Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Morphological and Compositional Analysis of Neutrophil Extracellular Traps Induced by Microbial and Chemical Stimuli
W tym Artykule
Podsumowanie
Presented here is a protocol for the induction and analysis of in vitro neutrophil extracellular traps (NETs). Quantification of DNA, cathelicidin (LL37), and enzyme activity yielded data that show the variability in the composition and morphology of NETs induced by microbial and chemical stimuli under similar controlled conditions.
Streszczenie
Neutrophils function as the first line of cellular defense in an innate immune response by employing diverse mechanisms, such as the formation of neutrophil extracellular traps (NETs). This study analyzes the morphological and compositional changes in NETs induced by microbial and chemical stimuli using standardized in vitro methodologies for NET induction and characterization with human cells. The procedures described here allow the analysis of NET morphology (lytic or non-lytic) and composition (DNA-protein structures and enzymatic activity), and the effect of soluble factors or cellular contact on such characteristics. Additionally, the techniques described here could be modified to evaluate the effect of exogenous soluble factors or cellular contact on NET composition.
The applied techniques include the purification of polymorphonuclear cells from human peripheral blood using a double density gradient (1.079-1.098 g/mL), guaranteeing optimal purity and viability (≥ 95%) as demonstrated by Wright's staining, trypan blue exclusion, and flow cytometry, including FSC versus SSC analysis and 7AAD staining. NET formation is induced with microbial (Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans) and chemical (phorbol myristate acetate, HOCl) stimuli, and the NETs are characterized by DNA-DAPI staining, immunostaining for the antimicrobial peptide cathelicidin (LL37), and quantification of enzymatic activity (neutrophil elastase, cathepsin G, and myeloperoxidase). The images are acquired through fluorescence microscopy and analyzed with ImageJ.
Wprowadzenie
Neutrophils are the most abundant leukocytes in the bloodstream, playing an essential role during the clearance of pathogenic agents by several mechanisms, including the release of large chromatin structures composed of DNA and several nuclear, cytoplasmic, and granular antibacterial proteins1,2. The direct antecedent describing this antimicrobial role of neutrophils was made by Takei et al.3 in 1996. These authors reported a new form of death different from apoptosis and necroptosis in neutrophils, showed morphological changes exhibiting nuclear rupture, followed by spilling out of the nucleoplasm into the cytoplasm, and an increase in membrane permeability from 3 h of incubation with phorbol myristate acetate (PMA)2,3. However, it was not until 2004 that the term "neutrophil extracellular traps (NETs)" was used4.
NET formation has been observed in various conditions, such as bacterial, fungal5, viral6, and parasitic infections, for neutralizing, killing, and preventing microbial dissemination7. Other studies show that it can also occur in non-pathogenic conditions by sterile stimuli, such as cytokines, monosodium uric acid or cholesterol crystals, autoantibodies, immune complexes, and activated platelets7. Lipopolysaccharide (LPS), interleukin-8 (IL-8), and PMA were among the first in vitro stimuli described as NET inducers, and the in vivo NET involvement in pathogenic processes was demonstrated in two models of acute inflammation: experimental dysentery and spontaneous human appendicitis4. DNA is an essential NET component. Its appropriate structure and composition are necessary for the sequestration and killing of microorganisms by delivering a high local concentration of antimicrobial molecules toward the caught microbes, as demonstrated by a brief deoxyribonuclease (DNase) treatment that disintegrates NETs and their microbicidal properties4. Besides DNA, NETs comprise attached proteins such as histones, neutrophil elastase (NE), cathepsin G (CG), proteinase 3, lactoferrin, gelatinase, myeloperoxidase (MPO), and antimicrobial peptides (AMPs) such as the cationic pro-inflammatory peptide cathelicidin LL-37 among others8,9. Such aggregates may form larger threads with diameters up to 50 nm. These factors can disrupt the microbial virulence factors or the integrity of the pathogen cell membrane; additionally, the AMPs can stabilize the NET-derived DNA against degradation by bacterial nucleases10.
The specific mechanisms regulating NET formation have not yet been completely clarified. The best-characterized pathway leading to NET release is through ERK signaling, which leads to NADPH oxidase activation and reactive oxygen species (ROS) production, as well as increased intracellular calcium that triggers activation of the MPO pathway. This in turn transforms hydrogen peroxide into hypochlorous acid, activating NE by oxidation11,12. NE is responsible for degrading the actin filaments of the cytoskeleton to block phagocytosis and translocating them to the nucleus for processing by proteolytic cleavage and deamination by PAD4 that drive the desensitization of chromatin fibers, which associate with granule and cytoplasmic proteins, and are then released extracellularly7. These proteases include those released from the azurosome complex of the azurophil granules and other proteases such as cathepsin G13.
Depending on the morphological changes in neutrophils, NETs are classified into two types: suicidal or lytic NET formation leading to cell death4, and vital or non-lytic NET formation produced by viable cells mediated by a vesicular release of nuclear or mitochondrial DNA, with a remnant of an anucleated cytoplast with phagocytic capability14,15. Generally, NETs composed of mitochondrial DNA present an elongated fiber14 morphology, while those structured of nuclear DNA have a cloud-like appearance3. However, it is not known how the neutrophil chooses its DNA origin. Contrary to previous studies that described the canonical pathways of NETs as requiring several hours, the vital pathway is rapidly activated in just 5-60 min15.
Despite these advances, the NET composition varies depending on the stimulus; for example, different mucoid and non-mucoid strains of P. aeruginosa induce the formation of NETs containing 33 common proteins and up to 50 variable proteins7. Thus, it is necessary to homogenize techniques that allow the generation of objective conclusions in research groups. This paper describes a protocol with various techniques that allow comparison and evaluation of the composition, structure, and morphology of NETs induced with different microorganisms: Staphylococcus aureus (gram-positive bacterium), Pseudomonas aeruginosa (gram-negative bacterium), and Candida albicans (fungus), as well as chemical stimuli (PMA, HOCl) in human neutrophils from healthy individuals. The representative results demonstrate the heterogeneity of NETs depending on their inducing stimulus under comparable in vitro conditions, characterized by DNA-DAPI staining, immunostaining for LL37, and quantification of enzymatic activity (NE, CG, and MPO).
Protokół
The blood samples were obtained as donations from clinically healthy participants after informed consent. All experiments were performed with the permission of the Human Research Ethics Committee of the Faculty of Biochemical Sciences, Universidad Autónoma 'Benito Juárez' of Oaxaca.
NOTE: The inclusion criteria in the study were indistinct sex and age, and clinically healthy according to participant responses to a questionnaire prior to taking a blood sample. A hematological analysis was performed to determine the cell count and rule out infections or anemia, as well as the C-reactive protein test to rule out inflammation in the donor.
1. Peripheral blood collection and obtaining the erythrocyte and leukocyte package
- Collect 10 mL of peripheral blood by venipuncture in tubes with 1.8 mg/mL of K2·EDTA as anticoagulant (see Table of Materials) from clinically healthy individuals after obtaining informed consent. Then, perform standard blood biometry and C-reactive protein test to rule out infection or inflammation, ensuring the quality of the sample.
- Centrifuge the peripheral blood sample at 82 x g for 15 min to remove the platelet-rich plasma, followed by a second centrifugation at 630 x g for 5 min. Discard the remaining plasma to obtain the erythrocyte and leukocyte package.
- Dilute it in 1:1 ratio (v/v) with 1x Dulbecco's phosphate-buffered saline (DPBS).
2. Polymorphonuclear neutrophil (PMN) purification using a double-density gradient
NOTE: Perform neutrophil purification immediately after the blood is collected, because they have a limited in vitro lifetime of about 8 h.
- Deposit the following in a sterile 10 mL glass tube (see Table of Materials) in order: 1 mL of 1.098 g/mL density solution, 1 mL of 1.079 g/mL density solution (see Table of Materials), and then 4 mL of the diluted erythrocyte and leukocyte package. Pour over the walls without breaking the surface tension between the layers to prevent them from mixing.
- Centrifuge at 320 x g for 20 min at 4 °C, avoiding acceleration/deceleration so that the high forces of the centrifuge do not disturb the gradient.
- Aspirate the phase that corresponds to granulocytes (Figure 1A) by pipetting, and transfer to another sterile 10 mL glass tube. Wash with 4 mL of 1x DPBS at 300 x g for 10 min at 4 °C.
- Discard the supernatant and treat the cells with osmotic shock to remove the remaining erythrocytes. Add 4 mL of 0.2% saline solution for 2 min at 4 °C, and centrifuge at 300 x g for 10 min at 4 °C. Discard the supernatant. Then add 4 mL of the isotonic solution (0.65% saline) for 5 min at 4 °C to restore membrane integrity, and centrifuge at 300 x g for 10 min at 4 °C.
NOTE: The 0.2% saline solution is a hypotonic medium, with a lower solute concentration relative to that of the RBC intracellular medium. Contact with the hypotonicmedium allows water to diffuse into the RBC, leading to their swelling and hemolysis. This removal of RBC from the supernatant was confirmed by microscopic observation. - Remove the supernatant. Resuspend the cells in 4 mL of 1x DPBS to remove cellular debris, and then centrifuge at 300 x g for 10 min at 4 °C. Finally, resuspend the cell pellet in 2 mL of cold Hank's balanced salt solution (HBSS) buffer.
3. Neutrophil morphology and viability (Figure 1B)
- Trypan blue exclusion test
- Dilute 5 μL of the cell suspension in 20 μL of 0.4% trypan blue (1:5 ratio). Count the cells in a Neubauer chamber and determine cell viability using an exclusion test. Consider the cells that maintain the integrity of their membrane without permeabilizing the dye as viable.
- Mount 5 μL of the cell suspension on a slide; dry and stain with Wright's stain for 15 s. Immediately fix the sample with phosphate buffer pH 6.4 for 30 s. Wash with sufficient distilled water and observe the morphology under an optical microscope (100x).
- 7AAD-staining and flow cytometry analysis
- Add 1 x 105 cells to flow cytometry tubes, and stain with 1 μL of 7AAD in 100 μL of FACS buffer (1x DPBS, 0.1% sodium azide, and 10% autologous decomplemented plasma) for 15 min at 4 °C in the dark.
- Wash with 500 μL of FACS buffer at 300 x g for 10 min. Fix the cells with 500 μL of 2% paraformaldehyde, and store at 4 °C until their analysis in the flow cytometer.
- For a dead cell control, fix 1 x 105 cells with 200 μL of 4% paraformaldehyde for 30 min, and wash with 500 μL of 1x PBS at 300 x g for 10 min at 4 °C. Draw off the supernatant and discard. Then add 200 μL of 0.1% Triton X-100 for 1 h at 4 °C. Wash with 500 μL of 1x PBS and stain with 7AAD as in step 3.2.1.
- Using a flow cytometer (see Table of Materials), perform FSC versus SSC analysis to analyze cell purity and SSC versus 7AAD staining to analyze the cell viability. Read 3 x 104 events in 100 μL of uptake volume at medium flow (1,000 cells/s) in the polymorphonuclear settings (FSC, 400-490 and SSC, 300-320).
- Analyze the captured data in the flow cytometer software (see Table of Materials), and determine the percentage of purity and positive cells for 7AAD in the polymorphonuclear population, presented through dot plots and histograms.
4. CFSE staining of microorganisms
- Add 1 x 108 bacteria or 1 x 106 fungal pseudohyphae in 1.5 mL microtubes, and stain with 200 µL of 5 µM carboxyfluorescein succinimidyl ester (CFSE) dissolved in 1x PBS. Mix for a few seconds, and incubate at 37 °C for 10 min in the dark.
- Stop the reaction by adding 500 µL of decomplemented plasma, and centrifuge at 620 x g for 10 min for pseudohyphae or at 1,800 x g for 10 min for bacteria.
- Discard the supernatants and wash the pellets with 1 mL of 1x PBS with centrifugation as in step 4.2. Finally, resuspend the microorganisms in 250 µL of 1x PBS.
- Prepare 50 µL aliquots in microtubes of 1.5 mL with 2 x 107 bacteria (MOI: 100) or 2 x 105 pseudohyphae (MOI:1) for NET induction.
5. NET induction
- Place 10 mm x 10 mm sterile glass coverslips in a 24-well plate and cover with 10 µL of 0.001% poly-L-lysine for 1 h at room temperature. Wash twice with 100 μL of 1x PBS, air dry, and irradiate with UV light for 15 min.
- Replace the HBSS solution of the neutrophil suspension in step 2.5 with RPMI 1640 medium supplemented with 10% autologous plasma. To the 24-well plate (step 5.1), add 350 μL of this cell suspension, for a final concentration of 2 x 105 neutrophils/well.
- Allow the cells to adhere to the bottom of the wells by incubating for 20 min at 37 °C with 5% CO2.
- Add the stimuli to induce NET formation in 50 µL: microbial stimuli-gram-positive bacterium S. aureus (ATCC 25923), gram-negative bacterium P. aeruginosa (ATCC 10145) at MOI 100, and pseudohyphae of C. albicans (ATCC 10231) at MOI:1; biochemical stimuli-PMA (200 nM) and HOCl (4.5 mM), and control with stimulus absent (50 µL of HBSS).
- Obtain a final volume of 400 µL per well. Mix on a plate shaker at 140 rpm for 30 s, and incubate for 4 h at 37 °C and 5% CO2.
6. Visualization of NETs by fluorescence microscopy
- DNA and LL37 immunostaining
- After NET induction, remove the supernatants from the wells by pipetting carefully, and fix the cells with 300 µL of 4% paraformaldehyde for 30 min.
- Wash the cells with 200 μL of 1x PBS without centrifuging, and add 200 µL of blocking buffer (10% decomplemented plasma in 1x PBS) for 30 min.
- For LL-37 stain, permeabilize the cells with 200 µL of 0.2% Triton X-100 in 1x PBS for 10 min to allow the antibody to enter the cells. Wash 2x carefully with 1x PBS to remove the excess detergent.
- Mount the coverslips on glass slides (four coverslips on each slide). DNA stain the cells with 2 µL of DAPI (see Table of Materials), seal the coverslips, and store at -20 °C until their analysis by confocal fluorescence microscopy.
- Acquisition and analysis of fluorescent images
- Take NET images to quantify their components, and use the corresponding filters in the confocal fluorescence microscope (see Table of Materials) to acquire the images with the computer's software.
NOTE: Consider that the DNA is stained with DAPI (blue color), showing excitation at 360 nm and emission at 460 nm. The microorganisms are stained with CFSE (green color), which has an excitation of 492 nm and an emission of 521 nm. The LL37 peptide is labeled with anti-LL37 Alexa Fluor 594 antibody (red color), which has an excitation of 594 nm and an emission of 614 nm. - Calibrate the microscope. Place the slide and focus using differential interference contrast (DIC) with normal light on. Choose Live to project the image on the monitor.
- Turn off the light and select the fluorochrome corresponding channel. For example, select filter 365 nm/blue for DAPI, 43 HE DsRed for Alexa 594, or 38 HE GFP for CFSE.
- Adjust the settings with the isotype control antibody for LL37 and unstained cells for DAPI and CFSE. Set the same exposure time, voltage, contrast, and lens settings to capture all the images under the same conditions.
NOTE: In this study, the exposure time, voltage, and contrast were set at 1.0 ms, 4.0 V, and 0.0, respectively, with a 40x objective. These values can be adjusted to facilitate the best image capture for the samples. - Select Snap to capture the image. Save five images (four extremes and the center) per well, and of the colocalization (merge) of DNA/LL37/CFSE.
- Define the three classes of pixels as background with the independent images of each color and analyze the Mean Gray Signal value per area with the Image J software.
- Take NET images to quantify their components, and use the corresponding filters in the confocal fluorescence microscope (see Table of Materials) to acquire the images with the computer's software.
7. Enzymatic activity quantification
- In a 96-well plate, add 90 μL of cell suspension in HBSS containing 1 x 105 neutrophils for NET induction, and incubate for 20 min at 37 °C and 5% CO2.
- Immediately, add 10 μL of the corresponding stimuli (concentration as in step 5.4) and incubate for 4 h at 37 °C with 5% CO2.
- Discard the supernatants and wash the cells with 100 μL of HBSS. Treat with 1 U/mL of DNase for 10 min at 37 °C to favor the release of DNA-protein structures, and centrifuge at 1,800 x g for 10 min.
- Recover the supernatants and evaluate the enzyme activity in the supernatant using colorimetric reactions as previously described by White et al.17.
- Determine the maximum enzyme activity of NE, CG, and MPO in neutrophils under the same experimental conditions without adding any stimuli for NET induction. Then, freeze the cell sample at -70 °C and thaw at 37 °C in a water bath, generating a temperature shock to favor the release of intracell proteins by cell lysis. Centrifuge at 1,800 x g for 10 min and recover the supernatants.
- Add 50 μL of the supernatant to each well in 96-well plates, and then add 50 μL of each substrate as indicated in step 7.7.
- Add 0.5 M of N-methoxysuccinyl-Ala-Ala-Pro-Val-p-nitro aniline as the substrate for NE, and 1 mM of N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide for CG. Incubate for 3 h at room temperature. For MPO, add 1.6 mM of 3,3', 5,5'-tetramethylbenzidine (TMB) and incubate for 30 min at room temperature.
- Post-incubation, add 50 μL of the stop solution (0.5 M H2SO4) for MPO and measure the absorbance at 405 nm for NE and CG and 450 nm for MPO, using a spectrophotometer.
- Compare the values obtained with the corresponding calibration curves and show the results of each condition relative to the maximum enzyme activity (100%).
8. Statistical analysis
- Analyze the measurement data in triplicate for each independent experiment (n = 10) and perform an ANOVA for statistical analysis by comparing groups with a 95% confidence level.
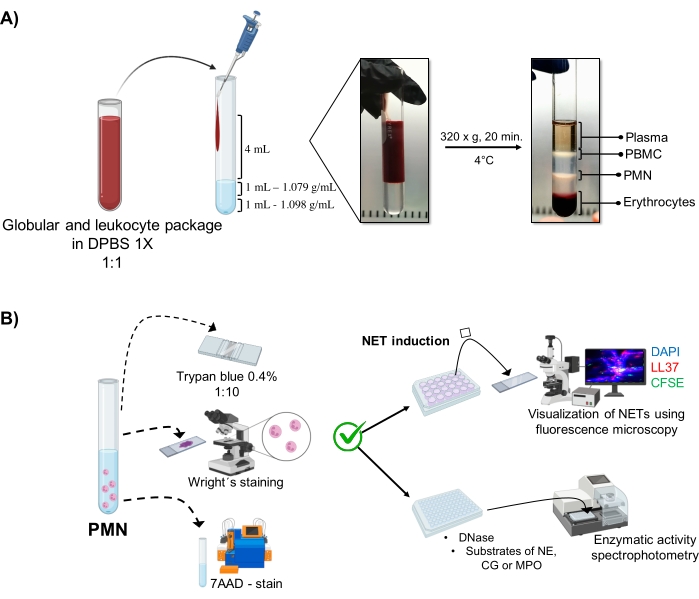
Figure 1: PMN purification and NET induction protocol. (A) Plasma was removed from the peripheral blood to obtain the erythrocyte and leukocyte package and diluted 1:1 (v/v) with 1x DPBS. Then, 4 mL of the dilution was added along the wall to the double-density gradient tube, and centrifuged at 320 x g for 20 min at 4 °C, obtaining the separation of different cell layers and recovering the one corresponding to PMN. (B) The purified cells were counted, and their morphology was analyzed by Wright's staining. Viability was determined by trypan blue exclusion and 7AAD staining using flow cytometry. Once optimal neutrophil purity and viability were verified, NET formation was induced by adding microbes (S. aureus, P. aeruginosa, and C. albicans) or chemicals (PMA, HOCl) in 24-well plates for analysis by fluorescence microscopy with DAPI-DNA, anti-LL37 Alexa Fluor 594, and microorganism-CFSE staining. For enzyme quantification, NETs were induced in 96-well plates for 3 h and treated with DNase, followed by the addition of substrates for each enzyme: NE, CG, and MPO; color changes were quantified by spectrophotometry. DPBS = Dulbecco's phosphate-buffered saline; PBMC = Peripheral blood mononuclear cells; PMN = Polymorphonuclear neutrophils; NE = Neutrophil elastase; CG = Cathepsin G; MPO = Myeloperoxidase; PMA = Phorbol myristate acetate; HOCl = Hypochlorous acid. Please click here to view a larger version of this figure.
Wyniki
Purity and viability of neutrophils
The dynamic cellular phases are visualized in the tube from the double-density gradient purification. Within these layers, the layer corresponding to granulocytes is above the 1.079 g/mL density layer, distinguished from the phases of peripheral blood mononucleocytes (PBMCs) and erythrocytes (Figure 1A). The morphology of the purified cells was verified with Wright's staining by observing cells with segmented nuclei connected wit...
Dyskusje
A highly pure population of viable neutrophils must be obtained to induce the release of NETs since these cells have a limited ex vivo lifetime of 8 h on average, a period within which all the experiments must be performed. To this end, the ideal methodology is the double-density gradient to optimize the purification time by isolating nonactivated cells more responsive to exogenous stimulation, in contrast to Ficoll-Histopaque gradient or Dextran sedimentation techniques17. Another advant...
Ujawnienia
The authors declare that they have no conflicts of interest.
Podziękowania
This work was supported by a basic science grant (#285480) from CONACyT and by the Department of Clinical Immunology Research of the Biochemical Sciences Faculty, Universidad Autónoma 'Benito Juárez' de Oaxaca. A.A.A, S.A.S.L, and W.J.R.R. have doctoral fellowships of CONACyT numbers #799779, #660793, and #827788, respectively.
Materiały
| Name | Company | Catalog Number | Comments |
| 24 Well plate for cell culture | Corning | 3526 | |
| 7-aminoactinomycin D (7-AAD) | BD Pharmingen | 51-668981E | |
| 96 Well plate for cell culture | Costar | 3596 | Flat bottom |
| Agitator | CRM Globe | CRM-OS1 | |
| Antibody LL37 | Santa Cruz Biotechnology | sc-166770 | |
| Blood collection tubes | BD VACUTAINER | 368171 | K2 EDTA 7.2 mg |
| Carboxyfluorescein succinimidyl ester (CFSE) | Sigma-Aldrich | 21878 | |
| Centrifuge | Hettich | 1406-01 | |
| Coverslip | Madesa | M03-CUB-22X22 | 22 mm x 22 mm |
| Dulbecco´s phosphate-buffered saline (DPBS) | Caisson | 1201022 | |
| Falcon tubes 50 mL | CORNING | 430829 | |
| Flow Cytometry Tubes | Miltenyi Biotec | 5 mL - Without caps | |
| FlowJo Software | BD Biosciences | Analyze flow cytometry data | |
| Fluorescence microscope | DM 2000 | LEICA | |
| Fluoroshield with DAPI | Sigma-Aldrich | F6057 | |
| Incubator | NUAIRE | UN-4750 | |
| MACSQuant Analyzer | Miltenyi Biotec | Flow cytometer | |
| Microplate reader photometer | Clarkson Laboratory - CL | ||
| Microtubes 1.5 mL | Zhejiang Runlab Tech | 35200N | wire snap |
| Minitab Software | Minitab | Statistical analysis | |
| Needles | BD VACUTAINER | 301746 | Diameter 1.34 mm |
| Optical microscope | VELAB | VE-B50 | |
| Percoll | GE Healthcare | 17-0891-01 | Solution for density gradient |
| Phosphate Buffered Saline (10x) | Caisson | PBL07-500ML | |
| Pyrex culture tubes | CORNING | CLS982025 | N°9820 |
| RPMI 1640 1x | Corning | 10-104-CV | contains Glutagro |
| Slides | Madesa | PDI257550 | 22 mm x 75 mm |
| Trypan Blue solution 0.4% | SIGMA | T8154-100ML |
Odniesienia
- De Buhr, N., Maren, K. B. How neutrophil extracellular traps become visible. Journal of Immunology Research. 2016, 4604713 (2016).
- Karlsson, A., Nixon, J. B., McPhail, L. C. Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. Journal of Leukocyte Biology. 67 (3), 396-404 (2000).
- Takei, H., Araki, A., Watanabe, H., Ichinose, A., Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. Journal of Leukocyte Biology. 59 (2), 229-240 (1996).
- Brinkmann, V., et al. Neutrophil extracellular traps kill bacteria. Science. 303 (5663), 1532-1535 (2004).
- Kenny, E. F., et al. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 6, 24437 (2017).
- Schultz, B. M., Acevedo, O. A., Kalergis, A. M., Bueno, S. M. Role of extracellular trap release during bacterial and viral infection. Frontiers in Microbiology. 13, 798853 (2022).
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nature Reviews Immunology. 18 (2), 134-147 (2018).
- Delgado-Rizo, V., et al. Neutrophil extracellular traps and its implications in inflammation: An overview. Frontiers in Immunology. 8, 81 (2017).
- Petretto, A., et al. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS One. 14 (7), 0218946 (2019).
- Neumann, A., et al. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. Journal of Innate Immunity. 6 (6), 860-868 (2014).
- Hakkim, A., et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nature Chemical Biology. 7 (2), 75-77 (2011).
- Sabbatini, M., Magnelli, V., Renò, F. NETosis in wound healing: When enough Is enough. Cells. 10 (3), 494 (2021).
- Metzler, K. D., Goosmann, C., Lubojemska, A., Zychlinsky, A., Papayannopoulos, V. A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Reports. 8 (3), 883-896 (2014).
- Clark, S. R., et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature Medicine. 13 (4), 463-469 (2007).
- Pilsczek, F. H., et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of Immunology. 185 (12), 7413-7425 (2010).
- Sosa, S. A., et al. Structural differences of neutrophil extracellular traps induced by biochemical and microbiologic stimuli under healthy and autoimmune milieus. Immunologic Research. 69 (3), 264-274 (2021).
- White, P. C., et al. Characterization, quantification, and visualization of neutrophil extracellular traps. Methods in Molecular Biology. 1537, 481-497 (2017).
- Boeltz, S., et al. To NET or not to NET: current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death and Differentiation. 26 (3), 395-408 (2019).
- Yousefi, S., Mihalache, C., Kozlowski, E., Schmid, I., Simon, H. U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death and Differentiation. 16 (11), 1438-1444 (2009).
- Brinkmann, V., Zychlinsky, A. Neutrophil extracellular traps: is immunity the second function of chromatin. The Journal of Cell Biology. 198 (5), 773-783 (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone