected, depicted as mean and standard deviation. Please click here to view a larger version of this figure.
to:
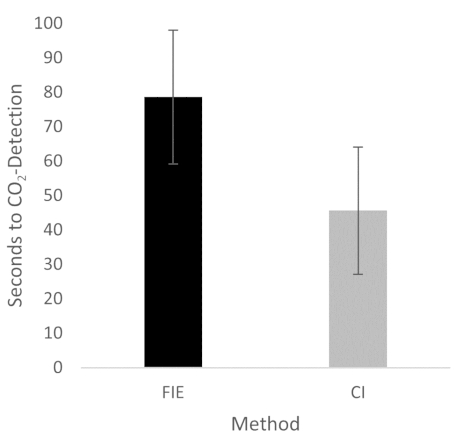
Figure 2: Time until CO2 detection in group comparison. For the group that was intubated using a flexible intubation endoscope, it took significantly longer until end-tidal CO2 could be detected, depicted as mean and standard deviation. n = 5 (for each group). Please click here to view a larger version of this figure.
In the Discussion, the fifth paragraph was updated from:
The increased duration had no clinical significance in this cohort. At no time was the termination criterion-a saturation of less than 93%-reached. This is shown in the results because a procedure change was unnecessary at any time. Prior adequate mask ventilation is a critical step to allow sufficient time for fiberoptic endotracheal tube placement to avoid rapid desaturation34. These results are consistent with previous studies comparing conventional intubation and endoscopically assisted intubations with inexperienced providers35.
to:
The increased duration had no clinical significance in this cohort. At no time was the termination criterion-a saturation of less than 93%-reached. This is shown in the results because a procedure change was unnecessary at any time. Prior adequate mask ventilation is a critical step to allow sufficient time for fiberoptic endotracheal tube placement to avoid rapid desaturation34. These results are consistent with previous studies comparing conventional intubation and endoscopically assisted intubations with inexperienced providers35. We attribute the prolonged duration of fiberoptic intubation to the fact that one must first reorient again after insertion, whereas with conventional intubation, one retains a view of the glottis. It is also important to avoid contact with the mucosa with the flexible intubation endoscope during advancement. This requires occasional corrective maneuvers. Last but not least, after successful placement, retraction of the relatively long endoscope is required, which increases the time to CO2 detection slightly.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone