A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
In Vivo Electrophysiological Measurements on Mouse Sciatic Nerves
In This Article
Summary
Measurements of nerve conduction properties in vivo exemplify a powerful tool to characterize various animal models of neuromuscular diseases. Here, we present an easy and reliable protocol by which electrophysiological analysis on sciatic nerves of anesthetized mice can be performed.
Abstract
Electrophysiological studies allow a rational classification of various neuromuscular diseases and are of help, together with neuropathological techniques, in the understanding of the underlying pathophysiology1. Here we describe a method to perform electrophysiological studies on mouse sciatic nerves in vivo.
The animals are anesthetized with isoflurane in order to ensure analgesia for the tested mice and undisturbed working environment during the measurements that take about 30 min/animal. A constant body temperature of 37 °C is maintained by a heating plate and continuously measured by a rectal thermo probe2. Additionally, an electrocardiogram (ECG) is routinely recorded during the measurements in order to continuously monitor the physiological state of the investigated animals.
Electrophysiological recordings are performed on the sciatic nerve, the largest nerve of the peripheral nervous system (PNS), supplying the mouse hind limb with both motoric and sensory fiber tracts. In our protocol, sciatic nerves remain in situ and therefore do not have to be extracted or exposed, allowing measurements without any adverse nerve irritations along with actual recordings. Using appropriate needle electrodes3 we perform both proximal and distal nerve stimulations, registering the transmitted potentials with sensing electrodes at gastrocnemius muscles. After data processing, reliable and highly consistent values for the nerve conduction velocity (NCV) and the compound motor action potential (CMAP), the key parameters for quantification of gross peripheral nerve functioning, can be achieved.
Introduction
Electrophysiological measurements are an indispensable tool for investigating the functional integrity of peripheral nerves in both clinical and laboratory environments. In humans, a large number of neuromuscular disorders and neuropathies diagnostically rely on electrophysiological measurements. By measuring nerve properties as conduction velocity or potential amplitudes of the signal, it is possible to characterize the rough origin of peripheral nerve diseases.
The nerve conduction velocity is highly dependent on rapid signal propagation enabled by myelination. Therefore, demyelinating processes generally show decreased conduction velocities4. The compound motor action potential (CMAP) - correlating with the number of functional axons - is an indicator for axonal damage when significantly reduced5.
Thus, by means of electrophysiological methods the etiology of peripheral nerve damage can be discriminated, such as for hereditary neuropathies6,7, diabetic neuropathy8,9, chronic inflammatory demyelinating polyneuropathies (CIDP)10, or metabolic neuropathies11.
Normally, in the human application noninvasive recordings on the sural or ulnar nerve are preferred. In mice, it is straightforward to analyze nerve properties of sciatic nerves, the largest nerve of the peripheral nervous system (PNS) containing both large - and small-caliber axons of the motoric and sensory system.
The procedure as demonstrated here is a quick, easy and reliable method to measure all standard values relevant for electrophysiology on peripheral nerves in the intact mouse. By taking recordings from a preserved organism, physiological conditions of the nerve environment are guaranteed.
Protocol
The present study was performed according to the Protection of Animals Act of the Federal Republic of Germany (Tierschutzgesetz der Bundesrepublik Deutschland) and was approved by the Thuringian State Office for Food Safety and Consumer Protection (Thüringer Landesamt für Lebensmittelsicherheit und Verbraucherschutz).
1. Setting Up the Measurements
- Anesthetize the mice by isoflurane/O2 inhalation - for initiation of anesthesia 3%, for maintenance 2% isoflurane in 100% oxygen (Figure 1). Confirm sufficient anesthesia by testing simple reflexes such as movement reflexes and testing of sensitivity for low-grade pain. Use of ointment on the eyes to prevent dryness during anesthesia is recommended but not indispensable because the procedure typically takes just 30 min/animal in total. In case of survival experiments, administer timely longer acting analgesics for managing postoperative pain.
- Shave the fur covering the hind limbs with an electric razor and perform depilation with a commercially available hair-removal cream while animals are already under analgesia. In order to maintain pathogen-free status throughout the procedure, wear aseptic gloves and always use instruments carefully cleaned with 70% ethanol.
- Control body temperature stability by a feedback controlled heating plate and rectal thermo probe (Figure 2). If necessary, please use a sterile drape to cover the heating plate between the animals in order to keep a sterile experimental environment. Furthermore, it is recommended to use a heating plate, which is electronically controlled via an integrated sensor to limit the heating temperature to ≤40 °C in order to avoid tissue damage.
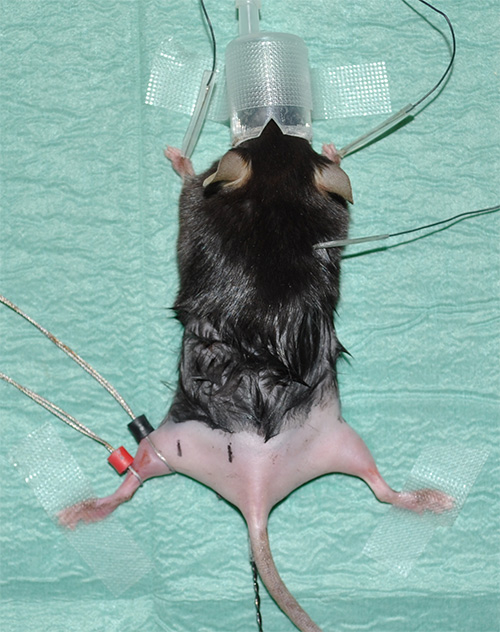
Figure 1. Experimental setup showing an anesthetized mouse with shaved hind limbs. - Take electrocardiography (ECG) recordings to monitor the heart rate as a vital parameter. Install three electrodes for ECG recordings as follows: one electrode under the skin of each forelimb and one electrode under the skin in the neck area.
- Place ring electrodes using contact gel for optimal conductivity / transfer resistance. The sensing electrode (labeled in black) is placed at the position where the gastrocnemius muscle has its maximum diameter. The reference electrode (indicated in red) is placed just beneath the sensing electrode.
Note: Please see 'Material table' for equipment details. - Mark the correct positions of measurement with predetermined but consistent distances between the proximal and distal sciatic nerve stimulation and the lead electrode. Experimental proposal: At a distance of 4 mm from the sensing electrode, the distal stimulation will take place. In a distance of 16 mm to the sensing electrode, the proximal stimulation will be carried out (Figure 2).

Figure 2. Representative picture showing the experimental situation just prior to the beginning of the measurements. The white arrow indicates the position of the sensing (black) and reference (red) electrode at the gastrocnemius muscle of the left hind limb. The stimulation by needle electrodes will be performed at defined positions in relation to the black sensing electrode. The point of distal stimulation (black mark with "d.s." at the left hind limb) has a distance of 4 mm from the sensing electrode; the place of proximal stimulation (black mark with "p.s.") is 16 mm away. The red line on the right hind limb shows the approximate anatomical course of the sciatic nerve. Furthermore, the rough positions of relevant hind limb muscles are shown as landmarks. The asterisk indicates the rectal thermal probe.
2. Measurement
- Principle: Perform a series of nerve stimulations with repetitively generated single square-wave pulses of 0.1 msec duration by monopolar disposable 28 G needle electrodes (repetition rate 200 msec; see Figure 3A). For off-line data analysis, it is recommended to simultaneously acquire stimulation signals together with the neuromuscular function response curve (Figure 3B) due to nerve stimulation. Average a series of maximal responses ("representative neuromuscular function response") for subsequent data analysis. In order to produce reliable data, record and later average at least 3 independent, optimal response curves per stimulation site and animal.
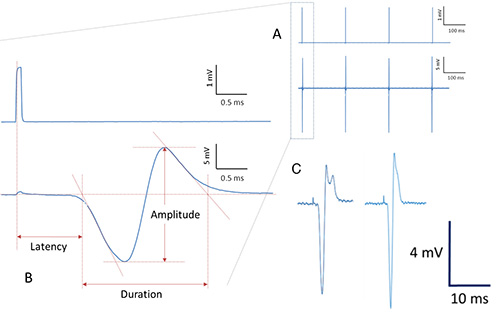
Figure 3. Procedure for data acquisition and analysis (schematic presentation). Repetitively generated pulses are applied to the sciatic nerve via needle electrodes (upper row in Figure 3A). Simultaneously, several corresponding neuromuscular response curves are recorded (lower row in Figure 3A). When averaged and magnified, neuromuscular response curves due to stimulation pulses (upper row in Figure 3B) show the following characteristic properties (lower row in Figure 3B): Latency of the signal response as well as duration and amplitude of the signal are indicated and can be obtained for subsequent calculations and statistics. Irregular signal conduction and / or suboptimal recordings usually result in various signal deformations with more than just one positive and one negative deflection or signal deformation (bimodal shape) with reduced amplitude (Figure 3C). - Perform proximal stimulation with a needle electrode at determined position ("p.s." in Figure 2).
- In order to achieve best recording conditions with maximum amplitudes, visualize the actual response curves simultaneous to the stimulation process. By doing so, the experimenter is able to immediately assess the shape of the response curves as well as the size of the amplitude.
- If necessary, slightly and carefully manipulate the position and / or the angle of the stimulation needle with respect to the sciatic nerve. This gentle optimization of stimulation conditions allows reaching constant amplitudes with the largest possible value and a response curve of typical biphasic shape (Figure 4).
- Perform distal stimulation with a needle electrode at determined position ("d.s." in Figure 2).
- After completion of measurement, transfer the tested mouse to a separate cage until it has regained sufficient consciousness to maintain sternal recumbency. Do not leave an animal unattended and in the company of other animals until it has fully recovered from anesthesia. Administer timely longer acting analgesics for managing postoperative pain. Systemic administration of nonsteroidal anti-inflammatory drugs (NSAIDs) and opioids are recommended for 1-3 days.
- Alternatively, sacrifice the still anesthetized mouse in a quick and humane fashion without any further pain for the animal, e.g. by neck dislocation.
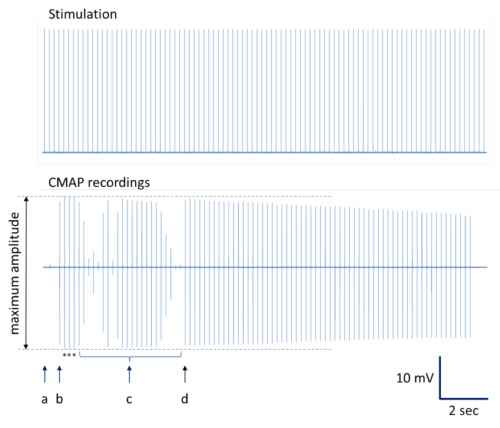
Figure 4. Illustration to determine the CMAP recordings with maximum amplitudes. A complete registration series is presented. (a) Insertion point with minimal CMAP response. (b) Slight stimulation needle movement results in CMAP recordings with maximum amplitudes. (c) Additional changes in needle placement produce CMAP recordings with different amplitudes including near-maximum amplitudes. (d) Stimulation needle replacement with serial CMAP recordings of near-maximum amplitudes. Note: Typical decrement in CMAP amplitudes can occur during repetitive stimulation at optimal stimulation site12,13. Asterisks indicate CMAP recordings with maximum amplitudes depicted for averaging.
3. Analysis
- Extract nerve conduction parameters based on the representative neuromuscular function response data set using an appropriate software package (e.g. AtisaPro).
Note: Please handle time-related data determination with special care because inflection point determination of compound motor action potential (CMAP) onset and termination can be difficult. A procedure with verified reproducibility is given in Figure 3B, where tangents on signal deflections after onset and before termination are used. - Calculate 'signal latency' and 'CMAP' values.
- Latency' represents the time delay between stimulation and CMAP onset, whereas the time span between CAMP onset of initial negative deflection to initial return to baseline is called 'CMAP duration'. Use the difference between distal and proximal latencies to calculate conduction velocity together with the distance between distal and proximal stimulation sites.
conduction velocity = latency / distance between stimulation sites - CMAP' (compound motor action potential) amplitude represents the magnitude between maximum positive and negative turnaround point of the CMAP signal (given in mV).
CMAP = value 'positive turnaround point' - value 'negative turnaround point'
- Latency' represents the time delay between stimulation and CMAP onset, whereas the time span between CAMP onset of initial negative deflection to initial return to baseline is called 'CMAP duration'. Use the difference between distal and proximal latencies to calculate conduction velocity together with the distance between distal and proximal stimulation sites.
Results
We conducted a series of in vivo electrophysiological measurements on sciatic nerves of 12 mice in total for this study: 6 animals of each gender. The measurements were performed with the presented protocol and delivered the following results:
Both male and female mice display a mean sciatic nerve conduction velocity of approximately 20 m/sec (Figure 5). This is consistent with other measurements in the literature. Furthermore, it shows that there are no relevant diff...
Discussion
The described protocol provides an easy and reliable method to determine sciatic nerve conduction properties on anesthetized mice without the need to expose the nerve of interest. Nevertheless, this experimental procedure causes tissue injury by needle puncture. It is therefore a reasonable option to sacrifice the animals after finishing the recordings. However, compared to other more invasive procedures, which require the exposure of the nerve prior to recordings, tissue damage is comparably small 3,14. There...
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by SFB 604, DFG MO 1421/2-1 and Krebshilfe 107089 (to H.M.). A.S. is recipient of a Young Investigator Award from the Children’s Tumor Foundation (New York, USA).
Materials
| Name | Company | Catalog Number | Comments |
| Concentric Needle Electrodes (Stimulation) | Natus Medical Incorporated, San Carlos, CA, USA | 9013S0901 | |
| Digital Ring Electrodes (Recording) | Natus Medical Incorporated, San Carlos, CA, USA | 9013S0302 | |
| ToM - Tower of Measurement (A/D converter) | GJB Datentechnik GmbH, Langewiesen, Germany | ||
| AtisaPro, data acquisition and analysis software | GJB Datentechnik GmbH, Langewiesen, Germany | ||
| HSE-Stimulator T | Hugo Sachs Elektronik, Hugstetten, Germany |
References
- Kimura, J. 3rd ed. Electrodiagnosis in Diseases of Nerve and Muscle. , (2001).
- Rutkove, S. B. Effects of temperature on neuromuscular electrophysiology. Muscle Nerve. 24, 867-882 (2001).
- Xia, R. H., Yosef, N., Ubogu, E. E. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle Nerve. 41, 850-856 (2010).
- Zielasek, J., Martini, R., Toyka, K. V. Functional abnormalities in P0-deficient mice resemble human hereditary neuropathies linked to P0 gene mutations. Muscle Nerve. 19, 946-952 (1996).
- Raynor, E. M., Ross, M. H., Shefner, J. M., Preston, D. C. Differentiation between axonal and demyelinating neuropathies: identical segments recorded from proximal and distal muscles. Muscle Nerve. 18, 402-408 (1995).
- Pareyson, D., Scaioli, V., Laura, M. Clinical and electrophysiological aspects of Charcot-Marie-Tooth disease. Neuromol. Med. 8, 3-22 (2006).
- Schulz, A., et al. Merlin isoform 2 in neurofibromatosis type 2-associated polyneuropathy. Nat. Neurosci. 16, 426-433 (2013).
- Lamontagne, A., Buchthal, F. Electrophysiological studies in diabetic neuropathy. J. Neurol. Neurosurg. Psychiatry. 33, 442-452 (1970).
- Andersen, H., Nielsen, J. F., Nielsen, V. K. Inability of insulin to maintain normal nerve function during high-frequency stimulation in diabetic rat tail nerves. Muscle Nerve. 17, 80-84 (1994).
- Magda, P., et al. Comparison of electrodiagnostic abnormalities and criteria in a cohort of patients with chronic inflammatory demyelinating polyneuropathy. Arch. Neurol. 60, 1755-1759 (2003).
- Lindberg, R. L., et al. Motor neuropathy in porphobilinogen deaminase-deficient mice imitates the peripheral neuropathy of human acute porphyria. J. Clin. Invest. 103, 1127-1134 (1999).
- Massey, J. M. Electromyography in disorders of neuromuscular transmission. Sem. Neurol. 10, 6-11 (1990).
- Stalberg, E., Falck, B. The role of electromyography in neurology. Electroencephalogr. Clin. Neurophysiol. 103, 579-598 (1997).
- Osuchowski, M. F., Teener, J., Remick, D. Noninvasive model of sciatic nerve conduction in healthy and septic mice: reliability and normative data. Muscle Nerve. 40, 610-616 (2009).
- Oh, S. S., Hayes, J. M., Sims-Robinson, C., Sullivan, K. A., Feldman, E. L. The effects of anesthesia on measures of nerve conduction velocity in male C57Bl6/J mice. Neurosci. Lett. 483, 127-131 (2010).
- Dilley, A., Lynn, B., Pang, S. J. Pressure and stretch mechanosensitivity of peripheral nerve fibres following local inflammation of the nerve trunk. Pain. 117, 462-472 (2005).
- Vleggeert-Lankamp, C. L., et al. Electrophysiology and morphometry of the alpha- and beta-fiber populations in the normal and regenerating rat sciatic nerve. Exp. Neurol. 187, 337-349 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved