A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Complaint Matrices for Quantifying Cellular Contraction
In This Article
Summary
In this video, we demonstrate the experimental techniques used to fabricate compliant, extracellular matrix (ECM) coated substrates suitable for cell culture, and which are amenable to traction force microscopy and observing effects of ECM stiffness on cell behavior.
Abstract
The regulation of cellular adhesion to the extracellular matrix (ECM) is essential for cell migration and ECM remodeling. Focal adhesions are macromolecular assemblies that couple the contractile F-actin cytoskeleton to the ECM. This connection allows for the transmission of intracellular mechanical forces across the cell membrane to the underlying substrate. Recent work has shown the mechanical properties of the ECM regulate focal adhesion and F-actin morphology as well as numerous physiological processes, including cell differentiation, division, proliferation and migration. Thus, the use of cell culture substrates has become an increasingly prevalent method to precisely control and modulate ECM mechanical properties.
To quantify traction forces at focal adhesions in an adherent cell, compliant substrates are used in conjunction with high-resolution imaging and computational techniques in a method termed traction force microscopy (TFM). This technique relies on measurements of the local magnitude and direction of substrate deformations induced by cellular contraction. In combination with high-resolution fluorescence microscopy of fluorescently tagged proteins, it is possible to correlate cytoskeletal organization and remodeling with traction forces.
Here we present a detailed experimental protocol for the preparation of two-dimensional, compliant matrices for the purpose of creating a cell culture substrate with a well-characterized, tunable mechanical stiffness, which is suitable for measuring cellular contraction. These protocols include the fabrication of polyacrylamide hydrogels, coating of ECM proteins on such gels, plating cells on gels, and high-resolution confocal microscopy using a perfusion chamber. Additionally, we provide a representative sample of data demonstrating location and magnitude of cellular forces using cited TFM protocols.
Protocol
1. Activating the coverslip surface
- Coverslips (#1.5, 22x40 mm) are cleaned using a series of soap and ethanol washes in a previously described protocol (Waterman-Storer, 1998) to clean and remove dust.
- Place coverslips in a stainless steel holder rack, such that coverslips are spaced apart and not touching.
- In chemical fume hood (nitrile gloves and goggles recommended), dilute full strength 3-aminopropyltrimethoxysilane in isopropanol for a final concentration of 2% (2ml silane /100 ml isopropanol) to fill a square glass dish (~350ml volume). Due to reactivity with plastic, use a glass Pasteur pipette to apply 3-aminopropyltrimethoxysilane to the isopropanol.
- Fully immerse coverslips from 1.2 into this solution for 10 min while gently stirring on a stir plate in the fume hood.
- Wash coverslips by immersing in ddH2O (4 exchanges of water). Allow 10 min soaking time for the final exchange, with stirring. Amino-silane containing solutions should be disposed as hazardous waste.
- Dry coverslips in incubator at warm temperature (~37°C) for 10 minutes in a dust free environment.
- Cool to room temperature.
- In the fume hood, immerse coverslips in 1% glutaraldehyde solution in ddH2O in a glass square dish on stir plate for 30 minutes.
- Wash by 3 exchanges of ddH2O for 10 minutes per exchange, with stirring. Dispose of glutaraldehyde as hazardous waste.
- Dry at room temperature, covering with aluminum foil to avoid dust from sticking to the coverslips.
- Store in a dry place, away from dust, for up to 2 months.
2. Preparation of polyacrylamide (PAA) gel
- Prepare stock solutions of acrylamide/bis-acrylamide mix from 40% acrylamide and 2% bis-acrylamide, following Table 1. We maintain several stock solutions that are optimized for PAA gels of different stiffness; examples are listed in Table 1 and 2. Stock solutions can be kept for several years, as long as they are maintained in a darkened bottle at 4 C.
- Working solutions containing the final desired concentrations of acrylamide/bis-acrylamide are obtained from stock solutions. For example, we prepare a working solution of 7.5% acrylamide/0.10% bis-acrylamide in ddH2O for making 2.8kPa PAA gels.
- Degas acrylamide solution in a vacuum chamber for 20 min, to reduce oxygen within the solution which prevents PAA polymerization.
- Prepare 10% ammonium persulfate (APS) solution (0.5g/5mL). Use fresh working stock within 3 days. Alternatively, stocks can be frozen to be used at later dates.
- While acrylamide is degassing, wipe a 1x3" microscope glass slide with Rain-X wipes vigorously to make glass slide surface hydrophobic. To remove excess Rain-X, wipe glass slide with damp Kimwipe. Set glass slide aside, covered.
- Remove acrylamide solution from vacuum chamber and add fluorescent beads (1% by volume, 5 μl for the working solution listed in Table 1). Add 0.75μl TEMED and 2.5 μl 10% APS, which will initiate gel polymerization. Mix well by pipetting for ~5 sec, to minimize the introduction of bubbles,
- Apply 10-12 μl of the acrylamide solution to hydrophobic microscope slide (prepared in step 2.4) and place activated 22x40mm coverslip on top of the droplet. Gel solution should coat entire coverslip. Smooth out any bubbles that may appear within the solution. Allow the gel solution to polymerize at room temperature for ~10 min.
- The completion of polymerization can be assessed by inverting remaining working solution in microcentrifuge tube. Also, the polymerized gel may pull away from coverslip edges. Immediately after macroscopic polymerization is observed, separate the coverslip from the glass slide. Using the fine tip of a pair of tweezers or a razor blade edge, carefully remove coverslip, with gel attached, from microscope slide surface and immerse gel in ddH2O, to maintain hydration.
3. Coupling extracellular matrix (ECM) proteins to the PAA gel
Three distinct methods can be used to attach ECM protein either to the top surface of the PAA gel (3.1 and 3.2) or incorporating ECM protein within the gel volume (3.3). Here, we discuss the coupling of fibronectin to PAA gels to result in a surface ligand density that is equivalent to the amount adsorbed on glass after incubation with 10 μg/mL fibronectin solution for 1 hour. Considerations for choosing a method are detailed in the discussion.
- Cross-linking ECM protein to PAA gel surface by Sulfo-SANPAH
Reactive amines on proteins are covalently attached to the PAA gel surface by the heterobifunctional cross-linker Sulfo-SANPAH
- Prepare 40 μl working aliquots of Sulfo-SANPAH by dissolving Sulfo-SANPAH powder in anhydrous dimethyl sulfoxide (DMSO) (20 μl per mg of Sulfo-SANPAH). Flash freeze stocks in liquid nitrogen and store at -80°C for later use.
- Remove ddH2O from gel surface using a coverslip spinner (< 2sec). Avoid drying the gel.
- Dilute Sulfo-SANPAH-DMSO aliquots in ddH2O (2mg/ml, pH 7) immediately before use and coat gel surface (~200 μl). Note that the reactivity half-life of Sulfo-SANPAH is short (~5 min) at room temperature in water; therefore, these steps should be done at a rapid pace.
- Expose gel surface to UV light in a UV cross-linker oven (8W, 254 nm wavelength at a distance of 2-3 inches for 1.5 min). Sulfo-SANPAH will change in color from orange to brown.
- Dip UV-treated coverslips in a beaker with fresh ddH2O and remove excess water from gel surface using a coverslip spinner (<2sec).
- Pipette ~50 μL of cold 1mg/ml Fibronectin (FN) (in PBS, pH 7.4) on Parafilm in Petri dish container. Invert coverslip on top of FN drop, gel side exposed to FN.
- React at room temperature for 1-2 hours or at 4°C overnight.
- Place coverslips in 6 cm tissue culture dishes containing PBS (pH 7.4), enough to cover coverslip, under sterile conditions in tissue culture hood.
- Wash extensively with several washes (3-5) of PBS (pH 7.4), under sterile conditions.
- Sterilize the coverslips by use of germicidal lamp in tissue culture hood for 30 min.
- Incubate coverslips in cell media for 30-45 min prior to plating cells.
- Cross-linking ECM to the PAA gel surface by hydrazine hydrate
Carbohydrate groups on proteins are oxidized and coupled to the gel using hydrazine hydrate.
- Prepare polyacrylamide gels as described in section 2.
- Place PAA gel coverslips in plastic Petri dish in a fume hood and using gloves pipette approximately 1 ml of undiluted hydrazine hydrate onto the surface of each PAA gel and incubate for at least two hours, but no longer than 24 hours
- Add ddH2O to the Petri dish; Remove hydrazine hydrate solution and dispose as hazardous waste.
- Add 5% acetic acid to the Petri dish to immerse coverslip. Cover and incubate for one hour.
- Remove the acetic acid and wash with ddH2O. Incubate in ddH2O for one hour. The coverslips are now activated and ready to cross-link oxidized Fibronectin (FN).
- Dilute 10 μl of 1 mg/ml FN solution in 940 μl of 50 mM sodium acetate buffer (pH 4.5) in a dark micro centrifuge tube, making a final concentration of 10 μg/ml.
- Make stock of 20X sodium meta-periodate by adding 80 mg of sodium meta-periodate to 1 ml of 50 mM sodium acetate buffer (pH 4.5).
- Add 50 μl of 20X sodium meta-periodate stock to the FN solution prepared in 3.2.6, such that final working concentration are 10 μg/ml FN and 4 μg/ml sodium meta-periodate. Incubate in the dark tube at room temperature for 30 minutes.
- Remove excess ddH2O from activated gel surface prepared in 3.2.5 using a coverslip spinner (< 2 sec). Avoid drying the gel.
- Pipet ~500 μl of FN solution onto activated gel surface and incubate for 1 hr at room temperature.
- Place coverslips in dishes containing PBS (pH 7.4), enough to cover coverslip.
- Wash extensively with several washes (3-5) of PBS (pH 7.4).
- Sterilize the coverslips by use of germicidal lamp in tissue culture hood for 30 min.
- Incubate coverslips in cell media for 30-45 min prior to plating cells.
- Bulk conjugation of ECM protein in PAA gel by Acryloyl-X, succinimidyl ester
This protocol is done prior to 2.5. Reactive amines on proteins are coupled to an acrylamide monomer with NHS ester chemistry and then co-polymerized into the bulk of the PAA gel.
- Conjugate ECM protein of choice to Acryloyl-X per the manufacturer's instructions. Stock solutions of conjugated protein should be stored at 4°C.
- Calculate the volume of PAA working solution required for gel fabrication (e.g. 10 uL per coverslip).
- Subtract 50 μL (e.g. 10% volume) of water from amount listed in the working solution recipe in Table 1 and initiate polymerization.
- Remove volume calculated in 3.3.2 and add ECM/Acryloyl-X solution to 10% volume. (e.g. 1 μL of ECM/Acryloyl-X to 9 μL of PAA solution). This step should be performed rapidly, as gel is polymerizing.
- Complete steps 2.6 and 2.7 as described previously.
- Wash extensively with several washes (3-5) of PBS (pH 7.4), under sterile conditions.
- Sterilize the coverslips by use of germicidal lamp in tissue culture hood for 30 min.
- Incubate coverslips in cell media for 30-45 min prior to plating cells.
4. Loading coverslip into confocal imaging chamber
These steps are conducted after cells have been allowed to spread on ECM-coated gel substrate (~6-12 hrs). To assemble the confocal imaging chamber (RC-30WA), it is useful to consult the Warner Instruments website for guidance.
- Warm cell media and 0.5% or 0.25% trypsin and load into a 5ml or 10ml syringe.
- Load a 22x30mm coverslip onto the Top Coverslip holder of a Warner Instruments confocal imaging chamber (RC-30WA) using vacuum grease to keep the coverslip in place.
- Place a chamber forming rubber gasket on top of the coverslip, allowing access to both inlet and outlet polyethylene tubing. This will allow a spacing of 150-1000 μm between the top coverslip and the gel-coated 22x40mm coverslip, depending on the size of gasket used.
- Load syringes onto inlet tubing, via connector kit, and check that media flows through tubing and onto Top Coverslip and no bubbles are apparent in the flow lines.
- Apply vacuum grease onto base of chamber and load gel-coated 22x40mm coverslip, cell-side up. Apply warm media to cells.
- Place Top Coverslip holder onto chamber base, with Chamber Gasket separating it from the gel-coated coverslip. Make sure that the Locating Pins within the chamber base sit within the Locating Holes in the Top Coverslip.
- Apply the Pressure Plate to the chamber base and use the Pressure Plate Wrench to screw in and secure the Pressure Plate.
- Check the flow of media through the tubing and chamber to monitor any potential leaks within the chamber and to eliminate any media-free zones on the cell surface. Note: using a small gauge needle to draw a slight vacuum while infusing with media can help eliminate media-free zones.
- Apply the confocal imaging chamber to the Stage Adapter situated within a microscope holder for imaging.
- Image fluorescently-labeled protein and fluorescent beads embedded within the gel substrate on a confocal fluorescence microscope.
- To obtain an image of unstrained bead positions within the gel, perfuse trypsin to detach cellular adhesions from the gel, and take an image of the fluorescent beads in the same imaging field where the cell adhered. Comparison of strained and unstrained bead positions allows for the quantification of gel substrate displacement under contraction.
Representative Results:
The above protocol describes the experimental procedure for preparing compliant PAA gels for studying cell contractility and is illustrated in Figure 1. The gel surface obtained with this protocol is relatively flat and smooth, with fluorescent beads embedded evenly throughout (Figure 2A).
If measuring gel contraction at the location of focal adhesions, imaging of the cell (Figure 3A) and gel surface (Figure 3B) should be done at the confocal optical plane of focal adhesions. The contraction of a gel can be visualized by displacement of embedded fluorescent beads (Figure 3B) at the gel surface when cells are adherent (strained) versus detached (unstrained). The use of computational algorithms can yield traction stresses associated with bead displacement and corresponding elastic modulus of the gel (Figure 3C and 3D) (Sabass et al., 2008). If imaging takes place deeper within the gel, then bead displacements will be smaller and not representative of traction forces exerted at focal adhesions.
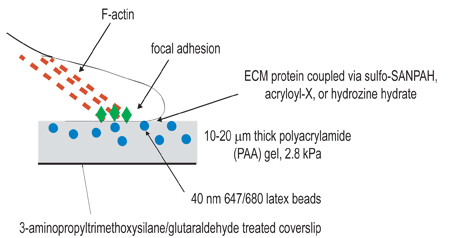
Figure 1. Schematic illustration of experimental setup. The overall goal of this procedure is to create compliant matrices for the purpose of studying cellular contraction. The first step of the experimental procedure is to activate coverslips by amino-silane/glutaraldehyde treatment for the purpose of anchoring polymerized gels. The second step is to polymerize a polyacrylamide gel, containing fluorescent beads, onto the activated coverslip. The third step involves the chemical cross-linking of extracellular ligand to the surface of the polyacrylamide gel, using one of the three coupling techniques listed in step 3. Cells are then plated onto the gel and allowed to adhere and spread. Under active cellular contraction, beads embedded in the gel displace.

Figure 2. Optical confocal slice of top surface of PAA gel, as visualized by (A.) fluorescent 40nm beads embedded within gel and (B.) fibronectin immunofluorescence.
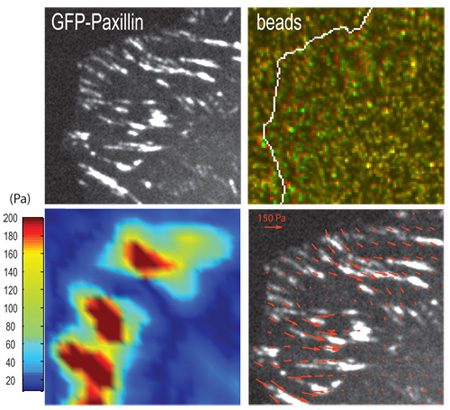
Figure 3. Representative result for a traction force experiment. (A.) Focal adhesions in a human osteosarcoma U2OS cell are marked by GFP-paxillin and (B.) positions of fluorescent beads embedded in the PAA gel underlying focal adhesions in the strained (green) and unstrained (red) states. Arrows indicate examples of bead displacement. (C.) Traction stress vectors and (D.) corresponding heat-scale map of traction stresses derived from the contraction of the gel, using computational algorithms (Sabass et al., 2008). Scale bar = 5 μm.
Table 1:
Example Stock and Working PAA Solutions (Data in table 1 was first obtained from Yeung et. al. and independently confirmed in our laboratory.)
| Stock PAA Solution | ||||
| Shear Modulus of PAA Gel (Pa) | 230 | 2833 | 8640 | 16344 |
| 40% Acrylamide (mL) | 1.25 | 3.12 | 2.34 | 2.50 |
| 2% Bis-Acrylamide (mL) | 0.50 | 0.83 | 1.88 | 0.60 |
| Water (mL) | 3.25 | 1.04 | 0.78 | 1. 90 |
| Total Volume (mL): | 5 | 5 | 5 | 5 |
| Working PAA Solution | ||||
| Stock Solution Used (Pa) | 230 | 2833 | 8640 | 16344 |
| Stock Solution Volume (μL) | 150 | 150 | 200 | 300 |
| Water (μL) | 341.75 | 341.75 | 291.75 | 191.75 |
| Beads (μL) | 5 | 5 | 5 | 5 |
| TEMED (μL) | 0.75 | 0.75 | 0.75 | 0.75 |
| 10% APS (μL) | 2.5 | 2.5 | 2.5 | 2.5 |
| Total Volume (μL): | 500 | 500 | 500 | 500 |
| Final Acrylamide % | 3 | 7.5 | 7.5 | 12 |
| Final Bis-Acrylamide % | 0.06 | 0.1 | 0.3 | 0.15 |
Table 2:
Shear Modulus of PAA substrates of various final acrylamide and bis-acrylamide percentages
| 12% Acrylamide | 7.5% Acrylamide | |||
| % Bis-Acrylamide | Shear Modulus (Pa) | % Bis-Acrylamide | Shear Modulus (Pa) | |
| 0.145 | 16344 | 0.01 | 689 | |
| 0.28 | 30067 | 0.03 | 1535 | |
| 0.45 | 34263 | 0.05 | 2286 | |
| 0.55 | 42375 | 0.075 | 2833 | |
| 0.575 | 50873 | 0.1 | 4069 | |
| 0.6 | 55293 | 0.2 | 5356 | |
| 0.3 | 8640 | |||
| 5% Acrylamide | 3% Acrylamide | |||
| % Bis-Acrylamide | Shear Modulus (Pa) | % Bis-Acrylamide | Shear Modulus (Pa) | |
| 0.05 | 430 | 0.02 | 1.3 | |
| 0.075 | 600 | 0.04 | 54 | |
| 0.1 | 1431 | |||
Discussion
The procedure described here for the setup of a traction force microscopy (TFM) experiment, along with the implementation of computational tracking routines (Sabass et al., 2008), allows for the quantification of cellular forces with micron-scale spatial resolution. To optimize the experimental protocol, it is critical to form a pure and uniform gel substrate with uniform coating of ECM ligand. We discuss potential pitfalls below:
Non-uniform Gel Surface or Tears:
Disclosures
No conflicts of interest declared.
Acknowledgements
We thank the lab of Ulrich Schwarz for computational tracking software used in quantification of cellular traction forces (Sabass et al., 2008). This work was supported by a Burroughs Wellcome Career Award and NIH Director's Pioneer Award (DP10D00354) to M.L. Gardel and Medical Scientist National Research Service Award (5 T32 GM07281) to S.P. Winter.
Materials
| Name | Company | Catalog Number | Comments |
| 3-aminopropyltrimethyoxysilane | Aldrich | 28, 177-8 | |
| 40% Acrylamide | Bio-Rad | 161-0140 | |
| 2% Bis-acrylamide | Fisher Scientific | BP1404 | |
| TEMED | Fisher Scientific | BP 150-20 | |
| Ammonium persulfate | Fisher Scientific | BP179 | |
| 40nm fluorescent micro-spheres | Invitrogen | F8789 | |
| Sulfo-SANPAH | Pierce, Thermo Scientific | 22589 | |
| Confocal imaging chamber (RC-30) | Warner Instruments | 64-0320 | |
| Coverslip spinner | Home made | NA | |
| Ultraviolet lamp CL1000 | UVP Inc. | 95-0228-01 | |
| Stainless steel rack | Electron Microscopy Sciences | 72239-04 | |
| acryloyl-X, SE (6-((acryloyl)amino)hexanoic acid) | Invitrogen | A-20770 | |
| Hydrazine hydrate | Sigma-Aldrich | 225819 | |
| Sodium meta-periodate | Thermo Fisher Scientific, Inc. | 20504 | |
| Isopropanol | Fisher Scientific | A416-4 | |
| Fibronectin | Sigma-Aldrich | F2006 | |
| Collagen | BD Biosciences | 354236 | |
| Coverslips (#1.5) | Corning | 2940‐224 | |
| Glutaraldehyde | Electron Microscopy Sciences | 16120 | |
| Rain-X | SOPUS Products | www.rainx.com | |
| Acetic Acid | Acros Organics | 64-19-7 |
References
- Damljanovic, V., Lajerholm, B. C., Jacobson, K. Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamid substrates for cell mechanotransduction assays. Biotechniques. 39 (6), 847-851 (2005).
- Engler, A., Bacakova, L. N. e. w. m. a. n., Hategan, C., Griffin, A., M, D. D. i. s. c. h. e. r. Substrate compliance versus ligand in cell on gel responses. Biophys J. 86 ((1 Pt 1)), 617-628 (2004).
- Gardel, M. L., Sabass, B., Ji, L., Danuser, G., Schwarz, U. S., Waterman, C. M. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 183, 999-1005 (2008).
- Rajagopalan, P., Marganski, W. A., Brown, X. Q., Wong, J. Y. Direct comparison of the spread area, contractility, and migration of balb/c 3T3 fibroblasts adhered to fibronectin- and RGD-modified substrata. Biophys J. 87 (4), 2818-2827 (2004).
- Reinhart-King, C. A., Dembo, M., Hammer, D. A. The dynamics and mechanics of endothelial cell spreading. Biophys J. 89, 676-689 (2005).
- Stricker, J., Sabass, B., Schwarz, U. S., Gardel, M. L. Optimization of traction force microscopy for micron-sized focal adhesions. J. Phys: Condensed Matter. 22, 194104-194114 (2010).
- Sabass, B., Gardel, M. L., Waterman, C. M., Schwarz, U. S. High resolution traction force microscopy based on experimental and computational advances. Biophys J. 94, 207-220 (2008).
- Yeung, T. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 60 (1), 24-34 (2005).
- Waterman-Storer, C. M. Microtubule/organelle motility assays. Curr Protoc Cell Biol. , 13.1.1-13.1.21 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved