A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Retrograde Perfusion and Filling of Mouse Coronary Vasculature as Preparation for Micro Computed Tomography Imaging
In This Article
Summary
Visualization of the coronary vessels is critical to advancing our understanding of cardiovascular diseases. Here we describe a method for perfusing murine coronary vasculature with a radiopaque silicone rubber (Microfil), in preparation for micro-Computed Tomography (μCT) imaging.
Abstract
Visualization of the vasculature is becoming increasingly important for understanding many different disease states. While several techniques exist for imaging vasculature, few are able to visualize the vascular network as a whole while extending to a resolution that includes the smaller vessels1,2. Additionally, many vascular casting techniques destroy the surrounding tissue, preventing further analysis of the sample3-5. One method which circumvents these issues is micro-Computed Tomography (μCT). μCT imaging can scan at resolutions <10 microns, is capable of producing 3D reconstructions of the vascular network, and leaves the tissue intact for subsequent analysis (e.g., histology and morphometry)6-11. However, imaging vessels by ex vivo μCT methods requires that the vessels be filled with a radiopaque compound. As such, the accurate representation of vasculature produced by μCT imaging is contingent upon reliable and complete filling of the vessels. In this protocol, we describe a technique for filling mouse coronary vessels in preparation for μCT imaging.
Two predominate techniques exist for filling the coronary vasculature: in vivo via cannulation and retrograde perfusion of the aorta (or a branch off the aortic arch) 12-14, or ex vivo via a Langendorff perfusion system 15-17. Here we describe an in vivo aortic cannulation method which has been specifically designed to ensure filling of all vessels. We use a low viscosity radiopaque compound called Microfil which can perfuse through the smallest vessels to fill all the capillaries, as well as both the arterial and venous sides of the vascular network. Vessels are perfused with buffer using a pressurized perfusion system, and then filled with Microfil. To ensure that Microfil fills the small higher resistance vessels, we ligate the large branches emanating from the aorta, which diverts the Microfil into the coronaries. Once filling is complete, to prevent the elastic nature of cardiac tissue from squeezing Microfil out of some vessels, we ligate accessible major vascular exit points immediately after filling. Therefore, our technique is optimized for complete filling and maximum retention of the filling agent, enabling visualization of the complete coronary vascular network – arteries, capillaries, and veins alike.
Protocol
1. Preparations before starting
- Fill each side of the pressure perfusion apparatus with Vasodilator buffer (4mg/L Papaverine + 1g/L Adenosine in PBS) or 4% Paraformaldehyde (PFA) in PBS, respectively.
- Prepare a 1/2cc Insulin Syringe (with a permanently attached 29G ½" needle) by filling it with 0.1 ml of 1:100 Heparin (5000U/ml stock) and bending the needle to ~120 degree angle with the bevel up. Do the same with a 1 ml syringe (with a 26G ½" needle) filled with 0.3 ml saturated KCl solution.
2. Exposing the heart and cannulating the aorta
- Anesthetize the mouse using your anesthetic of choice. (We use an overdose of a Ketamine/Xylazine mixture: IP injection of 130 mg/kg Ketamine and 8.8 mg/kg Xylazine in saline.)
- Pin the anesthetized mouse onto the dissecting tray, ventral side up. Open the abdominal cavity with a midline incision and retract the skin to expose the organs. Move the intestines to one side to expose a region of the Posterior Vena Cava (PVC).
- Inject Heparin solution into the PVC. As you extract the needle, cover the needle hole with a cotton-tipped applicator to prevent leaking and hold it for a few seconds until the PVC wall clots and seals. Wait 2-3 minutes for Heparin to disperse throughout the mouse circulation.
- Dissect the diaphragm and rib cage so you can observe the beating heart. Slowly inject KCl solution in the PVC until the heart arrests.
- Remove all organs below the diaphragm and excise the posterior portion of the mouse, leaving the region anterior of the diaphragm intact. Remove the diaphragm, being careful to cut the PVC near the diaphragm so the portion proximal to the heart is easy to locate in subsequent steps.
- Locate the cut end of aorta. Place one long length of 6-0 braided silk suture underneath the aorta a few millimeters anterior from the cut end such that the suture is doubled back on itself. Cut this longer suture in half so there are 2 pieces of suture under the aorta. Insert the angiocatheter into the cut end of the aorta (Fig 1A, B) and tie each suture with a double-knot to hold the angiocatheter in place and prevent any back-pressure within the aorta from leaking out.
3. Perfusion and Microfil injection
- Connect the angiocatheter to the pressure perfusion apparatus (Fig 2) and begin perfusing the vessels with vasodilator buffer (Fig 1C) by pumping the perfusion apparatus to a driving pressure of 100-110 mmHg. Double check that buffer is perfusing through the coronaries by ensuring liquid is exiting from the PVC. Continue to perfuse for at least 3 minutes or until the fluid exiting the PVC is clear. (Continue with the next steps while perfusing.)
- Dissect the ribs and pin back (or remove) the ribcage to expose the heart. Once exposed, be careful not to let the heart dry out by squeezing drops of buffer onto the heart from a buffer-soaked piece of gauze. Clear away the thymus to expose the aortic arch. Ligate the three major aortic branches using 6-0 braided silk suture to ensure fluid is diverted through the coronaries rather than through these larger, low resistance vessels (Fig 1D).
- Perfuse the heart with fixative for 15 minutes, then rinse with Vasodilation buffer for at least 2 minutes. Meanwhile, ligate both Anterior Vena Cavae to prevent Microfil from leaking out of the heart after injection (Fig 1E). Place sutures around the PVC and the aorta but do not tighten them until after filling.
- Prepare the Microfil (as specified in table of reagents) and load it into a 1 ml syringe. Fill the dissection tray with enough water to cover the catheter (so as to prevent the introduction of air bubbles when switching from perfusion tubing to the Microfil syringe). Disconnect the perfusion apparatus from the catheter and connect the prepared Microfil syringe.
- Inject the Microfil into the aorta until good filling of the coronary arteries is evident (Figs 1F, 3A): the arteries will fill first, and then the Microfil will "spill" into the capillaries as the tissue flushes with the color of the Microfil. Once on the venous side, the hydrophobic nature of the Microfil causes it to initially appear as independent spheres as it emerges from the smaller vessels. Continue to inject the Microfil until a continuous column fills the veins. Complete filling will be evident when the Microfil is continuous within the vessels, and it is exiting through the PVC.
- After filling is complete, quickly tighten the sutures that were previously placed around the PVC and aorta to prevent the elastic nature of the cardiac tissue from squeezing the Microfil out of the vessels.
- Cover the heart with wet gauze (soaked with water from the dissecting tray) to prevent its drying out, and let it sit for approximately 1 hour at room temperature until the Microfil has polymerized. Avoid any external pressures on the heart during the polymerization process, such as lifting or turning the heart in an attempt to get an early view of the filled vessels in the back of the heart. This may squeeze Microfil from some vessels into a more elastic area of the heart, causing breaks in the Microfil.
- Remove the heart and post-fix it in 4% PFA overnight at 4°C. Then store in 70% ethanol at 4 °C. The heart vasculature is now ready for μCT imaging.
4. Representative Results
Vessels which are effectively perfused by Microfil will have continuous, unbroken Microfil throughout the vessels (Fig. 3A). The extent of filling of the coronary vessels can be judged by eye; veins are epicardially located18, and can be easily observed (Fig 3A, arrowhead); arteries, which are more intramyocardial18, are also visible through the surface of the heart (Fig 3A, arrow). Capillary filling is also evident, as cardiac tissue has a very high density of capillaries, and therefore, when the capillaries fill, the cardiac tissue will flush with the color of the Microfil (Fig. 3A, star). Thus, any vascular networks that failed to fill will be noticeable due to the lack of Microfil (Fig. 3B, C).
Discontinuities in the Microfil (asterisks in Fig 3B) often appear because the hydrophobic nature of the Microfil will cause it to contract into itself and cause "breaks" within filled vessels. These "breaks" can be reduced if pressure within the vessels is maintained through proper tie-offs of the vascular exit points from the heart. Other discontinuities can be caused by air bubbles within the microfil. To prevent the introduction of air, make sure the angiocatheter is fully submerged in water when switching from the perfusion apparatus to the Microfil syringe. If an air bubble is introduced, it can often be removed simply by continuing the Microfil perfusion until the bubble has been pushed through and out of the coronary vessels.
Vascular networks may not fill completely if a portion of the vascular bed is blocked (Fig. 3B, arrow). While Heparin inhibits the formation of blood clots, occasional blockages may still occur due to incomplete Heparin perfusion prior to beginning the procedure, or due to other unknown factors. If a blockage occurs, there is, to our knowledge, no method for dislodging the blockage to complete the vascular fill. Incomplete filling can also result if too little pressure is used during filling, as the Microfil will not be forced into all the vascular beds and capillary networks (Fig. 3C). Conversely, too much pressure can cause the capillaries to burst and extravasate Microfil into the surrounding tissue (Fig. 3D).
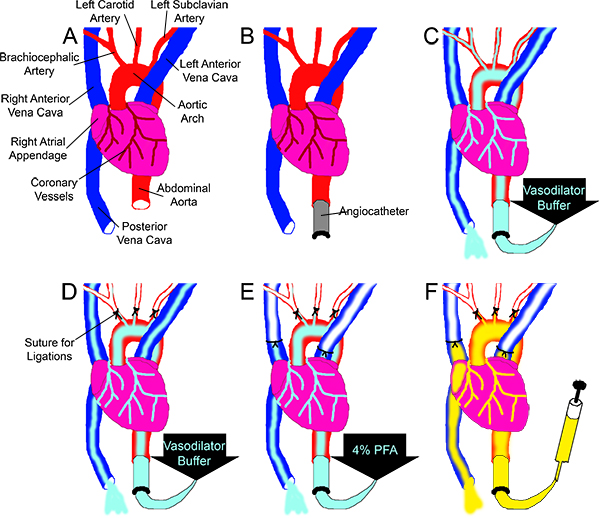
Figure 1. Overview of the Microfil perfusion scheme. (A) The aorta and the PVC are cut at approximately the level of the diaphragm. (B) The ascending aorta is cannulated with an angiocatheter. (C) Vasodilation buffer is perfused through the vessels, driven by the pressure perfusion apparatus (not pictured), while (D) the three main branches off the aortic arch are ligated. (E) 4% PFA is perfused through the coronaries while both Anterior Vena Cavas are ligated. (F) Using a syringe, Microfil is perfused through the coronaries until it is observed exiting from the PVC.
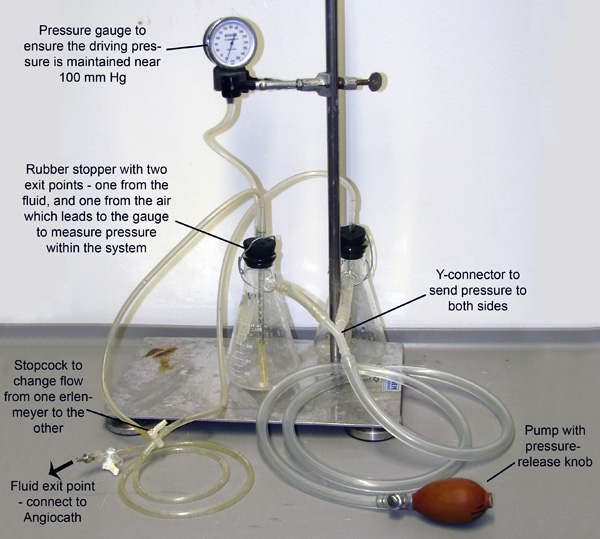
Figure 2. Perfusion Apparatus. Two Erlenmeyer flasks, each filled with either Vasodilation buffer or 4% PFA, are joined and pressurized through tubes connected to their sidearms. The system is pressurized through manual pumping of the bulb, and a pressure gauge is connected to one of the flasks to allow monitoring and maintenance of pressure. Small tubes extend through rubber stoppers and down into the fluid in each flask. Pressure entering from the sidearms pumps the fluid from each flask out these smaller tubes. The tubes then merge at a stopcock which only allows fluid to flow from one flask at a time.
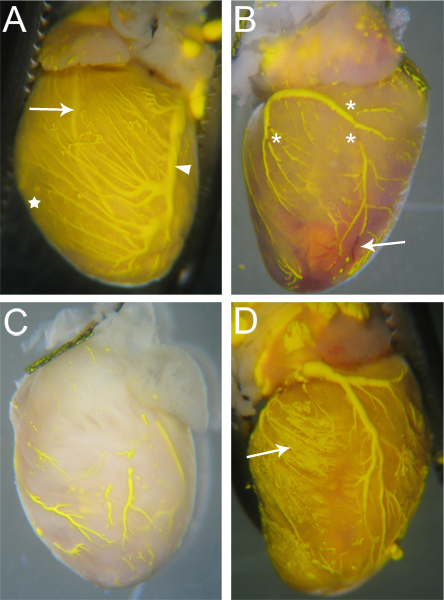
Figure 3. Sample Microfilled hearts. (A) Vessels that are filled well will have few (if any) breaks in the Microfil, and the heart tissue will be tinged the color of the Microfil due to the filled capillaries (star, and compare with C). Both arteries (arrow – Left Anterior Descending Artery) and veins (arrowhead – Left Coronary Vein) are visible through the heart surface. (B) A heart with breaks in the microfil (asterisks) as well as blockages in some vessels that prevented complete Microfil penetration. The blocked vessels remain red (arrow), as the blood was not flushed out during the perfusion process. (C) A heart with vessels that were incompletely filled. Notice the tissue has not taken on the yellow color of the Microfil, indicating the Microfil did not penetrate into the capillaries. (D) A heart where the capillaries burst during filling, causing the Microfil to leak into the surrounding tissue (arrow).
Discussion
Cardiac tissue has a very high metabolic demand, and therefore requires a constant supply of nutrients and oxygen from the blood delivered by the coronary vasculature. Diseases of the coronary vessels, which decrease coronary function due to vessel stenosis and blockage, can lead to tissue hypoxia and ischemia, and put affected patients at risk for myocardial infarction and irreparable damage to the heart muscle. A better understanding of the diseased state of these vessels is necessary, and critical to our ability to st...
Disclosures
Mice were handled with methods approved by the Institutional Animal Care and Use Committee of the University of Washington and in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Acknowledgements
We thank Dr. Kelly Stevens for initial trials of the protocol, Dr. Michael Simons, Dr. Kip Hauch, and members of both of their labs for general discussion.
This work is support by NIH grants HL087513 and P01 HL094374.
Materials
| Name | Company | Catalog Number | Comments |
| 1 ml syringes |  BD Biosciences BD Biosciences | BD-309602 | |
| 1/2cc insulin syringes with permanently attached 29G ½’ needles |  BD Biosciences BD Biosciences | BD-309306 | |
| 2" x 2" Gauze pads | Med101store.com | SKU 2208 | |
| 24G ¾" Angiocath IV catheter |  BD Biosciences BD Biosciences | BD-381112 | |
| 26G ½"gauge needles |  BD Biosciences BD Biosciences | BD-305111 | |
| Adenosine |  Sigma-Aldrich Sigma-Aldrich | A9251 | 1g/L in PBS for Vasodilation Buffer (with Papaverine) |
| Angled Graefe Forceps |  Fine Science Tools Fine Science Tools | 11052-10 | |
| Cotton-tipped applicators: 6" non-sterile |  Cardinal Health Cardinal Health | C15055-006 | |
| Curved Surgical Scissors |  Fine Science Tools Fine Science Tools | 14085-09 | |
| Dissecting stereoscope and light source |  Nikon Instruments Nikon Instruments | NA | NA |
| Dissecting Tray, 11.5 x 7.5 inches |  Cole-Parmer Cole-Parmer | YO-10915-12 | Filled with tar for pinning down the mouse |
| Fine Curved Forceps |  Aesculap Aesculap | FD281R | Need two |
| Heparin, 5000 U/ml stock |  APP Pharmaceuticals APP Pharmaceuticals | NDC 63323-047-10 | 1:100 dilution in water |
| KCl |  Fisher Scientific Fisher Scientific | P217 | Saturated solution in H2O |
| Ketamin (Ketaset), 100 mg/ml stock | Fort Dodge Animal Health | NDC 0856-2013-01 | Mixed as 130 mg/kg body weight, with Xylazine in 0.9% saline |
| Microfil |  FlowTech FlowTech | MV-122 (yellow). Other color options are also available. | Mix 1:1 by weight, with 10% by volume of curing agent. Prepare just before injection, and vortex to ensure it is well mixed |
| Non-sterile Suture: 6-0, braided silk |  Harvard Apparatus Harvard Apparatus | 723287 | |
| Papaverine |  American Regent Inc. American Regent Inc. | NDC 0517-4010-01 | 4mg/L in PBS for Vasodilation Buffer (with Adenosine) |
| Paraformaldehyde |  Sigma-Aldrich Sigma-Aldrich | P6148 | Prepared as 4% solution |
| Perfusion Apparatus | See figure 2 | ||
| Spring Scissors |  Fine Science Tools Fine Science Tools | 15018-10 | |
| Xylazine (Anased), 20 mg/gl stock |  Lloyd, Inc. Lloyd, Inc. | NADA #139-236 | Mixed as 8.8 mg/kg body weight, with Ketamin in 0.9% saline |
References
- Couffinhal, T., Dufourcq, P., Barandon, L., Leroux, L., Duplaa, C. Mouse models to study angiogenesis in the context of cardiovascular diseases. Front. Biosci. 14, 3310-3325 (2009).
- Zagorchev, L., Mulligan-Kehoe, M. J. Molecular imaging of vessels in mouse models of disease. Eur. J. Radiol. 70, 305-311 (2009).
- Krucker, T., Lang, A., Meyer, E. P. New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc. Res. Tech. 69, 138-147 (2006).
- Murakami, T. Blood flow patterns in the rat pancreas: a simulative demonstration by injection replication and scanning electron microscopy. Microsc. Res. Tech. 37, 497-508 (1997).
- Icardo, J. M., Colvee, E. Origin and course of the coronary arteries in normal mice and in iv/iv mice. J. Anat. 199, 473-482 (2001).
- Beighley, P. E., Thomas, P. J., Jorgensen, S. M., Ritman, E. L. 3D architecture of myocardial microcirculation in intact rat heart: a study with micro-CT. Adv. Exp. Med. Biol. 430, 165-175 (1997).
- Bentley, M. D., Ortiz, M. C., Ritman, E. L., Romero, J. C. The use of microcomputed tomography to study microvasculature in small rodents. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R1267-R1279 (2002).
- Jorgensen, S. M., Demirkaya, O., Ritman, E. L. Three-dimensional imaging of vasculature and parenchyma in intact rodent organs with X-ray micro-CT. Am. J. Physiol. 275, H1103-H1114 (1998).
- Marxen, M. MicroCT scanner performance and considerations for vascular specimen imaging. Med. Phys. 31, 305-313 (2004).
- Zagorchev, L. Micro computed tomography for vascular exploration. J. Angiogenes. Res. 2, 7-7 (2010).
- Heinzer, S. Hierarchical microimaging for multiscale analysis of large vascular networks. Neuroimage. 32, 626-636 (2006).
- Dedkov, E. I. Synectin/syndecan-4 regulate coronary arteriolar growth during development. Dev. Dyn. 236, 2004-2010 (2007).
- Gossl, M. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 272, 526-537 (2003).
- Rodriguez-Porcel, M. Altered myocardial microvascular 3D architecture in experimental hypercholesterolemia. Circulation. 102, 2028-2030 (2000).
- Bell, R. M., Mocanu, M. M., Yellon, D. M. Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion. J. Mol. Cell. Cardiol. 50, 940-950 (2011).
- Skrzypiec-Spring, M., Grotthus, B., Szelag, A., Schulz, R. Isolated heart perfusion according to Langendorff---still viable in the new millennium. J. Pharmacol. Toxicol. Methods. 55, 113-126 (2007).
- Toyota, E. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 112, 2108-2113 (2005).
- Lavine, K. J., Long, F., Choi, K., Smith, C., Ornitz, D. M. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 135, 3161-3171 (2008).
- Cheema, A. N. Adventitial microvessel formation after coronary stenting and the effects of SU11218, a tyrosine kinase inhibitor. J. Am. Coll. Cardiol. 47, 1067-1075 (2006).
- Lametschwandtner, A., Lametschwandtner, U., Weiger, T. Scanning electron microscopy of vascular corrosion casts--technique and applications: updated review. Scanning Microsc. 4, 889-941 (1990).
- Schneider, P. Simultaneous 3D visualization and quantification of murine bone and bone vasculature using micro-computed tomography and vascular replica. Microsc. Res. Tech. 72, 690-701 (2009).
- Manelli, A., Sangiorgi, S., Binaghi, E., Raspanti, M. 3D analysis of SEM images of corrosion casting using adaptive stereo matching. Microscopy Research and Technique. 70, 350-354 (2007).
- Alanentalo, T. Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat. Meth. 4, 31-33 (2007).
- Quintana, L., Sharpe, J. . Optical projection tomography of vertebrate embryo development. , 586-594 (2011).
- Walls, J. R., Coultas, L., Rossant, J., Henkelman, R. M. Three-Dimensional Analysis of Vascular Development in the Mouse Embryo. PLoS ONE. 3, e2853-e2853 (2008).
- Chalothorn, D., Clayton, J. A., Zhang, H., Pomp, D., Faber, J. E. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol. Genomics. 30, 179-191 (2007).
- Behm, C. Z. Molecular Imaging of Endothelial Vascular Cell Adhesion Molecule-1 Expression and Inflammatory Cell Recruitment During Vasculogenesis and Ischemia-Mediated Arteriogenesis. Circulation. 117, 2902-2911 (2008).
- Carr, C. L., Lindner, J. R. Myocardial perfusion imaging with contrast echocardiography. Curr. Cardiol. Rep. 10, 233-239 (2008).
- Leong-Poi, H. Assessment of Endogenous and Therapeutic Arteriogenesis by Contrast Ultrasound Molecular Imaging of Integrin Expression. Circulation. 111, 3248-3254 (2005).
- Villanueva, F. S. Microbubbles Targeted to Intercellular Adhesion Molecule-1 Bind to Activated Coronary Artery Endothelial Cells. Circulation. 98, 1-5 (1998).
- Wei, K. Quantification of Myocardial Blood Flow With Ultrasound-Induced Destruction of Microbubbles Administered as a Constant Venous Infusion. Circulation. 97, 473-483 (1998).
- Beckmann, N., Stirnimann, R., Bochelen, D. High-Resolution Magnetic Resonance Angiography of the Mouse Brain: Application to Murine Focal Cerebral Ischemia Models. Journal of Magnetic Resonance. 140, 442-450 (1999).
- Kobayashi, H. 3D MR angiography of intratumoral vasculature using a novel macromolecular MR contrast agent. Magnetic Resonance in Medicine. 46, 579-585 (2001).
- Nezafat, R. B1-insensitive T2 preparation for improved coronary magnetic resonance angiography at 3 T. Magn. Reson. Med. 55, 858-864 (2006).
- Wagner, S., Helisch, A., Ziegelhoeffer, T., Bachmann, G., Schaper, W. Magnetic resonance angiography of collateral vessels in a murine femoral artery ligation model. NMR in Biomedicine. 17, 21-27 (2004).
- Cochet, H. In vivo MR angiography and velocity measurement in mice coronary arteries at 9.4 T: assessment of coronary flow velocity reserve. Radiology. , 254-441 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved