A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation of Human Hepatocytes by a Two-step Collagenase Perfusion Procedure
In This Article
Erratum Notice
Summary
A modified two-step collagenase perfusion procedure for isolation of human hepatocytes is described. This method can also be applied to other mammalian livers. The isolated hepatocytes are available in high yield and viability, making them a suitable model for scientific research in areas such as liver regeneration, pharmacokinetics and toxicology.
Abstract
The liver, an organ with an exceptional regeneration capacity, carries out a wide range of functions, such as detoxification, metabolism and homeostasis. As such, hepatocytes are an important model for a large variety of research questions. In particular, the use of human hepatocytes is especially important in the fields of pharmacokinetics, toxicology, liver regeneration and translational research. Thus, this method presents a modified version of a two-step collagenase perfusion procedure to isolate hepatocytes as described by Seglen 1.
Previously, hepatocytes have been isolated by mechanical methods. However, enzymatic methods have been shown to be superior as hepatocytes retain their structural integrity and function after isolation. This method presented here adapts the method designed previously for rat livers to human liver pieces and results in a large yield of hepatocytes with a viability of 77±10%. The main difference in this procedure is the process of cannulization of the blood vessels. Further, the method described here can also be applied to livers from other species with comparable liver or blood vessel sizes.
Introduction
A liver cell suspension can be prepared from the liver by mechanical or enzymatic methods. Mechanical methods used to prepare whole liver cells include forcing the liver through cheesecloth 2, shaking a liver piece with glass beads in a Kahn shaker 3, using glass homogenizers with loose pestles 4,5 etc. Over the years, mechanical methods have fallen out of favor due to the damage to cell membranes and the loss of function of the isolated hepatocytes 6,7. Consequently, the use of an enzymatic method is currently the main method for isolation of hepatocytes.
Isolation of hepatocytes using an enzymatic method was greatly improved when Berry and Friend 8 perfused collagenase and hyaluronidase through the liver via the portal vein in rats. This perfusion process utilized the vasculature to allow the enzymes to come into close contact with the majority of the cells, leading to a 6-fold increase in yield of hepatocytes 8. Further, this method yielded cells that retained their structural integrity, with virtually no transformation of endoplasmic reticulum into isolated vesicles and no mitochondrial damage 8.
This method was modified by Seglen 1, who pioneered a two-step perfusion procedure for liver cell isolation. In this procedure, the rat liver is perfused with a Ca2+ free buffer followed by perfusion with a collagenase buffer containing Ca2+ 1. The removal of Ca2+ in the first step helps to disrupt desmosomes, while the addition of Ca2+ in the second step is required for optimum collagenase activity 1,9.
Given that the published work described above has been performed in rats, this article aims to demonstrate a modified procedure that can be used for isolation of hepatocytes with high viability from human livers. The use of human hepatocytes remains important for translational research and for validating experiments using animal models. The human liver pieces used in this study were acquired with consent for governance through the Human Tissue and Cell Research Foundation, a state-controlled non-profit foundation 10. After a pathologist removed what was required for diagnosis, liver pieces were collected from the remaining tissue. The tissue sectioned off by the pathologist was morphologically healthy tissue obtained from resection margins after liver resection.
Protocol
1. Preparation of Perfusion and Isolation Solutions
- Prepare the solutions required for the perfusion of the liver piece and the isolation of hepatocytes according to Table 1. Solutions can be stored at 4 °C until use.
- Sterile filter all solutions using a 0.22 μm filter.
- All solutions that come into contact with the liver should be sterile.
2. Preparation of Perfusion Equipment and Solutions
- The equipment for the perfusion of the liver piece should be set up as shown in Figure 1.
- The water bath should be set at an appropriate temperature, which is different in each particular experimental set-up, such that the solutions are at the temperature of 37 °C when they reach the liver piece. In this case, the water bath is set at 41 °C to warm the Solutions 1, 2 and 3 and the jacketed glass condenser. Solution 4 should be warmed up to 37 °C in a separate water bath for use to reduce the loss of collagenase activity.
- Shortly before liver perfusion, turn on the regulator of the gas tank containing 95% O2/5% CO2 to gas the oxygenation apparatus (Figure 1E).
3. Perfusion of the Liver
- A liver piece with as much intact Glisson's capsule as possible and ideally with only 1 cut surface should be obtained from a pathologist for perfusion.
- Place this liver piece on the Büchner funnel that contains a perforated filter disc (Figure 1B).
- The perfusion system should be primed with Solution 1.
- With a low flow rate, curved irrigation cannulae with olive tips should be inserted into the larger blood vessels on the cut surface of the liver piece. As blood flushes out from the liver, the tissue becomes lighter in areas with good perfusion. The number of cannulae used for various sizes of livers is shown in Figure 2A. The gauge size chosen should result in a snug fit that will hold the cannulae in place. The smaller blood vessels should be left open for the perfusion buffer to drain out of the liver piece.
- Increase the flow rate on the peristaltic pump to between 110-460 ml/min depending on the size of the liver (Figure 2B). This results in an average flow rate of 44±16 ml/min per cannula (Figure 2C). The speed chosen depends on the liver piece and should result in a slight plumping up of the liver piece. In some cases, it may be necessary to clamp shut some of the open vessels with micro vascular clamps to achieve the slight plumping mentioned above. A good perfusion can be observed when the liver piece is a lighter color throughout.
- Keep the liver piece moist during perfusion by covering it with a piece of gauze soaked in saline.
- Perfuse with 1 L of Solution 1 to flush out any remaining blood in the liver piece.
- Change the perfusion fluid to Solution 2 and perfuse for 10 min.
- Switch the perfusion fluid to Solution 3 and perfuse with 0.5 L.
- Change the perfusion fluid to Solution 4, which contains 0.1-0.15% of collagenase (Table 2).
- For this step, perfusion should be carried out in a recirculating manner for 9-12 min or until the liver is sufficiently digested; the liver tissue should appear to break apart slightly under the Glisson's capsule and feel softened when probed with the blunt side of a scalpel.
4. Isolation of Hepatocytes
- Turn off the peristaltic pump and remove cannulae from the liver piece.
- Place the liver piece in a crystallizing dish containing 100-200 ml of Solution 5.
- Remove the Glisson's capsule carefully and gently shake out the cells. If there are regions that are not well perfused, a scalpel can be used to cut through these regions to release cells contained within. Add more Solution 5 as needed during the process.
- Add more Solution 5 until a final volume of 500 ml is reached.
- Filter cell suspension twice; first through a 210 μm nylon mesh followed by a 70 μm nylon mesh. Next, pour the cell suspension into 200 ml centrifuge tubes.
- Centrifuge the cell suspension at 72 g for 5 min at 4 °C. Aspirate supernatant and gently resuspend cell pellet gently in 200 ml of Solution 5.
- Repeat the washing step number 4.6 three times. On the final centrifuge step, resuspend cells in cold storage solution (see list of materials). Cells should be approximately 2-5 million hepatocytes per milliliter for assessment of yield and viability using a hemocytometer-based trypan blue exclusion assay.
- To carry out a trypan blue exclusion assay, add 0.1 ml of appropriately diluted cells (≈2-5 million/ml) to a microfuge tube containing 0.5 ml of trypan blue solution (0.4% trypan blue dissolved in phosphate-buffered saline (PBS)) and 0.4 ml of PBS. After mixing the cell suspension thoroughly, load a hemocytometer with the suspension and examine under a microscope at 100X magnification. Under microscopy, the dead cells will be stained blue while the live cells appear unstained. Count the number of live and total cells in each of the 1 mm2 grids marked on the hemocytometer. Viability (%), yield of live cells (million hepatocytes per ml cold storage solution (CSS) or million hepatocytes per g liver) can be calculated using the formulae below.
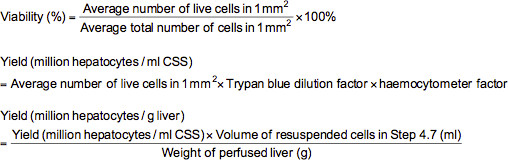
Note: In this case, the trypan blue dilution factor is 10 and the hemocytometer factor is 10,000.
Results
Perfusion Setup
The equipment required for liver perfusion should be set up according to Figure 1.
Viability and Yield of Isolated Human Hepatocytes
The average viability of isolated human hepatocytes was 77±10% and the average yield of hepatocytes was 13±11 million hepatocytes/g liver, with values expressed as means ± standard deviation. The number of hepatocyte isolations carried out to obtain these averages wa...
Discussion
This protocol results in the isolation of human hepatocytes with high viability and purity. In order to achieve these results, it is important to start with a suitable piece of liver. The piece of liver should have intact Glisson's capsule on all surfaces except for 1 cut surface. Another important factor is the particular batch of collagenase used, as different batches can result in marked differences in viabilities of hepatocytes after digestion 11. Therefore, different batches of collagenase should be teste...
Disclosures
Optimization of this protocol was partially funded by a grant from Hepacult GmbH. Dr. Wolfgang Thasler is one of the founders of Hepacult GmbH and remains one of the members of the board in this company. The employment of Maresa Demmel is partially by Hepacult. Maria Hauner is employed by Hepacult GmbH. Hepacult is a spin-off biotechnological firm from the University, which offers human hepatocytes with consent and open access for research purposes.
Acknowledgements
This work was made possible by the Human Tissue and Cell Research Foundation, which makes human tissues available for research. Financial support for this work was received from the Federal Ministry of Education and Research (grant name: Virtual Liver Network, grant number: 0315759) and Hepacult GmbH. Our thanks also go to the technical assistants from the Grosshadern Hospital Tissue bank for the collection of the liver samples and the technical assistants from the Cell Isolation Core Facility for carrying out the liver perfusion and hepatocyte isolation. In particular, we would like to thank Natalja Löwen for demonstrating this procedure in the video. Finally, we would like to thank Natalja Löwen and Edeltraud Hanesch for creating the illustrations for Figure 1 and the figures in the schematic overview of the video.
Materials
| Name | Company | Catalog Number | Comments |
| Bubble trap | Gaßner Glastechnik | ||
| Glass jacketed condenser | Gaßner Glastechnik | ||
| 41 °C Water bath | Julabo | 35723-H24/EG | |
| 37 °C Water bath | GFL | 1083 | |
| Compressed gas cylinder (95 % O2/5 % CO2) | Linde | ||
| Gas permeable tubing | Neolab | 2-4440 | |
| Peristaltic pump | Ismatec | IP65 | |
| Scalpel | Feather | 320010 | |
| Forceps | Omnilab | 5171014 | |
| Conical flasks 1 L | Schott Duran | 2121654 | |
| Conical flasks 5 L | Schott Duran | 2121673 | |
| Beakers | Schott Duran | 2110654 | |
| 200 ml centrifuge tubes | Becton Dickinson | 352075 | |
| Crystallizing dish | Omnilab | 5144063 | |
| Curved irrigation cannulae with ball tips | Ernst Kratz GmbH | 1464LL/ 1465LL A+B/ 1472LL | |
| Micro vascular clamps | Ernst Kratz GmbH | ||
| Büchner funnel | Carl Roth | HT38.1 | |
| Nylon mesh 210 μm | Neolab | 4-1413 | |
| Nylon mesh 70 μm | Neolab | 4-1419 | |
| 0.22 μm sterile filters | Peske | 99505 | |
| 500 ml bottles | Schott Duran | 2180144 | |
| 1 L bottles | Schott Duran | 2180154 | |
| Hemocytometer | Peske | 06-0001 | |
| 1.5 ml tubes | Eppendorf | 0030 120,086 | |
| 50 ml conical tubes | BD Biosciences | 352070 | |
| Ice bucket | Neolab | 1508454 | |
| Sterile Pasteur pipettes | Brand | 747715 | |
| Motorised pipette filler (Pipette boy acu) | Integra | 155017 | |
| Refridgerated centrifuge | Eppendorf | 5810R | |
| Laminar flow | Kendro | Hera safe-KS9 | |
| Aspirator (Low-flow surgical suction pump) | Atmos | C361 | |
| Laboratory Gas Burner | Integra | Fire Boy eco | |
| Disposable laboratory coat | Paperlynen GmbH | MD0202414 | |
| Surgical mask with visor | Kimberly-Clark | 48247 | |
| Surgical hood | Barrier | 42072 | |
| Latex gloves | Semper Care | CE0321 | |
| Collagenase (Batch number NB 4G) | Serva | 17465 | |
| Calcium chloride dihydrate | Merck | 2382 | |
| EGTA | Sigma | E4378 | |
| Sodium chloride | Roth | 9265.2 | |
| Hepes | Roth | 9105.3 | |
| Potassium chloride | Serva | 26868 | |
| Albumin | Biomol | 01400-2 | |
| Glucose | Serva | 22700 | |
| Sodium hydrogen carbonate | Serva | 30180 | |
| 0.4% Trypan blue solution | Lonza | 17-942E | |
| Cold storage solution | Hepacult GmbH |
References
- Seglen, P. O. Preparation of isolated rat liver cells. Methods in Cell Biology. 13, 29-83 (1976).
- Schneider, W. C., Potter, V. R. The assay of animal tissues for respiratory enzymes II. Succinic dehydrogenase and cytochrome oxidase. J. Biol. Chem. 149, 217-227 (1943).
- Aubin, P. M. G., Bucher, N. L. R. A Study of Binucleate Cell Counts in Resting and Regenerating Rat Liver Employing a Mechanical Method for the Separation of Liver Cells. Anat. Rec. 112, 797-809 (1952).
- Anderson, N. G. The Mass Isolation of Whole Cells from Rat Liver. Science. 117, 627-628 (1953).
- Jacob, S. T., Bhargava, P. M. New Method for Preparation of Liver Cell Suspensions. Experimental Cell Research. 27 (62), 453-467 (1962).
- Berry, M. N. Metabolic Properties of Cells Isolated from Adult Mouse Liver. Journal of Cell Biology. 15, 1-8 (1962).
- Laws, J. O., Stickland, L. H. Metabolism of Isolated Liver Cells. Nature. 178, 309-310 (1038).
- Berry, M. N., Friend, D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. The Journal of Cell Biology. 43, 506-520 (1969).
- Seglen, P. O. Preparation of Rat-Liver Cells .3. Enzymatic Requirements for Tissue Dispersion. Experimental Cell Research. 82 (73), 391-398 (1973).
- Thasler, W. E., et al. Charitable State-Controlled Foundation Human Tissue and Cell Research: Ethic and Legal Aspects in the Supply of Surgically Removed Human Tissue For Research in the Academic and Commercial Sector in Germany. Cell and Tissue Banking. 4, 49-56 (2003).
- Queral, A. E., DeAngelo, A. B., Garrett, C. T. Effect of different collagenases on the isolation of viable hepatocytes from rat liver. Analytical Biochemistry. 138, 235-237 (1984).
- Smedsrod, B., Pertoft, H. Preparation of Pure Hepatocytes and Reticuloendothelial Cells in High-Yield from a Single-Rat Liver by Means of Percoll Centrifugation and Selective Adherence. J. Leukocyte Biol. 38, 213-230 (1985).
- Cantrell, E., Bresnick, E. Benzpyrene Hydroxylase-Activity in Isolated Parenchymal and Nonparenchymal Cells of Rat-Liver. Journal of Cell Biology. 52, 316-321 (1972).
- Weiskirchen, R., Gressner, A. M. Isolation and culture of hepatic stellate cells. Methods in Molecular Medicine. 117, 99-113 (2005).
- Baccarani, U., et al. Steatotic versus cirrhotic livers as a source for human hepatocyte isolation. Transplant P. 33, 664-665 (2001).
- Renz, J. F., et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Annals of Surgery. 242, 556-563 (2005).
- Schemmer, P., et al. Extended donor criteria have no negative impact on early outcome after liver transplantation: a single-center multivariate analysis. Transplant Proc. 39, 529-534 (2007).
- Alexandre, E., et al. Influence of pre-, intra- and post-operative parameters of donor liver on the outcome of isolated human hepatocytes. Cell and Tissue Banking. 3, 223-233 (2002).
- Spotnitz, W. D., Burks, S. State-of-the-art review: Hemostats, sealants, and adhesives II: Update as well as how and when to use the components of the surgical toolbox. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 16, 497-514 (2010).
- Spotnitz, W. D., Burks, S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 52, 2243-2255 (2012).
- Toriumi, D. M., Raslan, W. F., Friedman, M., Tardy, M. E. Histotoxicity of cyanoacrylate tissue adhesives. A comparative study. Archives of otolaryngology--head & neck surgery. 116, 546-550 (1990).
- Vinters, H. V., Galil, K. A., Lundie, M. J., Kaufmann, J. C. The histotoxicity of cyanoacrylates. A selective review. Neuroradiology. 27, 279-291 (1985).
- Bhogal, R. H., et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PloS ONE. 6, e18222 (2011).
- Olson, H., et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharm. 32, 56-67 (2000).
- Koebe, H. G., Pahernik, S. A., Sproede, M., Thasler, W. E., Schildberg, F. W. Porcine hepatocytes from slaughterhouse organs. An unlimited resource for bioartificial liver devices. ASAIO Journal. 41, 189-193 (1995).
- Russell, W. M. S., Burch, R. L. . The principles of humane experimental technique. , (1959).
Erratum
Formal Correction: Erratum: Isolation of Human Hepatocytes by a Two-step Collagenase Perfusion Procedure
Posted by JoVE Editors on 7/01/2016. Citeable Link.
A correction was made to: Isolation of Human Hepatocytes by a Two-step Collagenase Perfusion Procedure
The changes listed below have been made to Table 1.
1). In the recipe for Solution 2, the Final Concentration of EGTA has been changed from:
| EGTA | 0.1mM |
to:
| EGTA | 1mM |
2). In the recipes for Solution 3 and 4, the Final Concentration of Calcium chloride dihydrate has been changed from:
| Calcium chloride dihydrate | 0.5µM |
to:
| Calcium chloride dihydrate | 5mM |
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved