Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Live Calcium Imaging of Virus-Infected Human Intestinal Organoid Monolayers Using Genetically Encoded Calcium Indicators
W tym Artykule
Podsumowanie
This protocol describes an approach for performing calcium imaging in virus-infected human intestinal organoids and offers an approach to analysis.
Streszczenie
Calcium signaling is an integral regulator of nearly every tissue. Within the intestinal epithelium, calcium is involved in the regulation of secretory activity, actin dynamics, inflammatory responses, stem cell proliferation, and many other uncharacterized cellular functions. As such, mapping calcium signaling dynamics within the intestinal epithelium can provide insight into homeostatic cellular processes and unveil unique responses to various stimuli. Human intestinal organoids (HIOs) are a high-throughput, human-derived model to study the intestinal epithelium and thus represent a useful system to investigate calcium dynamics. This paper describes a protocol to stably transduce HIOs with genetically encoded calcium indicators (GECIs), perform live fluorescence microscopy, and analyze imaging data to meaningfully characterize calcium signals. As a representative example, 3-dimensional HIOs were transduced with lentivirus to stably express GCaMP6s, a green fluorescent protein-based cytosolic GECI. The engineered HIOs were then dispersed into a single-cell suspension and seeded as monolayers. After differentiation, the HIO monolayers were infected with rotavirus and/or treated with drugs known to stimulate a calcium response. An epifluorescence microscope fitted with a temperature-controlled, humidified live-imaging chamber allowed for long-term imaging of infected or drug-treated monolayers. Following imaging, acquired images were analyzed using the freely available analysis software, ImageJ. Overall, this work establishes an adaptable pipeline for characterizing cellular signaling in HIOs.
Wprowadzenie
Calcium is a widely conserved second messenger that plays a critical role in regulating cellular physiology1. Given its strong charge, small size, and high solubility in physiological conditions, calcium is an ideal manipulator of protein conformation. This makes calcium a powerful means to transduce electrochemical signals into enzymatic, transcriptional, or post-transcriptional alterations. The strict calcium concentration gradients across the endoplasmic reticulum (ER) and plasma membranes create a high driving force that allows for rapid changes in cytosolic calcium concentration. Multiple mechanisms, including both buffering and active transport, tightly maintain this gradient. While necessary for normal cellular functions, this maintenance is energetically expensive, making it particularly susceptible in states of stress 2.
As such, dysregulation of calcium within the cytosol is a near-universal signal of many kinds of cellular stress. Metabolic disturbances, toxins, pathogens, mechanical damage, and genetic perturbations can all disrupt calcium signaling. Regardless of the stimulus, on a whole-cell level, sustained, uncontrolled rises in cytosolic calcium can promote apoptosis and eventually necrosis3,4. Alterations in cytosolic calcium levels of lower amplitude or higher frequency, however, have varying effects2. Likewise, the outcomes of calcium fluctuations may differ based on the spatial microdomain in which they occur5. Monitoring calcium levels can therefore offer insight into dynamic signaling processes, but this requires sampling with relatively high temporal and spatial resolution.
Genetically encoded calcium indicators (GECIs) are powerful tools for continuous sampling in live-cell systems6. Some of the most widely used GECIs are GFP-based calcium-responsive fluorescent proteins known as GCaMPs7. The canonical GCaMP is a fusion of three distinct protein domains: a circularly permuted GFP (cpGFP), calmodulin, and M136. The calmodulin domain undergoes a conformation change upon binding calcium, allowing its interaction with M13. The calmodulin-M13 interaction induces a conformational change in the cpGFP that increases its fluorescent emission upon excitation. As such, an increase in calcium concentration correlates with an increase in GCaMP fluorescence intensity. These sensors can be cytosolic or targeted to specific organelles8.
Similar to most tissues, calcium regulates a variety of functions within the gastrointestinal epithelium. The intestinal epithelium is integral for nutrient and fluid absorption but also must form a tight barrier and immune interface to avoid pathogen invasion or toxic insults. Calcium-dependent pathways influence nearly all of these vital functions9,10,11. However, calcium signaling within the intestinal epithelium remains an underexplored frontier with promising potential as a therapeutic target. While monitoring calcium dynamics within the intestinal epithelium in vivo continues to present challenges, human intestinal organoids (HIOs) offer an adaptable ex vivo system for experimentation12. HIOs are 3-dimensional (3D) spheroids derived from human intestinal stem cells and, upon differentiation, recapitulate much of the cellular diversity of the native intestinal epithelium12.
This protocol describes comprehensive methods to engineer HIOs that express GECIs and then prepare engineered HIOs as monolayers for live-cell calcium imaging. It offers viral infection as an example of a pathologic manipulation that disrupts calcium signaling and provides an analytic approach to quantify these changes.
Protokół
All of the human intestinal organoids (HIOs) used in this protocol and the representative experiments were derived from human tissue obtained and maintained by the Texas Medical Center Digestive Diseases Enteroid Core. All samples were collected in accordance with a protocol approved by the Institutional Review Board at Baylor College of Medicine.
1. Preparation of materials and reagents
- For organoid maintenance, gather cell-culture treated 24-well plates, basement membrane matrix (BMM), 15 mL conical tubes, and 1.5 mL conical tubes.
- To prepare complete media without growth factors (CMGF-), to 500 mL of advanced DMEM F12 add 5 mL of 1M HEPES, 5 mL of 100x antibiotic-antimycotic, 5 mL of 100x glutamine supplement.
- To prepare Wnt-, R-spondin-, and Noggin-containing (WRNE) media, mix equal parts CMGF- and Wnt-conditioned media, add Noggin-conditioned medium, 10% by volume, R-spondin conditioned medium, 20% by volume, 50 ng/mL human epidermal growth factor, 10 mM nicotinamide, 10 nM [Leu15]-Gastrin I, 500 nM A-83-01, 10 µM SB202190, 1x B27 supplement, 1x N2 supplement, and 1 mM N-acetylcysteine.
- For lentiviral transduction, prepare Fetal Bovine Serum (FBS), 0.05% Trypsin-EDTA in 1x phosphate buffered saline (PBS), CMGF- with 10% FBS, Sterile 1x PBS, Polybrene, 10 µM Y-27632, Lentivirus, High Wnt WRNE + 10 µM Y-27632
- For generating organoid monolayers, prepare glass bottom 10-well cell culture slide, FBS, Collagen IV (1 mg/mL in de-ionized (di)H2O), basement membrane matrix, 0.5 mM EDTA in 1x PBS, 5 mM EDTA in 1x PBS, enzymatic dissociation buffer, CMGF- with 10% FBS, WRNE + 10 µM Y-27632.
- For viral infection of organoid monolayers, prepare trypsin, rotavirus stock, CMGF-, 25G needle, Sterile 1x PBS, and phenol red-free differentiation media.
- To prepare phenol red-free differentiation media, take 500 mL of phenol red-free cell culture media, add 5 mL of 100x MEM non-essential amino acids, 5 mL of 100x L-Glutamine, 5 mL of 100 nM sodium pyruvate, and 7.5 mL of 1M HEPES.
- For immunofluorescence staining of organoids, prepare 4% formaldehyde (16% formaldehyde diluted in 1x PBS), Triton X-100 (0.1% Triton X-100 in 1x PBS), Bovine serum albumin (3% bovine serum albumin in 1x PBS), NH4Cl solution (50 mM), DAPI (1 µg/mL DAPI solution in 1x PBS).
2. Engineering organoids to express genetically encoded calcium sensors
NOTE: This protocol describes the steps to transduce a single well of 3-dimensional human intestinal organoids plated in 30 μL of Basement Membrane Matrix (BMM) on a 24-well plate13. Most lines will contain around 400,000 cells per well. A second, non-transduced well should be included as a control. Keep all reagents and cell suspensions on ice.
- After 2-5 days from the last passage, remove maintenance WRNE medium from two wells of HIOs. Replace with 300 μL of 0.05% Trypsin-EDTA per well and gently pipette up and down 5x to detach the BMM from the plate. Place in a 37 °C incubator for 4 min.
- Add 500 μL of CMGF- + 10% FBS per well. Pre-coat a 1 mL low-bind pipette tip with FBS by pipetting 1 mL of FBS up and down 2x. FBS can be re-used on multiple tips. With the pre-coated tip, pipette the HIOs up and down 10x.
- Transfer the contents of each well to its own pre-coated 1.5 mL microcentrifuge tube. Rinse each well with an additional 500 μL of CMGF- and add the wash to the respective tube.
- Centrifuge the tubes at 100 x g in a swinging bucket centrifuge for 5 min at 4 °C. Remove supernatant and any residual BMM.
- Resuspend in 1 mL of 1x PBS. Split each tube into two microcentrifuge tubes for 4 total tubes. Centrifuge the tubes at 100 x g for 5 min at 4 °C. Remove supernatant.
- Resuspend each tube one additional time in 1x PBS and centrifuge at 100 x g for 5 min at 4 °C.
- Prepare 400 µL of transduction medium and control medium (Table 1). Resuspend 2 tubes in 200 µL of the control medium and 2 tubes in 200 µL of the transduction medium.
- Incubate in a 37 °C cell culture incubator for 24 h. Resuspend by pipetting up and down with a coated tip periodically (e.g., 2 h post transduction (hpt), 12 hpt, 18 hpt) to encourage uniform transduction.
- After 24 h, centrifuge tubes at 100 x g for 5 min at 4 °C. Remove supernatant.
- Resuspend pellet in 500 µL of 1x PBS to wash. Centrifuge at 100 x g for 5 min at 4 °C. Remove supernatant.
- Using an ice-cold 200 µL pipette tip, resuspend each of the 4 pellets in 30 µL of BMM. Gently pipette up and down to disperse evenly.
- Plate the contents of each tube into its own well on a 24-well plate. Incubate for 10 min at 37 °C to allow BMM to solidify before adding 500 μL of HighWnt WRNE + 10μM Y-27632.
- Allow transduced HIOs to grow for 1 week, refreshing the media (HighWnt WRNE + 10 μM Y-27632) every other day. After 1 week, check for the expression of the fluorescent indicator by microscopy. If the signal is strong, begin drug selection. If the signal is weak, repeat the transduction as described above.
- Once the line is established, verify the function of the fluorescence indicator via agonist treatment. 100nM ADP is a reliable agonist for GCaMP validation. Test 3D organoids for initial validation as described below.
- After a passage, plate a well (or multiple) of organoids in BMM on a separate imaging-bottom plate. Plate the organoids at about 1/3 of the normal density to avoid excess overlap when imaging. Do not continue to passage these HIOs following agonist treatment, as it is difficult to ensure sterility.
- Allow 2-3 days for the HIOs to recover post-passage. Before imaging, switch the media to phenol red-free differentiation media.
- Using a fluorescent microscope, set up a 3 min run with images acquired every 5 s using 488 nm excitation and a FITC/GFP filter set. After 30 s of imaging, add 100 nM ADP or vehicle control. Continue imaging until the signal returns to near-baseline, ~2 min. A transient, ~2-fold increase in GCaMP fluorescence with ADP treatment indicates successful transduction and biosensor function. For more precise estimate of transduction efficiency, repeat agonist test with imaging using monolayers generated via the process described in part 3.
3. Preparation of HIO monolayers for live fluorescence imaging
- Coat all wells of a 10-well imaging bottom chamber slide with Collagen IV. To do this, mix 34 μL of 1 mg/mL Collagen IV with 960 μL of sterile deionized water. Add 95 μL of diluted collagen IV solution to each well and incubate at 37 °C for 0.5-2 h.
- Remove the WRNE maintenance medium from 4 wells of 3D HIOs. HIOs should be 5-7 days from their last passage.
NOTE: 1 10-well plate typically requires ~1.25 x 106 cells. This typically requires 2-4 wells of 3D HIOs plated in 30 μL of BMM each, but will vary based on density. - Add 500 μL of 1x PBS + 0.5 mM EDTA per well. With a pre-coated 1 mL tip, pipette up and down gently to detach BMM from the plate. Transfer the suspension to a pre-coated 15 mL conical tube, combining like wells into the same tube.
- Rinse each well with an additional 500 μL of PBS + 0.5 mM EDTA. Centrifuge at 300 x g for 5 min at 4 °C. Remove the supernatant and residual BMM.
- Using a pre-coated tip, resuspend the remaining pellet in 3 mL of PBS + 5 mM EDTA (note that this is 10x more EDTA than the first wash).
- Centrifuge at 300 x g for 5 min at 4 °C. Remove the supernatant and residual BMM. Resuspend the pellet in 2 mL of enzymatic dissociation buffer.
- Incubate in 37 °C bead/water bath for 5 min. Add 3 mL of CMGF- + 10% FBS and pipette gently to mix.
- Centrifuge at 300 x g for 5 min at 4 °C. Remove the supernatant. Add 1 mL of CMGF-.
- Vigorously pipette up and down with a pre-coated tip 80x-100x to mechanically break HIOs into single cells. Centrifuge at 300 x g for 5 min at 4 °C. Remove supernatant.
- Resuspend in 1 mL of WRNE + 10 µM Y-27632. Obtain a cell count for the 1 mL suspension.
- Dilute the cell suspension with WRNE + 10µM Y-27632 to achieve a concentration of 1.25 x 105 cells/100 μL (1.25 x 106 cells/mL).
- Using a 200 μL pipette, remove the collagen solution from the plate prepared in step 1, as the collagen has now settled to the bottom of the well. Avoid touching the bottom of the well with the pipette tip.
- Using a pre-coated 200 μL pipette tip, add 100 μL of the cell solution from step 3.11 (1.25 x 105 cells) per well.
- Incubate for 24 h in a 37 °C cell culture incubator. After 24 h, remove the culture medium from all wells and replace it with 100 μL of differentiation medium per well.
NOTE: By this point, the cells should be adhering to the plate. Note that the monolayer likely will not be confluent or fully planar. - Place the slide back into the 37 °C cell culture incubator. Refresh the differentiation medium every 24 h until the monolayer is confluent. This usually requires 3-5 days from plating. After this point, the monolayers are ready for downstream applications.
4. Viral infection of HIO monolayers
- Prepare the virus inoculum. If necessary, trypsin activate the virus stock. For rotavirus, add 10 µg/mL Worthington's Trypsin to the virus stocks and incubate at 37 °C for 1 h.
- Dilute the activated virus stock using one of the two following methods.
- If aiming for the maximum number of infected cells using only one viral strain, mix 50 μL of the activated viral stock with 50 μL CMGF-.
- If comparing multiple strains, prepare the inoculums to achieve equivalent multiplicities of infection (MOIs). Use the following formula to determine the volume of virus stock per well necessary for the desired MOI. Add CMGF- to the volume of virus stock calculated to achieve a final volume of 100 μL per well.
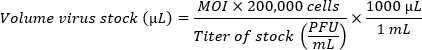
NOTE: A single HIO monolayer plated in a well with a diameter of 5.46 mm (96-well plate) contains about 200,000 cells. Because of the poor efficiency of infection in monolayers, a high MOI (100-1 million viral particles per cell) is preferred. The optimal MOI must be determined empirically.
- Infect the monolayers by gently removing the media from the HIO monolayer using a 200 mL pipette. Replace with 100 mL of virus inoculum or mock inoculum.
- Since most rotavirus stocks are propagated in MA104 cells, use an uninfected MA104 cell lysate for the mock inoculum. Use a volume equivalent to the volume of virus stock used for the infected wells and add CMGF- for a final volume of 100 mL per well.
- For viruses that infect cells basolaterally, such as rotavirus14, use a 25G needle to score the monolayer. It is easiest to make a single score across the length of the monolayer, from the bottom to the top of the well. Gently press the needle into the monolayer at an angle with the bevel side up, opposite the direction of motion, and drag it across the length of the monolayer to create a scar (Figure 2A).
- Incubate in a 37 °C incubator for 2 h. Remove the virus and mock inoculum. Wash monolayers once with 1x PBS.
- Add 100 μL of phenol red-free differentiation media. Place slide in a 37 °C incubator until ready to image. The optimal imaging window will vary based on the kinetics of the viral infection. For rotavirus, begin imaging at 6-8 h post infection.
- Dilute the activated virus stock using one of the two following methods.
5. Ca2+ Imaging of infected monolayers
- Pre-heat the stage-top incubator to 37 °C. Run humidified CO2 at 0.02 L/min. Place slide with the infected monolayers into the incubator chamber and seal the lid.
- Using brightfield (BF) illumination and a 20x objective, select the X and Y coordinates of the fields of view within the monolayer that will be imaged. It is best to select points with the scored region in view, as most infected cells will be near the scratch.
- Optimize imaging parameters and acquire an image using 488 nm excitation. To minimize phototoxicity, set the light source to 50% power with a 50 ms exposure time. The appropriate acquisition parameters may vary and should be optimized by acquiring multiple acquisitions. Ensure no pixels are saturated. Adjust exposure time and light power as needed to ensure the entire dynamic range of the fluorescent calcium sensor can be detected.
- If using other fluorophores (e.g., fluorescently tagged viruses), repeat optimization on these channels. Set up the imaging loop and acquire a 488 nm image at every selected X, Y coordinate in a 1 min loop. Image this loop continuously. When using a fluorescently tagged virus, acquire an image on the appropriate channel every 10th loop (i.e., 1 image/10 min).
- Collect images for ~18 h. If using viruses without fluorescent tags, fix monolayers and perform immunofluorescence staining to identify infected cells. Perform this after completing the imaging run as described below.
- Remove imaging media and replace with 100 μL of 4% formaldehyde in 1x PBS. Incubate at room temperature for 30 min.
- Remove fixative and quench with 100 μL of 50mM NH4Cl for 10 min. Remove NH4Cl. Permeabilize the monolayers with 100 μL of 0.1% Triton X-100 in 1x PBS for 1 h at room temperature or overnight at 4 °C.
- Remove permeabilization buffer. Block with 100 μL of 3% bovine serum albumin in 1x PBS for 1 h.
- Remove the blocking solution. Add primary antibodies diluted to the recommended/optimized concentration in 1x PBS. Add 100 μL of antibody solution per well.
- Incubate overnight at 4 °C with gentle rocking. Remove primary antibodies. Wash 3x with 1x PBS.
- Add fluorescent-conjugated secondary antibodies diluted to the recommended/optimized concentration in 1x PBS. Add 100 μL per well. Incubate for 2 h at room temperature.
NOTE: FPBase15 is a great resource to help select secondaries that will avoid bleed-through when multiplexing. Most secondaries are effective at a 1:1000 dilution in 1x PBS. - Remove secondary antibodies. Make a 1 µg/mL DAPI solution in 1x PBS and add 100 μL per well. Incubate for 20 min. Remove DAPI. Wash 3x with 1x PBS.
- Keep fixed monolayers in 100 μL of 1x PBS for imaging. Place the slide back onto the microscope stage, reload the X and Y coordinates from the live imaging run, and image each multi-point on the channel that corresponds to the secondary antibody that was used for staining. Reference the images from the live imaging run to ensure the same points are captured.
6. Quantitation of intercellular calcium waves
- Ensure Fiji is Just ImageJ (FIJI) is installed with the following plug-ins: Bio-Formats (should be pre-installed in FIJI but will not be in ImageJ); Cookbook: To install, from the FIJI menu, go to Help > Updates > Manage update sites. Check Cookbook. Restart FIJI.
- Split the data file from the live imaging run into multiple files, separating each X, Y coordinate. In Nikon NES Elements, select File > Import/Export > Split Multipoints. Select a folder for export and an appropriate prefix.
- Open FIJI. Load a single file from the split multipoints. If using a multi-channel image, split the channels. Select the window with the images from the 488 nm channel (GCaMP).
- Run the DeltaF Up function to determine the change in each pixel value from one timepoint to the next. To do this, select Cookbook > T-functions > Delta F Up.
- Scroll through the stack until an image with a calcium wave is found. With the calcium wave in view, set a threshold by clicking Image > Adjust > Threshold. Adjust the lower limit to minimize the amount of signal that is not part of the wave that makes it past the threshold. For 16-bit images acquired using the parameters described above, start with a lower threshold of 600 and adjust as needed.
- Run the particle analyzer to segment waves by clicking Analyze > Analyze Particles. Set the minimum size of wave in µm2 by adjusting the range. It is set to 0-infinity at baseline. For images of MA104 cells acquired using a 20x objective, start by increasing the lower limit to 10,000 µm2 and adjust as appropriate.
- Check the boxes for Display results, and Clear results. From the "Show" dropdown menu, select Outlines, then click OK. This should result in 2 outputs: One window, Results, will list each wave detected, the area of the wave, and its mean, minimum, and maximum intensity values (based on delta F). The other window, titled Drawing of [file name], will include a new stack with the outlines of segmented waves. Use this to verify wave detection. Adjust threshold values and size range if needed, and re-check the wave calls.
- For batch processing, use the macro script included (Supplementary Coding File 1). To utilize this, follow the steps described below.
- Split multi-points into single files as described in step 6.2. Open FIJI. Select Process > Batch > Macro. In the "input" box, provide the map to the folder containing the split multipoint files. Leave the "output" box empty.
- Paste the script from Supplementary Coding File 1 into the box. Adjust the intensity threshold and size threshold in the script to reflect the optimized parameters from steps 6.5 and 6.6.
- Click Process. Depending on the number of multi-points and processing speed of the computer, it can take 30 min or longer to process all images. Once complete, the data will be written into a new spreadsheet file named "Rename me after writing" on the Desktop. The output file should contain, for each multipoint, a wave count and wave metrics (area, mean intensity, minimum and maximum intensity, and intensity density).
Wyniki
Figure 1A shows a BMM dome containing 3-dimensional human intestinal organoids that have been transduced to stably express GCaMP6s. Figure 1B shows the same line of organoid replated as a monolayer at 24, 48, and 72 h post-seeding. To validate the function of GCaMP6s, the monolayer was imaged by fluorescence microscopy every 2 s for 4 min, and 100 nM ADP was added to the media after ~20 s. ADP elicits calcium release from the endoplasmic reticulum, increasi...
Dyskusje
Alterations in cytosolic Ca2+ levels can be both a cause and effect of pathologies within the epithelium10,16,17. Increases in cytosolic calcium can directly drive secretion via activation of the calcium-dependent chloride channel TMEM16A18,19. Activation of TMEM16A in response to Ca2+ allows for the apical efflux of chloride, establishing an osmoti...
Ujawnienia
The authors have no competing financial interests to disclose.
Podziękowania
This work was supported by grants R01DK115507 and R01AI158683 (PI: J. M. Hyser) from the National Institutes of Health (NIH). Trainee support was provided by NIH grants F30DK131828 (PI: J.T. Gebert), F31DK132942 (PI: F. J. Scribano), and F32DK130288 (PI: K.A. Engevik). We would like to thank the Texas Medical Center Digestive Diseases Enteroid Core for providing the organoid maintenance media.
Materiały
| Name | Company | Catalog Number | Comments |
| Advanced DMEM F12 | Gibco | 12634028 | |
| [Leu15]-Gastrin I | Sigma-Aldrich | G9145 | |
| 0.05% Trypsin EDTA | Gibco | 25300054 | |
| 0.05% Trypsin EDTA | Gibco | 25300054 | |
| 1.5mL microcentrifuge tubes | Fisherbrand | 5408137 | |
| 15mL conical tubes | Thermofisher Scientific | 0553859A | |
| 16% formaldehyde | Thermofisher Scientific | 28906 | |
| 1M HEPES | Gibco | 15630080 | |
| 1M HEPES | Gibco | 15630080 | |
| 1X PBS | Corning | 21-040-CV | |
| 25 gauge needle | Thermofisher Scientific | 1482113D | |
| A-83-01 | Tocris | 2939 | |
| ADP | Sigma-Aldrich | A2754 | |
| Advanced DMEM F12 | Gibco | 12634028 | |
| Antibiotic-antimycocytic | Gibco | 15240062 | |
| Antibiotic-antimycotic | Gibco | 15240062 | |
| B27 Supplement | Gibco | 17504-044 | |
| Bovine serum albumin | FisherScientific | BP1600100 | |
| CellView Cell Culture Slide, PS, 75/25 MM, Glass Bottom, 10 compartments | Greiner | 543979 | |
| Collagen IV | Sigma Aldrich | C5533 | |
| DAPI | Thermofisher Scientific | D1306 | |
| EDTA | Corning | 46-034-CI | |
| Fetal bovine serum | Corning | 35010CV | |
| Fetal bovine serum | Corning | 35010CV | |
| Fluorobrite | Gibco | A1896701 | |
| GlutaMAX | Gibco | 35050079 | |
| GlutaMAX | Gibco | 35050079 | |
| Human epidermal growth factor | ProteinTech | HZ-1326 | |
| Lentivirus | VectorBuilder | (variable) | |
| Matrigel | BD Biosceicen | 356231/CB40230C | |
| N2 Supplement | Gibco | 17502-048 | |
| N-acetylcysteine | Sigma-Aldrich | A9165-5G | |
| NH4Cl | Sigma-Aldrich | A9434 | |
| Nicotinamide | Sigma-Aldrich | N0636 | |
| Nunc Cell Culture Treated 24-well Plates | Thermofisher Scientific | 142475 | |
| Polybrene | MilliporeSigma | TR1003G | |
| SB202190 | Sigma-Aldrich | S70767 | |
| Triton X-100 | Fisher BioReagents | BP151100 | |
| TrypLE Express Enzyme, no phenol red | Thermofisher Scientific | 12604013 | |
| Trypsin | Worthington Biochemical | NC9811754 | |
| Y-27632 | Tocris | 1254 |
Odniesienia
- Bootman, M. D., Bultynck, G. Fundamentals of cellular calcium signaling: A primer. Cold Spring Harb Perspect Biol. 12 (1), a038802 (2020).
- Clapham, D. E. Calcium signaling. Cell. 131 (6), 1047-1058 (2007).
- Danese, A., et al. Cell death as a result of calcium signaling modulation: A cancer-centric prospective. Biochim Biophys Acta Mol Cell Res. 1868 (8), 119061 (2021).
- Harr, M. W., Distelhorst, C. W. Apoptosis and autophagy: Decoding calcium signals that mediate life or death. Cold Spring Harb Perspect Biol. 2 (10), a005579 (2010).
- Barak, P., Parekh, A. B. Signaling through Ca2+ microdomains from store-operated CRAC channels. Cold Spring Harb Perspect Biol. 12 (7), a035097 (2020).
- Nakai, J., Ohkura, M., Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 19 (2), 137-141 (2001).
- Erofeev, A. I., Vinokurov, E. K., Vlasova, O. L., Bezprozvanny, I. B. GCaMP, a family of single-fluorophore genetically encoded calcium indicators. J Evol Biochem Phys. 59 (4), 1195-1214 (2023).
- Suzuki, J., Kanemaru, K., Iino, M. Genetically encoded fluorescent indicators for organellar calcium imaging. Biophys J. 111 (6), 1119-1131 (2016).
- Nászai, M., Cordero, J. B. Intestinal stem cells: Got calcium. Curr Biol. 26 (3), R117-R119 (2016).
- Barrett, K. E. Calcium-mediated chloride secretion in the intestinal epithelium: Significance and regulation. Curr Top Membr. 53, 257-282 (2002).
- Xu, J., et al. Calcium-sensing receptor regulates intestinal dipeptide absorption via Ca2+ signaling and IKCa activation. Physiol Rep. 8 (1), e14337 (2020).
- Clevers, H. Modeling development and disease with organoids. Cell. 165 (7), 1586-1597 (2016).
- Lin, S. C., Haga, K., Zeng, X. L., Estes, M. K. Generation of CRISPR–Cas9-mediated genetic knockout human intestinal tissue–derived enteroid lines by lentivirus transduction and single-cell cloning. Nat Protoc. 17 (4), 1004-127 (2022).
- Crawford, S. E., Ramani, S., Blutt, S. E., Estes, M. K. Organoids to dissect gastrointestinal virus-host interactions: What have we learned. Viruses. 13 (6), 999 (2021).
- Lambert, T. J. FPbase: a community-editable fluorescent protein database. Nat Methods. 16 (4), 277-278 (2019).
- Lai, Y., et al. Inhibition of calcium-triggered secretion by hydrocarbon-stapled peptides. Nature. 603 (7903), 949-956 (2022).
- Chang-Graham, A. L., et al. Rotavirus induces intercellular calcium waves through ADP signaling. Science. 370 (6519), eabc3621 (2020).
- Lee, B., et al. Anoctamin 1/TMEM16A controls intestinal Cl− secretion induced by carbachol and cholera toxin. Exp Mol Med. 51 (8), 1-14 (2019).
- Saha, T., et al. Intestinal TMEM16A control luminal chloride secretion in a NHERF1 dependent manner. Biochem Biophys Rep. 25, 100912 (2021).
- Mroz, M. S., Keely, S. J. Epidermal growth factor chronically upregulates Ca2+-dependent Cl− conductance and TMEM16A expression in intestinal epithelial cells. J Physiol. 590 (8), 1907-1920 (2012).
- Sui, J., et al. Dual role of Ca2+-activated Cl− channel transmembrane member 16A in lipopolysaccharide-induced intestinal epithelial barrier dysfunction in vitro. Cell Death Dis. 11 (5), 404 (2020).
- Bellono, N. W., et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 170 (1), 185-198.e16 (2017).
- Paradis, T., Bègue, H., Basmaciyan, L., Dalle, F., Bon, F. Tight junctions as a key for pathogens invasion in intestinal epithelial cells. Int J Mol Sci. 22 (5), 2506 (2021).
- Samak, G., et al. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J. 465 (3), 503-515 (2015).
- Deng, H., Gerencser, A. A., Jasper, H. Signal integration by Ca2+ regulates intestinal stem cell activity. Nature. 528 (7581), 212-217 (2015).
- Saurav, S., Tanwar, J., Ahuja, K., Motiani, R. K. Dysregulation of host cell calcium signaling during viral infections: Emerging paradigm with high clinical relevance. Mol Aspects Med. 81, 101004 (2021).
- Chang-Graham, A. L., et al. Rotavirus calcium dysregulation manifests as dynamic calcium signaling in the cytoplasm and endoplasmic reticulum. Sci Rep. 9 (1), 10822 (2019).
- Hyser, J. M., Collinson-Pautz, M. R., Utama, B., Estes, M. K. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio. 1 (5), e00265-e00310 (2010).
- Pham, T., Perry, J. L., Dosey, T. L., Delcour, A. H., Hyser, J. M. The Rotavirus NSP4 viroporin domain is a calcium-conducting ion channel. Sci Rep. 7, 43487 (2017).
- Crawford, S. E., Hyser, J. M., Utama, B., Estes, M. K. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. Proc Natl Acad Sci U S A. 109 (50), E3405-E3413 (2012).
- Crawford, S. E., Criglar, J. M., Liu, Z., Broughman, J. R., Estes, M. K. COPII vesicle transport is required for Rotavirus NSP4 interaction with the autophagy protein LC3 II and trafficking to viroplasms. J Virol. 94 (1), e01341 (2019).
- Pando, V., Iša, P., Arias, C. F., Ló Pez, S. Influence of calcium on the early steps of Rotavirus infection. Virology. 295 (1), 190-200 (2002).
- Hyser, J. M., Estes, M. K. Pathophysiological consequences of calcium-conducting viroporins. Annu Rev Virol. 2 (1), 473-496 (2015).
- Strtak, A. C., et al. Recovirus NS1-2 has viroporin activity that induces aberrant cellular calcium signaling to facilitate virus replication. mSphere. 4 (5), e00506-e00519 (2019).
- In, J. G., Foulke-Abel, J., Clarke, E., Kovbasnjuk, O. Human colonoid monolayers to study interactions between pathogens, commensals, and host intestinal epithelium. J Vis Exp. (146), 59357 (2019).
- Hirota, A., AlMusawi, S., Nateri, A. S., Ordóñez-Morán, P., Imajo, M. Biomaterials for intestinal organoid technology and personalized disease modeling. Acta Biomater. 132, 272-287 (2021).
- Cevallos Porta, D., López, S., Arias, C. F., Isa, P. Polarized rotavirus entry and release from differentiated small intestinal cells. Virology. 499, 65-71 (2016).
- Mirabelli, C., et al. Human Norovirus efficiently replicates in differentiated 3D-human intestinal enteroids. J Virol. 96 (22), e0085522 (2022).
- Icha, J., Weber, M., Waters, J. C., Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays. 39 (8), 28749075 (2017).
- Li, J., et al. Engineering of NEMO as calcium indicators with large dynamics and high sensitivity. Nat Methods. 20 (6), 918-924 (2023).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone