A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Preparation of Segmented Microtubules to Study Motions Driven by the Disassembling Microtubule Ends
In This Article
Summary
Microtubules are inherently unstable polymers, and their switching between growth and shortening is stochastic and difficult to control. Here we describe protocols using segmented microtubules with photoablatable stabilizing caps. Depolymerization of segmented microtubules can be triggered with high temporal and spatial resolution, thereby assisting analysis of motions with the disassembling microtubule ends.
Abstract
Microtubule depolymerization can provide force to transport different protein complexes and protein-coated beads in vitro. The underlying mechanisms are thought to play a vital role in the microtubule-dependent chromosome motions during cell division, but the relevant proteins and their exact roles are ill-defined. Thus, there is a growing need to develop assays with which to study such motility in vitro using purified components and defined biochemical milieu. Microtubules, however, are inherently unstable polymers; their switching between growth and shortening is stochastic and difficult to control. The protocols we describe here take advantage of the segmented microtubules that are made with the photoablatable stabilizing caps. Depolymerization of such segmented microtubules can be triggered with high temporal and spatial resolution, thereby assisting studies of motility at the disassembling microtubule ends. This technique can be used to carry out a quantitative analysis of the number of molecules in the fluorescently-labeled protein complexes, which move processively with dynamic microtubule ends. To optimize a signal-to-noise ratio in this and other quantitative fluorescent assays, coverslips should be treated to reduce nonspecific absorption of soluble fluorescently-labeled proteins. Detailed protocols are provided to take into account the unevenness of fluorescent illumination, and determine the intensity of a single fluorophore using equidistant Gaussian fit. Finally, we describe the use of segmented microtubules to study microtubule-dependent motions of the protein-coated microbeads, providing insights into the ability of different motor and nonmotor proteins to couple microtubule depolymerization to processive cargo motion.
Introduction
Microtubules are highly conserved cytoskeletal structures that are important for cellular architecture, cell motility, cell division, and intracellular transport1. These dynamic polymers assemble from tubulin in the presence of GTP, and they switch spontaneously between growth and shortening2. Microtubules are very thin (only 25 nm in diameter) therefore special optical techniques to enhance contrast should be used to observe microtubules with a light microscope. Previous work with these polymers examined their dynamic behavior using differential interference contrast (DIC)3. This and similar studies in vitro revealed that under typical experimental conditions, the microtubules undergo catastrophe and switch to the depolymerization only rarely, once every 5-15 min (this frequency is for 7-15 mM soluble tubulin concentration examined at 28-32 °C)4. Different techniques have thus been proposed to induce microtubule depolymerization in a controlled manner. Microtubule shortening can be triggered by washing away soluble tubulin5,6, cutting microtubules with a laser beam7, or using segmented microtubules8, as described here. Previous work using segmented microtubules, as well as stochastically switching polymers, has found that small intracellular cargos, such as chromosomes, vesicles, and protein-coated beads, can move at the ends of the shortening microtubules9-13. This phenomenon is thought to have a direct implication for chromosome motions in mitotic cells, and the underlying mechanisms are currently under active investigation14-16.
Recently, fluorescent-based techniques, including the total internal reflection fluorescence (TIRF) microscopy, have been employed to study motility with dynamic microtubule ends17-24. The advantage of this approach is that it allows examination of interactions between microtubules and microtubule-binding proteins in real time using proteins labeled with different fluorophores. Several protein complexes were found to move processively with elongating and/or shortening microtubule ends. They include the microtubule-associated proteins Dam110,12,18, Ska119, and XMAP21520, as well as kinesin motors Kif18A21,22, MCAK23 and CENP-E24. These proteins exhibit processive tip-tracking, which is fundamentally different from that of the classic tip-tracking proteins like EB125. Although EB1 molecules and the associated partners appear to remain stably associated with dynamic microtubule ends, the individual molecules remain bound to the microtubule tip for only ~0.8 sec, rapidly exchanging with the soluble pool26. In contrast, processive tip-trackers, like Dam1, travel with microtubule ends for many microns, and their association with microtubule tips can last for many seconds. The tip association time, as well as the resulting rate of tracking, depends strongly on the number of molecules that form the tip-tracking complex27. Larger protein ensembles are usually much better tip-trackers. For example, such complex assemblies as the isolated yeast kinetochores can remain coupled to microtubule ends for hours28. Some microtubule-binding proteins, e.g. Ndc80 kinetochore protein complex, have been found to be unable to track with microtubule ends at a single molecule level, yet Ndc80 is very efficient in coupling the motion of bead cargo19,29-31. Thus, to understand the mechanism of tip-tracking by different protein complexes, as well as their biological roles, it is important to examine tip-tracking as a function of the number of molecules in the tip-tracking complex, as well as to determine the ability of these complexes to exhibit collective motility on the surface of bead cargo.
Below we provide detailed protocols to prepare and conduct experiments with segmented microtubules (Figure 1A). First, the commercially available glass slides are modified to attach short polyethylene tubing (Protocol 1). The reusable microscopy flow chamber is then assembled from such a slide and the chemically or plasma-cleaned and silanized coverslip (protocol 2)32-34. The resulting chamber volume is only 20-25 μl (or as small as 15 μl, see Note 3 in Protocol 1), including the volume of the inlet tubing. Commercially available flow chambers can also be used, but their volume is usually larger, leading to the unnecessary waste of proteins. If a larger chamber is employed, the volume of all solutions in the protocols below should be scaled proportionally. Microtubule seeds are then prepared, for example using slowly hydrolysable GTP analog, GMPCPP (Guanosine-5’-[(α,β)-methyleno]triphosphate) (protocol 3, see also Hyman et al.35). The seeds are immobilized on a cleaned coverslip and the surface is subsequently blocked to prevent nonspecific absorption of other proteins32 (protocol 4 describes seeds immobilization using digoxigenin). The segmented microtubules can then be prepared using Protocol 5. The main rationale for this approach is that dynamic microtubule polymers, which form in the presence of GTP, can be stabilized temporarily by adding the short “caps” of stable tubulin segments, which contain GMPCPP. These caps also contain Rhodamine-labeled tubulin, so they can be removed simply by illuminating the field of view with a 530-550 nm laser or mercury arc lamp (Protocol 6)36. Fluorescence intensity of the tip-tracking signal can then be used to estimate the number of molecules that travel with the disassembling microtubule ends, taking into account the unevenness of the microscope field illumination (Protocol 7). A similar approach can be used to study interactions between depolymerizing microtubules and protein-coated beads, prepared as described in27 (Protocol 8). Some proteins will readily bind to the walls of segmented microtubules, but laser tweezers can also be used to hold the bead near the microtubule wall, thereby promoting its binding.
Protocol
Required equipment: The experiments described below require a light microscope equipped for DIC and fluorescence imaging (Table 1). Bright field LED illumination can be used to significantly improve the detection of the coverslip-attached microtubule seeds37, which are difficult to observe with a regular Halogen lamp. To control liquid flow in microscopy chambers, the solutions should be exchanged with a peristaltic pump capable of flow speeds from 10-100 μl/min. A syringe pump can also be used but care should be taken to avoid air bubbles that may form when the flow speed is changed abruptly. For handling protein-coated beads, for example to bring them close to the segmented microtubule wall, a 1,064 nm continuous wave laser beam can be introduced into the microscope's optical axis and focused with a high numerical aperture objective (1.3 or higher) to produce a trap. For quantitative analysis of the fluorescent intensity of single molecules the excitation light should be provided by a laser-base source since the intensity of this light source is more stable than that generated by a mercury lamp. To minimize mechanical vibrations, the microscope should be placed on an optical table. More sophisticated equipment is required to study the movement of the beads with the depolymerizing microtubule ends under a constant force, and to measure the single-shot force signals11,38,39, these methods will be described elsewhere.
1. Manufacturing Reusable Flow Chambers
Glass slides for reusable flow chambers can be ordered from a local glass manufacturing facility using schematics in Figure 1B (see Table 2 for details about our supplier). With ultrasonic milling modify regular microscope slides (75 mm x 25 mm, 1.0 mm thick) to make two grooves 15±1 mm long, 1.0±0.1 mm wide and 0.8±0.05 mm deep. Distance between the closest ends should be 14±1 mm; this distance is optimal for a chamber assembled with 22 mm x 22 mm coverslip. See Table 2 for a list of other materials.
- Place a 100 mm long polyethylene tube (O.D. 0.61 mm, Table 2) in each groove in the slide, leaving ~5 mm overhangs at the inner ends of the grooves. Fix the tubes inside the grooves with cyanoacrylate adhesive, embedding the tubes completely inside the grooves.
- Fill the grooves with epoxy glue, while avoiding spilling the glue inside the tubes. Let the glue dry for ~1 day.
- With a sharp razor blade cut the solidified glue mass 3-4 mm from the distal end of each attachment site, removing the parts proximal to the center of the slide. The tubes should remain inside their grooves. Removing the proximal parts will also cut and remove the inner overhangs, creating a flat surface with two tube openings.
- Fill a syringe with water and test whether the tubes are working properly. If the liquid flows freely, put a drop of epoxy glue (~5 mm in diameter) at the outer ends of the groves, dry for 1 day (Figure 1D). This will make chambers more durable, so they can be used repeatedly for many months.
Note 1: To make a chamber for an inverted microscope, the slides should be modified additionally to make two small holes at the opposite ends of the grooves (Figure 1C). Insert the tubes through the holes in the slide, bend the tubes and fit them tightly inside the grooves (Figure 1E). Follow steps 1.2-1.4, but remove the epoxy glue from the entire surface, which will be used to make a flow chamber.
Note 2: To reduce chamber volume, use milling to make two indentations 0.050±0.005 mm deep, leaving the central part of the slide 5.0±0.5 mm wide and slightly elevated (see “etched areas” on Figures 1B and 1C). When the flow chamber is assembled (as described below), place the double-sided tape inside these indentations.
Note 3: To reuse these modified slides, after finishing the experiments remove the coverslip and double-sided tape using a razor blade. Remove the sealant by peeling it off and by wiping the slide with 70% ethanol. Place the slide in a container with 1-2% of a lab dishwashing detergent, attach tubing to a peristaltic pump and perfuse 50-70 ml, follow with equal volume of deionized water, dry and store in a dust-free compartment.
2. Preparation of Coverslips
This protocol takes 6-8 hr and will help to prepare 12 coverslips. You will need one ceramic coverslip holder and 3 coverslip staining jars with lids; jar volume should be 15 ml, so each will hold 4 coverslips stacked together. A glass jar with a lid (250 ml) should be used to incubate coverslips with silane. Use regular No.1 glass coverslips (22 mm x 22 mm or 22 mm x 30 mm, see Tables 2 and 3 for a list of materials). All steps should be carried out in a fume hood, while wearing gloves.
- Put the coverslips into the glass coverslip staining jars and fill the jars with acetone. Incubate for 1 hr, wash 10x with deionized water.
- Incubate the coverslips 10 min with ethanol and wash again 10x with deionized water.
- Prepare “piranha” solution. Put 60 ml of hydrogen peroxide solution (30% in water) in a heat-resistant glass vessel and slowly add 100 ml of sulfuric acid (final ratio of acid to hydrogen peroxide solution is 5:3). Solution will heat up, this is normal but use caution. Piranha solution is extremely corrosive! Use thick lab coat, gloves and goggles!
- Fill the coverslip staining jars with “piranha” solution, close the lids and place the jars in a water bath preheated to 90 °C for 1 hr.
- Pour off the “piranha” solution and discard as instructed by the safety regulations at your workplace. Wash coverslips 10x with deionized water.
- Fill the coverslip staining jars with 0.1 M KOH, incubate 10 min, and wash 10x with deionized water. This will neutralize any acid residues left on the coverslips after “piranha” treatment.
- Dry coverslips one at a time by holding each coverslip with Teflon coated flat-edged tweezers (to minimize damage to a glass surface) and while blowing compressed dry nitrogen. Make sure that coverslips are dried completely, because silane solution is highly reactive with water.
- Stack the dried coverslips in ceramic holders (12 coverslips per holder), which should be thoroughly predried with nitrogen. Keep the ceramic holders covered to avoid dust from sticking to the coverslip surface.
- Cover the bottom of 250 ml glass jar (6 cm in diameter) with Molecular Sieves, Grade 564, for water absorption.
- Fill the jar with 200 ml of PlusOne Repel Silane solution and slowly immerse a ceramic holder with coverslips in a jar, close the lid and incubate for 5 min at room temperature. This will create hydrophobic coating on the coverslip surface.
- Slowly remove the holder with coverslips from the jar and transfer coverslips one at a time into the coverslip staining jars filled with methanol.
- Place a metal or glass pedestal into the water reservoir of a sonic bath, such that the coverslip staining jar is immersed for 2/3 of its height. Sonicate at 70 W for 20 min, changing methanol solution every 5 min, then rinse 10x with deionized water. If the silanization worked properly, the coverslips will appear dry when removed from water.
- Thoroughly remove any residual water using nitrogen, as above.
- Interlay the coverslips with Kimwipes to avoid surface-to-surface contact between the coverslips. Coverslips can be stored in a sealed container for several weeks at room temperature.
Note 1: Steps 2.1-2.6 can be replaced by cleaning the coverslips with Plasma Cleaner for 15 min at 30 W, greatly reducing the total preparation time. Pressure inside the cleaning chamber is set at 100-200 mTorr. Both atmospheric and compressed oxygen can be used. Stack the plasma-cleaned coverslips in ceramic holders and proceed to step 2.7.
3. Preparation of GMPCPP-stabilized Microtubule Seeds
This procedure will take ~1 hr and the resulting microtubule seeds are stable for 1-2 days at room temperature. See Table 4 for a list of reagents.
- Mix on ice:
- 10 μl unlabeled tubulin (100 μM, Table 4) in BRB-80 buffer (80 mM Pipes, 1 mM EGTA, 4 mM MgCl2, pH 6.9 with KOH; supplement with 1-2 mM DTT using fresh aliquot for each experiment).
- 2.6 μl digoxigenin-labeled tubulin (Table 4). Adjust volume depending on preparation, such that the final ratio of labeled to unlabeled tubulin is ~1:10. Mix well by pipetting.
- 1.4 μl 10 mM GMPCPP (final concentration 1 mM)
- Incubate 15 min at 35 °C, the seeds will grow 2-3 μm long. Adjust time if different microtubule length is desired.
- Add 35 μl BRB-80 (prewarmed to 35 °C), mix by pipetting, and centrifuge for 15 min at 25,000 x g to pellet the seeds at room temperature.
- Discard supernatant, wash the pellet by gently adding and removing 50 μl of warm BRB-80.
- Resuspend pellet well in 25 μl BRB-80.
4. Attachment of Microtubule Seeds to the Coverslips
Protocols 4 and 5 will require 2-3 hr, so two flow chambers are used per day.
- Assemble flow chamber as per manufacturer's instructions using silanized coverslips and proceed to step 4.2. If using custom-made coverslips (Protocol 1), follow the steps below.
- Attach two pieces of double-sided tape (5 mm x 30 mm) along the central ~5 mm wide area, put silanized coverslip atop the tape, press firmly.
- Fill the chamber with BRB-80 through one of the tubes and plug both tubes with round toothpicks.
- Squeeze a small drop of two-colored Kwik Cast sealant on top of a small plastic Petri dish, and mix quickly but thoroughly using a toothpick. The sealant will turn green; apply immediately, carefully sealing all edges of the coverslip. If the sealant penetrates too deeply under the coverslip, open one of the tubes by removing the toothpick plug and apply gentle pressure to prevent sealant from leaking inside the tubes.
- Let the chamber dry for 10 min and confirm that the flow is not restricted before proceeding further.
- Place the chamber on a microscope stage prewarmed to 32 °C and attach one of the tubes to a pump, which will pump the liquid out. The length of the inlet tube should be minimized to avoid the unnecessary loss of reagents: the recommended length is 5-7 cm. Immerse this end in a 0.5 ml vial with BRB-80 buffer. This and all solutions below should be prewarmed to 32-35 °C.
- Apply a gentle pressure with a pump or simply lift the end of the outlet tube to squeeze out the air bubbles, which may form occasionally when the inlet tube plug is removed.
- Set the pump rate at 100 μl/min. Wash with 2 chamber volumes of anti-digoxigenin antibodies diluted 1:30 in BRB-80, incubate 15 min to allow antibody adsorption.
- Wash with 5-10 chamber volumes of warm BRB-80, incubate 10 min with 1% Pluronic F-127 in BRB-80 to block the hydrophobic surface of silanized coverslip.
- Wash with 5-10 chamber volumes of motility buffer (BRB-80 supplemented with 0.4 mg/ml of casein).
- Reduce the pump speed to 10 μl/min and perfuse microtubule seeds diluted 1:200-1:600 in 30-40 μl motility buffer. Incubate 15 min to promote binding of the seeds to the coverslip-adsorbed antibodies.
- Wash the chamber at 100 μl/min with 400 μl of motility buffer to remove any unbound material.
Note 1: The resulting density of seeds should be 10-30 per microscope field (Figure 2A). To troubleshoot, use fluorescently labeled tubulin during polymerization (step 3.1) for easier detection of the coverslip-attached seeds.
Note 2: Axonemes prepared from Chlamydomonas40 or other biological sources, as well as the pellicles of lysed and deciliated Tetrahymena cells41 can also be used as microtubule nucleators. These nucleators are useful for creating small microtubule arrays, and are preferred when microtubules with specific number of protofilaments are desired (GMPCPP seed nucleates one microtubule that contains ≥14 protofilaments42). These structures can be attached to the cleaned coverslips by nonspecific absorption, but the attachment is usually less stable compared with antibody-based attachment, especially when using the silanized coverslips.
5. Preparation of Segmented Microtubules
All solution volumes below are for chamber volume 15-20 μl; increase proportionally if larger chamber is used.
- Prewarm unlabeled tubulin mix (45 μl motility buffer supplemented with 1 mM Mg-GTP and with 10-15 μM unlabeled tubulin) for 30 sec at 35 °C. Perfuse at 30 μl/min.
- Monitor microtubule growth with DIC optics (Figure 2B, Video 1). During 5-7 min incubation the microtubules usually grow ~10 μm long.
- Prepare Rhodamine-tubulin mix (65 μl motility buffer supplemented with 0.5 mM GMPCPP and 2-5 μM of Rhodamine-labeled tubulin with 0.5-1 molar ratio of Rhodamine to tubulin) and warm the solution at 35 °C for 30 sec.
- Perfuse immediately at 30 μl/min. Incubate for 8-10 min to promote formation of stable fluorescent caps at the microtubule tips. Stable microtubule segments will also nucleate spontaneously and will be visible with DIC optics.
- Wash the chamber well with 100 μl of motility buffer at 20 μl/min to remove tubulins and nucleotides, as well as soluble microtubule fragments.
- With DIC, confirm that the microtubules are visible (Figure 2D); their number, however, should decrease because many microtubules disassemble during capping (Video 2 shows a typical field with segmented microtubules).
Note 1: Segmented microtubules are very stable and can be used for at least 2 hr. However, the lifetime of these microtubules decreases with excessive solution exchange, or if 2-mercaptoethanol is used in the imaging buffer.
6. Experimental Observation of the Protein Tracking with Depolymerizing Microtubule Ends
- Introduce 30-50 μl of fluorescent protein (0.1-20 nM) into the chamber at 10 μl/min. If protein sticking to the coverslip is evident, supplement the motility buffer with 4-8 mg/ml BSA. Alexa488-Dam1 tip-tracking additionally requires 10 mM DTT or 0.5-1% βME10.
- Limit the illumination field using a microscope field diaphragm to avoid the unnecessary bleaching and disassembling of the microtubules.
- Start video acquisition using GFP filter cube, then switch to Rhodamine filter cube without interrupting the image recording. The red segments at the microtubule ends should be clearly visible; they will begin to fade and disintegrate quickly (Video 3).
- Continue to illuminate until the caps almost disappear (usually for 10-20 sec but this time will be longer for the caps grown with a lower Rhodamine labeling ratio), and switch back to the GFP channel to record protein tracking with microtubule disassembly.
- Analyze the resulting sequences by constructing kymographs, i.e. two-dimensional images that show fluorescence intensity along microtubule axis for various times during observation) using MetaMorph, freely available ImageJ or other image processing software (Figure 2E).
Note 1: Acquisition rate should be adjusted depending on the timing of the observed events. The recommended rate is 2-3 frames per second (fps) for the slow-moving, ring-sized Dam1 complexes27 but acquisition time for single molecules should be >20 fps19.
Note 2: A highly sensitive EMCCD, e.g. ANDOR iXon3, is required for the fast recording of tip-tracking events with depolymerizing microtubules. The recommended settings for Andor iXon3 camera are: gain 5x, EM gain 200, 1 MHz readout speed, 16-bit sensor mode, 80 msec exposure time.
Note 3: Using TIRF microscopy will improve signal-to-noise ratio, however, shorter microtubules should be used, such that the fluorescent stabilizing caps remain within the reach of evanescent field.
7. Quantitative Analysis of the Molecular Size of Microtubule Tip-tracking Complexes
The rationale for this approach is to determine the number of molecules in a tip-tracking complex by finding the ratio of total fluorescent intensity of the tip-tracking complex to the intensity of a single fluorophore. This approach can be applied to GFP-protein fusions and proteins labeled with fluorescent dyes, but it may underestimate the number of molecules in the tip-tracking complexes if some protein molecules in the preparations are not fluorescent.
- Record photobleaching kinetics for the fluorescently labeled protein molecules.
- Assemble regular microscopy chamber using a nonmodified glass slide, two strips of double-sided tape, and a clean coverslip, which can be prepared using the entire Protocol 2, or only steps 2.1-2.6 of this protocol.
- Add approximately 50 nM protein in motility buffer, wash briefly with motility buffer and seal the chamber with VALAP (Table 4). Optimize protein concentration to obtain the field with evenly dispersed spots (Figure 3A), representing single molecules and their small aggregates (trimers and tetramers, which may form spontaneously in the solution or may appear when several single molecules are close together and cannot be resolved). This step is very important for obtaining a multi-peak distribution of photobleaching steps and accurate determination of a step size (see below).
- Minimize the illumination laser intensity at which the individual fluorescent spots are still visible; with lower illumination the photobleaching time is extended, so longer photobleaching traces can be obtained. Also minimize the exposure time to reduce the probability of more than one fluorophore bleaching during one frame. The recommended setting for Andor iXon3 camera: gain 5.0x, EM gain 999, 10 MHz readout speed, 50-100 msec exposure time.
- Focus at the surface of the coverslip, close the illumination shutter, move to a fresh field, open the illumination shutter and acquire images until all complexes have bleached (thereafter referred to as img(x,y)).
- Correct the acquired images for unevenness of illumination (Figure 3B).
- Prepare solution of any fluorophore, e.g. 1 µM Fluorescein isothiocyanate (FITC) in BRB-80. Such solution can be prepared in advance, aliquoted and stored at -20 °C.
- Assemble a chamber as in Section 7.1.1 but use a regular coverslip. Add fluorophore solution and seal the chamber using VALAP.
- Collect >50 images of the entire microscope field: move the stage to a new unbleached area while the illumination shutter is closed, and acquire the images immediately after opening the shutter.
- Create average projection of this stack and filter with Gaussian blur with 5 pixel radius using ImageJ or other software (Figure 3C). The resulting image represents the distribution of the illumination intensity of the field (Illum(x,y), where x and y correspond to pixel's coordinates).
- Determine the maximum pixel brightness of this image (Max(Illum)).
- With the closed illumination shutter and using same camera settings as in Section 7.2.3 acquire one image, determine the average pixel intensity of this image; this value corresponds to CN, camera noise.
- Use the above values and image (Illum(x,y)) to normalize the experimental image (img(x,y)) using the following expression:
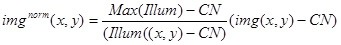
Use the resulting image imgnorm(x,y) for the quantitative analysis of the brightness of the stationary fluorescent complexes, and also to normalize the images with tip-tacking complexes (Figure 3D).
- Determine intensity of a single fluorophore.
- Using normalized images imgnorm(x,y) and any image processing software select a fluorescent spot with a circular region (5-6 pixels in diameter) and determine its integral intensity for all frames, generating the photobleaching traces. Avoid very bright dots (> 5-fold brighter than the dimmer ones).
- Using the same circular region tool, select at least 3 spot-free areas, generate the corresponding photobleaching traces, average and fit with exponential decay function.
- Tabulate this background intensity curve to match the experimental time points and subtract it from the photobleaching curves.
- Smooth the photobleaching curves (average with the sliding window of 3-5 points). Visually inspect the resulting curves and discard any curve that shows an abrupt increase in fluorescence or lack of obvious bleaching (Figure 3E).
- For each of the remaining curves (usually 50-70% of the total number of curves), visually select the final plateau, when the fluorescent spot has bleached. Shorten this segment to leave only ~100 points and average these intensities. Subtract this value from the shortened photobleaching curve to minimize small variations is the background levels and to reduce the size of the background peak (below).
- Plot a histogram of the intensities for all time points from 20 or more photobleaching curves (>1,000 time points). The histogram should exhibit at least 4 distinct peaks (see Note 2).
- Fit the histogram with equidistant Gaussian distribution10,43 using MATLAB, Mathematica or similar software (Figure 3F):
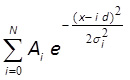
where Ai and σi , d and N are fitting parameters. Parameters Ai and σi correspond to amplitude and width of i-th peak; d is distance between peaks; N is integer number, which corresponds to the total number of peaks in the distribution. If centers of the first 3 or more peaks show a visually good match to the fitted line, the distance between these peaks (parameter d) corresponds to a fluorescent intensity of a single fluorophore.
Note 1: Number of examined dots (Section 7.3.6) should be increased if the microscopy system exhibits significant vibrations or there is another source of noise (e.g. unstable laser beam).
Note 2: It is essential to obtain >3 peaks for accurate analysis with equidistant Gaussian fit. If fewer peaks are obtained, the false (e.g. double) step size could be obtained when the illumination conditions are not optimal, e.g. when the dots bleach too fast and single steps are not resolved well.
- Determine the molecular size of the tip-tracking complex.
- Use images collected with Protocol 6 and select the first 2-4 frames, which were acquired immediately after opening the shutter. If the field was illuminated for some time before the tip-tracking was observed, estimate the original intensity from the kinetics of photobleaching under the same experimental conditions.
- Average the selected frames and normalize the resulting image as in Sections 7.2.5-7.2.7.
- Measure integral intensity of the fluorescent tip-tracking complex using the same region size as in Section 7.3.1.
- Measure integral intensity of 3 background areas located near the tip-tracking complex and using the same region, average these values and subtract from the intensity of the tip-tracking complex from Section 7.4.3.
- Calculate the number of fluorophore molecules in the complex by dividing the fluorescent intensity obtained in Section 7.4.4 by the intensity of the single fluorophore obtained in Section 7.3.5.
Note 1: It is desirable that the illumination and acquisition settings for protocol 7.4 are the same as in protocol 7.1. If either the exposure time or laser intensity was adjusted during these steps, the resulting fluorescent values should be scaled accordingly. However, the accuracy of such scaling should be verified by imaging the same sample (e.g. fluorescent time) under these different conditions and calculating the ratio of the resulting intensities.
8. Microtubule Tip-tracking by the Protein Coated Beads
- Carry out experiments with tip-tracking beads by triggering MT disassembly as in protocol 6. Intensity of DIC light source should be reduced to allow viewing Rhodamine fluorescence simultaneously with DIC imaging.
- Prepare the beads as in Grishchuk et al.10 and Asbury et al.11 Introduce 30-50 μl of bead suspension into the chamber at 10 μl/min. The suggested bead concentration is 10-16-10-17 M.
- If using upright microscope, remove the chamber from the microscope stage and invert it for 5-10 min to allow beads to sediment at the coverslip. This promotes a better binding of the bead to the microtubules tethered to the coverslip, but this procedure is not successful with 0.5 μm polystyrene beads, which show little gravity-based sedimentation during such a short time.
- Select a bead that is attached to the coverslip-tethered microtubule; the bead should move in a clear arc44 (Figure 4A). The tethered bead should be located 1-3 μm away from the coverslip surface, which is clearly visible owing to the occasional coverslip-attached beads that remain motionless.
- Switch to Rhodamine filter cube and begin collecting images while using DIC illumination.
- Open shutter to illuminate the imaging field (restricted with a field diaphragm) with a mercury lamp or 530-550 nm laser. Continue recording until the bead detaches or moves with the disassembling microtubule end (Figures 4D, 4F, and 4G).
Note 1: Optical trap can be used to promote interaction between the microtubule wall and protein-coated bead. This is especially useful when working with beads coated with kinesin motors (Figures 4E-G). Follow same protocols as above but supplement motility buffer with 2 mM Mg-ATP. In step 8.3, capture a free floating bead with the 1,064 nm laser beam, move the stage to bring the trapped bead closer to the segmented microtubule wall. Begin imaging with low light DIC and via the Rhodamine filter cube and wait for the bead to begin walking toward the capped microtubule end. After the directed bead motion is observed, close the shutter for trapping beam and open the shutter for fluorescent illumination. Continue recording until the bead detaches or tracks with the disassembling microtubule end.
Results
Protein tracking with depolymerizing microtubule ends. Yeast kinetochore component Dam1 is by far the best tip-tracker of the depolymerizing microtubule ends14. This 10-subunit complex labeled with GFP can be readily expressed and purified from bacterial cells18,38, so we recommend using it as a positive control for the tip-tracking assay. A fluorescent protein that tracks with the depolymerizing end of a microtubule is seen as a bright fluorescent spot steadily moving towards the c...
Discussion
Many single molecule assays nowadays routinely use specially treated coverslips to drastically reduce nonspecific protein sticking. The procedure we describe here is a modification of the original protocol developed in Howard lab32, and we find that silanizing the coverslips is well worth the effort even with DIC-based bead assays, which do not use fluorescence. Chambers assembled with such coverslips show much cleaner surfaces, and the results obtained in the presence of soluble microtubule-binding protein ar...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank F. I. Ataullakhanov for helping to design and manufacture reusable flow chambers, N. Dashkevich, N. Gudimchuk and A. Korbalev for providing images for figures, N. Gudimchuk and P. Zakharov for developing a protocol and providing reagents to prepare digoxigenin-labeled microtubule seeds, A. Potapenko for help with text editing and other members of Grishchuk lab for tips and discussions. This work was supported in part by NIH grant GM R01-098389 and a pilot grant from Pennsylvania Muscle Institute to E.L.G., who is a Kimmel Scholar, by RFBR grants 12-04-00111-a, 13-04-40190-H and 13-04-40188-H, Russian Academy of Sciences Presidium Grants (Mechanisms of the Molecular Systems Integration and Molecular and Molecular Cell Biology programs) to F. I. Ataullakhanov, NIH grant GM R01 GM033787 to J.R. McIntosh, and a Dmitry Zimin Dynasty Foundation postdoctoral fellowship to V.A.V.
Materials
| Name | Company | Catalog Number | Comments |
| Table 1. Microscopy and other equipment. | |||
| Microscope | Zeiss Nikon | Axio Imager 2 Eclipse Ti | other microscope models capable of DIC and epifluorescence-imaging can be used |
| Objective | Zeiss Nikon | 420490-9900-000 CFI Apo 100x Oil 1.49 | 100X, DIC, 1.3-1.49 NA |
| Objective heater | Bioptechs | 150803, 150819-19 | |
| Fluorescent filter cube | Chroma | 49004 or 49008 41017 or 49020 | optimized for Rhodamine fluorescence optimized for GFP fluorescence |
| Acquisition software | freeware MicroManager Molecular Devices | not applicable MetaMorph 7.5 | http://valelab.ucsf.edu/~MM/MMwiki/ other software can be used to acquire images and for a particle tracking |
| EMCCD camera | Andor | iXon3, DU-897E-cs0-#BV | Highly sensitive EMCCD camera |
| Trapping laser | IPG Photonics | YLR-10-1064-LP | 1,064 nm laser, 10 W |
| Fluorescence excitation lasers | Coherent, Inc. Coherent, Inc. | Sapphire 488 LP Sapphire 552 LP | excitation of green fluorophores excitation of red fluorophores |
| Plasma Cleaner | Harrick Plasma | PDC-001 | |
| Commercial flow chambers | Warner Instruments | RC-20 or RC-30 | |
| Perfusion pump | Cole Palmer Harvard Apparatus | Masterflex 77120-52 Pico Plus | Both pumps provide the required rate of liquid flow but a peristaltic pump may pulse at very slow speed. The flow with a syringe pump is more consistent for a wide range of rates but this pump has inertia. |
| Table 2. Microscopy chamber preparation. | |||
| Modified microscope slides for reusable chambers | Precision Glassblowing of Colorado | Custom order www.precisionglassblowing.com | Sonic slots in slides using schematics in Figure 1 |
| Polyethylene tubing | Intramedic | 427410 | I.D. 0.58 mm, O.D. 0.965 mm; use these tubes to connect assembled chamber to the pump and waste container |
| Polyethylene tubing | Intramedic | 427400 | I.D. 0.28 mm, O.D. 0.61 mm; use these tubes to make the reusable chamber |
| Regular microscope slides | VWR | 48312-003 | Other similar slides can be used |
| Coverslips | VWR | 48393-150, 48366-067 | Other similar coverslips can be used |
| Silicon sealant | World Precision Instruments | KIT, SILICON SEALANT 5 MIN CURE | |
| Epoxy glue | Loctite | 83082 | |
| Cyanoacrylate adhesive | Scotch 3M | AD114 | Or cyanoacrylate adhesive from other manufacturers |
| Table 3. Coverslips cleaning and coating. | |||
| Molecular Sieves, Grade 564 | Macron | 4490-04 | |
| Coverglass Staining Jar | Ted Pella, Inc. | 21036 | |
| Coverslip Ceramic Holder | Thomas Scientific | 8542e40 | |
| PlusOne Repel Silane | GE Healthcare Biosciences | 17-1332-01 | |
| Pluronic F-127 | Sigma-Aldrich | P2443 | |
| Anti-digoxigenin AB | Roche Applied Science | 11093274910 | |
| Table 4. Preparation of seeds and segmented microtubules. | |||
| Tubulin | purified from cow brains Cytoskeleton, Inc | T238P | For purification protocols see 49–51 Unlabeled porcine tubulin |
| Labeled tubulin | Cytoskeleton, Inc Invitrogen Invitrogen | TL590M C1171 (Rhodamine) A-2952 (Digoxigenin) | Rhodamine-labeled porcine tubulin Tubulin can be labeled with any amine-reactive dye as in reference52. |
| GMPCPP | Jena Biosciences | NU-405 | Aliquot and store at -70 °C |
| VALAP | Vaseline, lanolin, and paraffin at 1:1:2 by mass | see reference9 | |
References
- Desai, A., Mitchison, T. J. Microtubule polymerization dynamics. Ann. Rev. Cell Dev. Biol. 13, 83-117 (1997).
- Mitchison, T. M., Kirschner, M. W. Dynamic instability of microtubule growth. Nature. 312 (15), 237-242 (1984).
- Walker, R. A., Brien, O., et al. Dynamic Instability of Individual Microtubules Analyzed by Video Light Microscopy: Rate Constants and Transition Frequencies. J. Cell Biol. 107, 1437-1448 (1988).
- Gardner, M. K., Zanic, M., Gell, C., Bormuth, V., Howard, J. Depolymerizing Kinesins Kip3 and MCAK Shape Cellular Microtubule Architecture by Differential Control of Catastrophe. Cell. 147 (5), 1092-1103 (2011).
- Lombillo, V. A., Stewart, R. J., McIntosh, J. R. Minus-end-directed motion of kinesin-coated microspheres driven by microtubule depolymerization. Nature. 373, 161-164 (1995).
- Franck, A. D., Powers, A. F., Gestaut, D. R., Gonen, T., Davis, T. N., Asbury, C. L. Tension applied through the Dam1 complex promotes microtubule elongation providing a direct mechanism for length control in mitosis. Nat. Cell Biol. 9 (7), 832-837 (2007).
- Tran, P. T., Walker, R. A., Salmon, E. D. A metastable intermediate state of microtubule dynamic instability that differs significantly between plus and minus ends. J. Cell Biol. 138 (1), 105-117 (1997).
- Grishchuk, E. L., Molodtsov, M. I., Ataullakhanov, F. I., McIntosh, J. R. Force production by disassembling microtubules. Nature. 438, 384-388 .
- Coue, M., Lombillo, A., Richard, J. Microtubule Depolymerization Promotes Particle and Chromosome Movement In Vitro. J. Cell Biol. 112 (6), 1165-1175 (1991).
- Grishchuk, E. L., Spiridonov, I. S., et al. Different assemblies of the DAM1 complex follow shortening microtubules by distinct mechanisms. Proc. Natl. Acad. Sci. U.S.A. 105 (19), 6918-6923 (2008).
- Asbury, C. L., Gestaut, D. R., Powers, A. F., Franck, A. D., Davis, T. N. The Dam1 kinetochore complex harnesses microtubule dynamics to produce force and movement. Proc. Natl. Acad. Sci. U.S.A. 103 (26), 9873-9878 (2006).
- Westermann, S., Wang, H. -. W., Avila-Sakar, A., Drubin, D. G., Nogales, E., Barnes, G. The Dam1 kinetochore ring complex moves processively on depolymerizing microtubule ends. Nature. 440 (7083), 565-569 (2006).
- Grissom, P. M., Fiedler, T., Grishchuk, E. L., Nicastro, D., West, R. R., Mcintosh, J. R. Kinesin-8 from Fission Yeast A Heterodimeric , Plus-End – directed Motor that Can Couple Microtubule Depolymerization to Cargo Movement. Mol. Biol. Cell. 20, 963-972 (2009).
- McIntosh, J. R., Volkov, V., Ataullakhanov, F. I., Grishchuk, E. L. Tubulin depolymerization may be an ancient biological motor. J. Sci. 123, 3425-3434 (2010).
- Grishchuk, E. L., McIntosh, J. R., Molodtsov, M. I., Ataullakhanov, F. I. Force generation by dynamic microtubule polymers. Compr. Biophys. 4, 93-117 (2012).
- Asbury, C. L., Tien, J. F., Davis, T. N. Kinetochores' gripping feat: conformational wave or biased diffusion. Trends Cell Biol. (1), 38-46 (2011).
- Tien, J. F., Umbreit, N. T., et al. Cooperation of the Dam1 and Ndc80 kinetochore complexes enhances microtubule coupling and is regulated by aurora B. Cell Biol. 189 (4), 713-723 (2010).
- Gestaut, D. R., Graczyk, B., et al. Phosphoregulation and depolymerization-driven movement of the Dam1 complex do not require ring formation. Nat. Biol. 10 (4), 407-414 (2008).
- Schmidt, J. C., Arthanari, H., et al. The Kinetochore-Bound Ska1 Complex Tracks Depolymerizing Microtubules and Binds to Curved Protofilaments. Dev. Cell. 23 (5), 968-980 (2012).
- Brouhard, G. J., Stear, J. H., et al. XMAP215 is a processive microtubule polymerase. Cell. 132 (1), 79-88 (2008).
- Stumpff, J., Du, Y., et al. A Tethering Mechanism Controls the Processivity and Kinetochore-Microtubule Plus-End Enrichment of the Kinesin-8 Kif18A. Mol. Cell. 43 (5), 764-775 (2011).
- Su, X., Qui, W., Gupta, M., Pereira-Leal, J., Reck-Peterson, S. L., Pellman, D. Mechanisms underlying the dual-mode regulation of microtubule dynamics by Kip3/kinesin-8. Mol. Cell. 43 (5), 751-763 (2011).
- Helenius, J., Brouhard, G., Kalaidzidis, Y., Diez, S., Howard, J. The depolymerizing kinesin MCAK uses lattice diffusion to rapidly target microtubule ends. Nature. 441 (7089), 115-119 (2006).
- Gudimchuk, N., Vitre, B., et al. Kinetochore kinesin CENP-E is a processive bi-directional tracker of dynamic microtubule tips. Nat. Cell Biol. 15 (9), 1079-1088 (2013).
- Akhmanova, A., Steinmetz, M. Microtubule +TIPs at a glance. J. Sci. 20 (Pt 20), 3415-3419 (2010).
- Dixit, R., Barnett, B., Lazarus, J., Tokito, M., Goldman, Y., Holzbaur, E. Microtubule plus-end tracking by CLIP-170 requires EB1. Proc. Natl. Acad. Sci. U.S.A. 106 (2), 492-497 (2009).
- Grishchuk, E. L., Efremov, A. K., et al. The Dam1 ring binds microtubules strongly enough to be a processive as well as energy-efficient coupler for chromosome motion. Proc. Natl. Acad. Sci. U.S.A. 105 (40), 15423-15428 (2008).
- Akiyoshi, B., Sarangapani, K. K., et al. Tension directly stabilizes reconstituted kinetochore-microtubule attachments. Nature. 468 (7323), 576-579 (2010).
- McIntosh, J. R., Grishchuk, E. L., et al. Fibrils Connect Microtubule Tips with Kinetochores A Mechanism to Couple Tubulin Dynamics to Chromosome Motion. Cell. 135 (2), 322-333 (2008).
- Powers, A. F., Franck, A. D., et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell. 136 (5), 865-875 (2009).
- Umbreit, N. T., Gestaut, D. R., et al. The Ndc80 kinetochore complex directly modulates microtubule dynamics. Proc. Natl. Acad. Sci. U.S.A. 109 (40), 16113-16118 (2012).
- Gell, C., Bormuth, V., et al. Microtubule Dynamics Reconstituted In Vitro and Imaged by Single-Molecule Fluorescence Microscopy. Methods Biol. 95, 221-245 (2010).
- Dixit, R., Ross, J. L. Studying Plus-End Tracking at Single Molecule Resolution Using TIRF Microscopy. Methods Cell Biol. 95, 543-554 (2010).
- Beausang, F. J., Sun, Y., Quinlan, E. M., Forkey, N. J., Goldman, Y. Construction of Flow Chambers for Polarized Total Internal Reflection Fluorescence Microscopy (polTIRFM). Cold Spring Harbour Protoc. 6, 712-715 (2012).
- Hyman, A. A., Salser, S., Drechsel, D. N., Unwin, N., Mitchison, T. J. Role of GTP Hydrolysis in Microtubule Dynamics: Information from a Slowly Hydrolyzable Analogue, GMPCPP. Mol. Biol. Cell. 3, 1155-1167 (1992).
- Grishchuk, E. L., Ataullakhanov, F. I. In Vitro Assays to Study the Tracking of Shortening Microtubule Ends and to Measure Associated Forces. Methods Cell Biol. 95, 657-676 (2010).
- Gutiérrez-Medina, B., Block, S. M. Visualizing individual microtubules by bright field microscopy. Am. J. Phys. 78 (11), 1152-1159 (2010).
- Volkov, V. A., Zaytsev, A. V., et al. Long tethers provide high-force coupling of the Dam1 ring to shortening microtubules. Proc. Natl. Acad. Sci. U.S.A. 110 (19), 7708-7713 (2013).
- Laan, L., Pavin, N., et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 148 (3), 502-514 (2012).
- Myster, S. H., Knott, J. A., O'Toole, E., Porter, M. E. The Chlamydomonas Dhc1 gene encodes a dynein heavy chain subunit required for assembly of the I1 inner arm complex. Mol. Biol. Cell. 8, 607-620 (1997).
- Lombillo, V. A., Coue, M., McIntosh, J. R. In vitro motility assays using microtubules tethered to Tetrahymena pellicles. Methods Cell Biol. 39, 149-165 (1993).
- Hyman, A., Chrétien, D., Arnal, I., Wade, R. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(alpha,beta)-methylene-diphosphonate. J. Cell Biol. 128 (1-2), 117-125 (1995).
- Park, M., Kim, H., Kim, D., Song, N. W. Counting the Number of Fluorophores Labeled in Biomolecules by Observing the Fluorescence-Intensity Transient of a Single Molecule. Bull. Chem. Soc. Jap. 78, 1612-1618 (2005).
- Welburn, J. P. I., Grishchuk, E. L., Backer, C. B., Wilson-Kubalek, E. M., Yates, J. R., Cheeseman, I. M. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev. Cell. 16 (3), 374-385 (2009).
- Efremov, A., Grishchuk, E. L., Mcintosh, J. R., Ataullakhanov, F. I. In search of an optimal ring to couple microtubule depolymerization to processive chromosome motions. Natl. Acad. Sci. U.S.A. (48), 19017-19022 (2007).
- Itoh, T., Hisanaga, S., Hosoi, T., Kishimoto, T., Hotani, H. Phosphorylation states of microtubule-associated protein 2 (MAP2) determine the regulatory role of MAP2 in microtubule dynamics. Biochemistry. 36 (41), 12574-12582 (1997).
- Oguchi, Y., Uchimura, S., Ohki, T., Mikhailenko, S. V., Ishiwata, S. The bidirectional depolymerizer MCAK generates force by disassembling both microtubule ends. Nat. Biol. (6), 1-8 (2011).
- Kishino, A., Yanagida, T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 334, 74-76 (1988).
- Borisy, G. G., Marcum, J. M., Olmsted, J. B., Murphy, D. B., Johnson, K. A. Purification of tubulin and associated high molecular weight proteins from porcine brain and characterization of microtubule assembly in vitro. Ann. NY Acad. Sci. 253, 107-132 (1975).
- Weingarten, M. D., Lockwood, A. H., Hwo, S., Kirschner, M. W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. U.S.A. 72 (5), 1858-1862 (1975).
- Widlund, P. O., Podolski, M., et al. One-step purification of assembly-competent tubulin from diverse eukaryotic sources. Mol. Biol. Cell. 23 (22), 4393-4401 (2012).
- Hyman, A., Drechsel, D., et al. Preparation of Modified Tubulins. Methods Enzymol. 196, 478-485 (1991).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved