Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Microfluidic Co-culture of Renal Healthy and Tumor Epithelium to Model Kidney Cancer Progression
W tym Artykule
Podsumowanie
Here, we present protocols that detail instructions for implementing 3D cell cultures using collagen and collagen-agarose matrixes in a microphysiological system. These protocols support renal proximal tubule and renal cell carcinoma spheroids co-culture, simulating in vivo conditions and enabling advanced investigation of kidney cancer cell interactions.
Streszczenie
Microphysiological systems (MPS) have enabled the introduction of more complex and relevant physiological elements into in vitro models, recreating intricate morphological features in three-dimensional environments with dynamic interactions lacking in conventional models. We implemented a renal cell carcinoma (RCC) co-culture model to recreate the cross-talk between healthy and malignant renal tissue.
This model is based on the referenced multi-organ platform and consists of co-culturing a reconstructed renal proximal tubule with RCC spheroids. Custom-designed 3D-printed chambers were used to culture human renal epithelial proximal tubule cells (RPTEC) and facilitate their self-assembly into a renal tubule contained in a collagen type I matrix. Caki-1 RCC cells were embedded in an agar collagen matrix, subsequently forming cancer spheroids. Both collagen and agar/collagen gels were optimized to maintain their integrity during cyclic perfusion and withstand shear stress during a minimum culture period of 7 days.
The gels also enable adequate nutrient supply and cell secretions. Moreover, the agar/collagen gels limit the overproliferation of RCC cells, ensuring relatively homogeneous spheroid size. The MPS chip microfluidic circuits comprise two independent culture chambers with the size of a standard 96-microplate well. The renal tubule and RCC gels populate separate chambers and share the same culture media, which is recirculated approximately twice per minute. Under these conditions, we observed upregulation of immune factor expression and secretion in the renal tubules (interleukin-8 and tumor necrosis factor-alpha). The renal tubules also shift their metabolic activity towards glycolysis under the influence of RCC. This novel approach demonstrates that a co-culture-based MPS can amplify the complexity of RCC in vitro and be employed to study the impact of cancer on non-tumor cells.
Wprowadzenie
Advancements in 3D cell culture systems have revolutionized tissue engineering and regenerative medicine by offering more physiologically relevant models compared to traditional 2D cultures1,2. In this study, we used collagen and collagen-agarose gel matrixes, given their ability to mimic the extracellular matrix (ECM) environment, promoting more accurate cellular behavior and function, while being compatible with the dynamic culture conditions employed.
Collagen, the most abundant protein in the ECM, plays a crucial role in maintaining the structural integrity and biological activity of tissues3. Type I collagen, commonly sourced from rat tail, was favored for its biocompatibility and ability to be tailored to meet different matrix rigidity conditions, as well as providing a proven substrate for epithelial cells4. In the 3D collagen matrix, renal cells are provided with a scaffold that supports their adhesion, proliferation, and differentiation. This environment enables cells to exhibit more natural morphologies and behaviors, including gene expression patterns and cellular interactions that reflect in vivo conditions5. Consequently, collagen gels have been extensively utilized in applications ranging from cancer research to tissue engineering due to their capacity to replicate the ECM's supportive and regulatory roles.
The immortalized renal proximal tubular epithelial cell line RPTEC/TERT1 was used to represent healthy kidney epithelium. This cell line overexpresses the human telomerase reverse transcriptase, enabling it to proliferate in culture, while maintaining a representative RPTEC phenotype. Rat tail collagen type I gel matrix was employed to recreate the renal microenvironment. This collagen gel matrix supports RPTEC/TERT16 cells in maintaining their native characteristics and physiological functions. In contrast, a hybrid collagen-agarose gel matrix is utilized for culturing renal cell carcinoma (Caki-1) cells. Agarose, a polysaccharide derived from seaweed, adds another dimension to 3D cell culture systems. It is thermally reversible, allowing easy handling and manipulation during the preparation process7. Agarose gels provide a neutral supportive matrix that maintains cell viability and promotes the formation of complex structures by proliferating cancer cells. When combined with collagen, the resulting hybrid gel leverages the biochemical cues from collagen and the structural support from agarose. This combination creates a suitable microenvironment for the renal cell carcinoma (RCC) Caki-1 cells8 for growth into spheroids and is used as the kidney cancer model.
The use of these different 3D matrixes for RPTEC/TERT1 and Caki-1 cells underscores the importance of tailoring the ECM environment to the specific needs of different cell types. The gels comprising either RPTEC, representing healthy renal epithelium, or Caki-1 cells, representing RCC, were combined in a microfluidic system that recirculates culture media between the cells, effectively exposing the healthy renal cell model to the secretions of the RCC, without direct contact. The TissUse HUMUMIC platform9,10(henceforth referred to as the multi-organ chip platform) employed in this study consists of a microphysiology chip with two independent fluidic circuits, with flow driven by an external perfusion unit.
Access restricted. Please log in or start a trial to view this content.
Protokół
NOTE: These protocols outline the comprehensive steps for preparing 3D collagen-agarose gels, injecting cells, perfusing the samples, and extracting them for further analysis. Adjust incubation times and conditions based on specific experimental requirements.
1. Preparation of collagen and agarose gel matrix
- 3D printing
NOTE: To create the appropriate shape for the gel matrix, we developed a unique chamber design that shapes the gel into a physiologically relevant organoid form (see Supplemental File 1).- Design the chambers using computer-assisted design software. The chamber comprises four parallel compartments: two cell compartments, one matrix compartment, and a connecting canal (Figure 1 and Figure 2).
- Build the chambers by 3D printing; a heating nozzle melts a bioplastic filament and deposits layer by layer. To follow this protocol, use Polypropylene (PP), a non-toxic bioplastic filament, and a Cartesian-type 3D printer, named after the XYZ coordinate system.
- Add a reverse-cone shape feature to the cell compartments to direct cells into the canal.
- Give a round shape to the matrix compartment, which is crucial for forming collagen gel.
- Connect the cell and matrix compartments with a canal (diameter 0.04 mm, Figure 3B), allowing cells to flow from the cell compartment into the matrix compartment and attach to the tube surface.
- Preparation of collagen matrix for the cultivation of RPTEC-TERT1 cells
NOTE: This procedure ensures that the collagen matrix is prepared correctly and loaded into the chamber, creating a suitable environment for the subsequent injection and cultivation of RPTEC/TERT1 cells and the growth of the 3D cell structure. Ensure all steps are performed under sterile conditions to avoid contamination.- Prepare the crosslinking solution by mixing 50 µL of Genipin (30 mM) with 50 µL of NaOH (1 M) using a vortex to ensure thorough mixing.
- Add 1,000 µL of collagen type I to the prepared Genipin and NaOH solution while keeping the mixture on ice to prevent premature gelation. Mix the solution again using a vortex to achieve a homogeneous mixture.
NOTE: Keep the mixed solution on ice continuously to prevent premature gelation. - Using a 1,000 µL pipette tip, collect the entire prepared solution and add 150-200 µL of the solution into the matrix compartment of the 3D printed chamber.
- Let the matrix polymerize by keeping the chamber in the CO2 incubator at 37 ᵒC for 60-90 min. Add 150 µL of culture medium on the top of the matrix and leave it overnight in the incubator.
- Preparation of collagen-agarose matrix and embedding of Caki-1 cells
NOTE: Both RPTEC/TERT1 and Caki-1 cells were cultured using the same culture medium, consisting of DMEM F-12 high glucose media supplemented with ITS (10 µg/mL of insulin, 5.5 µg/mL of transferrin, and 5 ng/mL of sodium selenite), 10 ng/mL of epidermal growth factor (EGF), 36 ng/mL of hydrocortisone, 100 U/mL of penicillin, 100 µg/mL of streptomycin (5% v/v), and 10% (v/v) Fetal Calf Serum (FCS).- Dissolve agarose powder in either sterile water or DPBS to achieve a final concentration of 2%. Place the agarose solution on a heating block set to 85 ᵒC to ensure complete dissolution.
NOTE: Handle the agarose solution carefully due to its temperature sensitivity.
- Dissolve agarose powder in either sterile water or DPBS to achieve a final concentration of 2%. Place the agarose solution on a heating block set to 85 ᵒC to ensure complete dissolution.
- Caki-1 cells embedding
- Detach Caki-1 cells from their culture flasks by adding 1x trypsin-EDTA and incubating at 37 °C until the cells detach. Neutralize the 1x trypsin by adding an equal volume of culture medium.
- Collect the cell suspension, count cells, and centrifuge at 2,500 × g for 5 min. Discard the supernatant and resuspend the cell pellets in fresh culture medium to a final concentration of 1 × 103 cells/mL (Caki-1).
NOTE: Ensure the cell suspension is thoroughly mixed and free of clumps before loading into the chamber. - Add 25 µL of cell suspension into a 1.5 mL microcentrifuge tube.
NOTE: Ensure the syringe or pipette is sterilized and appropriate for the handling of cells. - Add 75 µL of collagen type I into the tube with the cell suspension and vortex.
NOTE: Keep the mixture on ice continuously to prevent premature gelation. - Add 100 µL of melted agarose gel 2% and mix carefully on vortex until the gel becomes homogeneous.
NOTE: Ensure even distribution of the cells within the gel. Avoid introducing air bubbles during injection, as they can disrupt cell distribution and gel integrity! - After 1 h, add 150 µL of cell media at the top of the matrix and remove it from the chamber using a spatula. Place the 3D matrixes with cells in a 24-well plate with 1,000 µL of culture medium and incubate at 37 °C, 5% CO2.
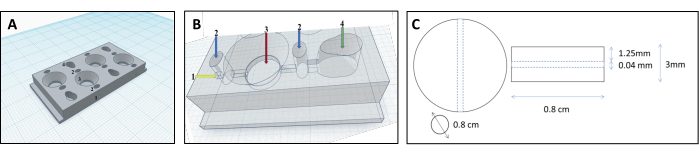
Figure 1: 3D printed chamber design for the reconstruction of renal tubules. (A,B) Representation of the 3D printed chamber designed to accommodate a crosslinked collagen matrix to reconstruct renal tubules. Each chamber consists of four individual compartments, that are used to produce an equal amount of gels. 1-insertion point for the filament, 2-pre-chambers to be filled with agar only to prevent leaks, 3-collagen chamber, 4-cell chamber. (C) Dimensions of the crosslinked collagen gel and embedded renal tubule. Please click here to view a larger version of this figure.
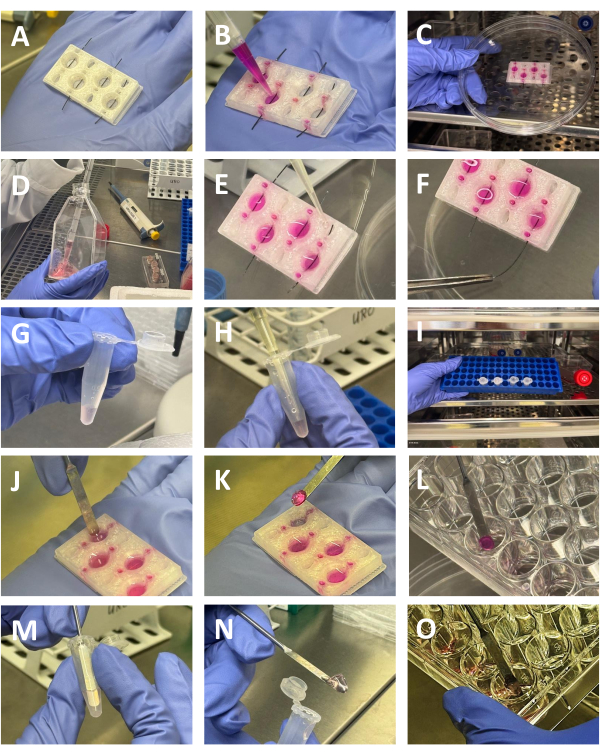
Figure 2: Preparation of collagen and agar-collagen gels. (A) 3D printed chamber with filaments inserted. (B) The crosslinked collagen solution is dispensed in the wells of the chamber (C) Chamber filled with collagen solution is placed in the incubator to facilitate the matrix polymerization (D) Collection of RPTEC-TERT1 cells before injection into the collagen matrix (E) Adding cell suspension the cell compartment of the chamber (F) Removing the filament with the aid of forceps, enabling the cells to populate the hollow tubular structure inside the matrix (G) Suspension of RCC cells mixed with collagen in a 1.5mL tube (H) Adding 2% agar solution the cells to generate the RCC gel. (I) The gels are placed in the incubator to facilitate matrix gelation (J,K,L) Removing the polymerized collagen gel with the renal tubules from the 3D printed chamber with the aid of a spatula, and placing them in a 24-well plate before further processing (M,N,O) Removing the polymerized collagen-agar gel with the RCC cells with the aid of a spatula, and placing them in a 24-well plate before further processing Please click here to view a larger version of this figure.
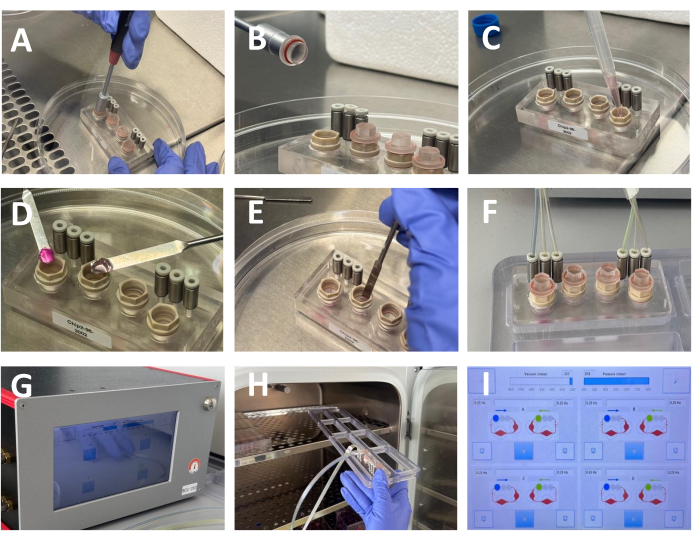
Figure 3: Assembly of the microfluidic culture system. (A,B) Opening the culture compartments of the Humimic chip2 using a dedicated tool (C) After removing the culture media used to wash the chip, add 400 μL of fresh culture media. (D,E) After removing the renal and RCC gels from the respective 24 well plates place each gel in the culture compartment of a single perfusion circuit of the Humimic chip using spatulas to handle the gels. (F,G) After positioning the gels in the chip, the culture chambers are sealed, and the chip is linked to the perfusion unit through three micro-pumps in each microfluidic circuit, connected by specialized tubing. (H) The chips are placed in the incubator for the duration of the culture period (I) Perfusion parameters set in the control unit. Please click here to view a larger version of this figure.
2. Injection of RPTEC/TERT1 cells
- RPTEC/TERT1 cells injection
- Detach RPTEC/TERT1 cells from their culture flasks by adding 1x trypsin-EDTA and incubating at 37 °C until the cells detach.
- Neutralize the 1x trypsin by adding an equal volume of culture medium with supplements. Collect the cell suspension and centrifuge at 2,500 × g for 5 min.
- Discard the supernatant and resuspend the cell pellets in fresh culture medium to a final concentration of 1 × 106 cells/mL (RPTEC/TERT1). Ensure the cell suspension is thoroughly mixed and free of clumps before loading into the chamber.
- Load 10 µL of the prepared cell suspension into a micro-pipette with a 10µL tip. Ensure the pipette is sterilized and appropriate for the handling of cells. Carefully inject the cell suspension into the solidified collagen (step 1.2.4) through the cell compartment within the chamber.
- Gently remove the filament using forceps, allowing the cells to populate the hollow tubular structure within the matrix
NOTE: Ensure even distribution of the cells within the gel. Avoid introducing air bubbles during injection, as they can disrupt cell distribution and gel integrity. - Place the chambers in a 37 °C, 5% CO2 incubator. After 60 min, add 150 µL of cell media on the top of the matrix to avoid drying. Incubate for 24 h to allow cells to acclimate and integrate into the gel matrix.
- On the next day, remove the matrix from the chamber using a spatula. Place the 3D matrixes with cells in a 24-well plate with 1,000 µL of culture medium and incubate at 37 °C with 5% CO2.
NOTE: The gels prepared with either the reconstructed renal tubule and the embedded RCC spheroids will be added to the microfluid system for co-culture
3. Perfusion of 3D cell samples
- 3D cell perfusion in the multi-organ chip system
- Connect the chip with the 3D cell to the perfusion system with tubing.
- Set the system: Frequency: 0.25 Hz, Vacuum: -220 mbar, Pressure: 800 mbar, and connect chip 2 to the system (Figure 3).
- Cultivate the dynamic 3D cell culture for 5 days in an incubator at 37 °C with 5% CO2.
4. 3D cell samples post perfusion collection
- Post perfusion collection and analysis
- Remove the gels from the perfusion system and rinse them with PBS.
- Fix the gels by placing the samples in 2% paraformaldehyde for 2-3 h at room temperature. Store fixed samples at 4 ºC.
- Stain the gels with dyes (e.g., Hoechst33342, phalloidin, ZO1, tubulin).
- After 5 days of perfusion using the multi-organ chip system, various assays, and methods were performed on the 3D cell cultures and collected media to assess cell viability, morphology, gene expression levels, and functional adjustments. The RPTEC/TERT1 cells in the collagen gel matrix and the Caki-1 cells in the collagen-agarose matrix maintain high viability; confirm this using viability assays such as live/dead staining.
Access restricted. Please log in or start a trial to view this content.
Wyniki
The HUMIMIC system provides a dynamic environment that enables continuous nutrient and oxygen supply while removing metabolic waste, thus maintaining cell viability and function over extended periods. These systems are particularly beneficial for creating complex, organ-on-a-chip models that replicate the microenvironment of specific tissues. It is specifically designed for organ-on-a-chip applications and allows for the precise control of fluid flow and shear stress, which are critical factors in simulating the physiolo...
Access restricted. Please log in or start a trial to view this content.
Dyskusje
The protocol described in this study represents the development of a complex kidney cancer model, leveraging the integration of two distinct cell types-renal proximal tubular epithelial cells (RPTEC/TERT1) and renal cell carcinoma (Caki-1) cells-within specific collagen and collagen-agarose gel matrixes in a microfluidic system. The preparation of collagen gels is critical to the success of this model. The precise concentration of collagen and agarose is necessary to maintain the structural integrity of the matrix throug...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have no conflicts of interest to declare.
Podziękowania
Maryna Somova was supported by the University of Greifswald Doctoral Scholarship - Landesgraduiertenförderungsgesetzes (Act on State Graduate Funding) of Mecklenburg-Vorpommern. The authors would like to thank Dr. Janosh Schoon and Dirk Stobe from the Center for Orthopedics, Trauma Surgery and Rehabilitation Medicine, University Medicine Greifswald for their insights in 3D cell culture and sample preparation.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 1.5-2.0 mL tubes | Eppendorf | 003012/10237-20205 | |
| 24-well plates | Sarstedt | 83.3922.500 | |
| 3D printer | Prusa | ||
| Agarose | Carl Roth | 3810.3 | |
| AutoDesk Tinker CAD software | computer-assisted design software | ||
| Caki-1 cells | ATCC | HTB-46 | |
| Caspase activity | Promega | G8090 | Caspase 3/7 assay |
| Cell viability | Promega | G7570 | Cell Titer Glo assay |
| Collagen Type I – rat tail, 3.0 mg/mL | Corning | 354236 | |
| DMEM/12F Medium | PAN Biotech | PO4-41650 | |
| DPBS solution | PAN Biotech | P04-53500 | |
| Epidermal Growth Factor | Merck | E4127 | |
| Fetal Calf Serum | PAN Biotech | P30-3033 | |
| Genipin (30 mM) | Merck | G4796 | |
| HUMIMIC chips 2 | TissUse | multi-organ chips | |
| HUMIMIC control unit | TissUse | multi-organ chip control unit | |
| Hydrocortisone | Merck | H6909 | |
| Incubator (37 °C, 5% CO2) | nd | ||
| Insulin,Sodium selenite,Transferrin (IST) | Merck | I1884 | |
| LDH release | Promega | J2380 | |
| Metal spatula | nd | ||
| NaOH (1 M) | Carl Roth | P031.2 | |
| Petri dishes | Sarstedt | 82.1135.500 | |
| Polypropylene (PP) filament | Verbatin | 55952 | |
| RNA-easy extraction kit | Qiagen | 74104 | |
| RPTEC/TERT cells | ATCC | CRL - 4031 | |
| TNF-alfa ELISA | R&D Systems | DY210-05 | |
| Triiodothyronine (T3 ) | Merck | 709611 | |
| Trypsin-EDTA | PAN Biotech | P10-021100 |
Odniesienia
- Ainslie, G. R., et al. Microphysiological lung models to evaluate the safety of new pharmaceutical modalities: A biopharmaceutical perspective. Lab Chip. 19 (19), 3152-3161 (2019).
- Van Ness, K. P., et al. Microphysiological systems to assess nonclinical toxicity. Curr Protoc Toxicol. 73, 14.18.1-14.18.28 (2017).
- Majo, S., et al. Impact of extracellular matrix components to renal cell carcinoma behavior. Front Oncol. 10, 625(2020).
- Caetano-Pinto, P., et al. Epidermal growth factor receptor mediates the basolateral uptake of phosphorothioate-modified antisense oligonucleotides in the kidney. Organs-on-a-Chip. 4, 100022(2022).
- Manduca, N., et al. 3D cancer models: One step closer to in vitro human studies. Front Immunol. 14, 1175503(2023).
- Simon, B. R., et al. The RPTEC/TERT1 cell line models key renal cell responses to the environmental toxicants, benzo[a]pyrene and cadmium. Toxicol Rep. 1, 231-242 (2014).
- Ogura, T., et al. Methods of high integrity RNA extraction from cell/agarose construct. BMC Res Notes. 8, 644(2015).
- Glube, N., et al. Caki-1 cells represent an in vitro model system for studying the human proximal tubule epithelium. Nephron Exp Nephrol. 107 (2), e47-e56 (2007).
- Kühnl, J., et al. Characterization of application scenario-dependent pharmacokinetics and pharmacodynamic properties of permethrin and hyperforin in a dynamic skin and liver multi-organ-chip model. Toxicology. 448, 152637(2021).
- Padmyastuti, A., et al. Microfluidic-based prostate cancer model for investigating the secretion of prostate-specific antigen and microRNAs in vitro. Sci Rep. 13 (1), 11623(2023).
- Zhang, X., et al. Cancer-on-a-Chip: Models for studying metastasis. Cancers (Basel). 14 (3), 648(2022).
- Sobczuk, P., et al. Choosing the right animal model for renal cancer research. Transl Oncol. 13 (3), 100745(2020).
- Ewart, L., et al. Performance assessment and economic analysis of a human Liver-Chip for predictive toxicology. Commun Med. 2 (1), 154(2022).
- Caetano-Pinto, P., et al. Kidney cancer and chronic kidney disease: Too close for comfort. Biomedicines. 9 (12), 1761(2021).
- Del Piccolo, N., et al. Tumor-on-chip modeling of organ-specific cancer and metastasis. Adv Drug Deliv Rev. 175, 113798(2021).
- Soo, J. Y. C., et al. Advances in predictive in vitro models of drug-induced nephrotoxicity. Nat Rev Nephrol. 14 (6), 378-393 (2018).
- Chapron, A., et al. An improved vascularized, dual-channel microphysiological system facilitates modeling of proximal tubular solute secretion. ACS Pharmacol Transl Sci. 3 (3), 496-508 (2020).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone