A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
A Novel Stretching Platform for Applications in Cell and Tissue Mechanobiology
In This Article
Summary
We present in this article a novel stretching platform that can be used to investigate single cell responses to complex anisotropic biaxial mechanical deformation and quantify the mechanical properties of biological tissues.
Abstract
Tools that allow the application of mechanical forces to cells and tissues or that can quantify the mechanical properties of biological tissues have contributed dramatically to the understanding of basic mechanobiology. These techniques have been extensively used to demonstrate how the onset and progression of various diseases are heavily influenced by mechanical cues. This article presents a multi-functional biaxial stretching (BAXS) platform that can either mechanically stimulate single cells or quantify the mechanical stiffness of tissues. The BAXS platform consists of four voice coil motors that can be controlled independently. Single cells can be cultured on a flexible substrate that can be attached to the motors allowing one to expose the cells to complex, dynamic, and spatially varying strain fields. Conversely, by incorporating a force load cell, one can also quantify the mechanical properties of primary tissues as they are exposed to deformation cycles. In both cases, a proper set of clamps must be designed and mounted to the BAXS platform motors in order to firmly hold the flexible substrate or the tissue of interest. The BAXS platform can be mounted on an inverted microscope to perform simultaneous transmitted light and/or fluorescence imaging to examine the structural or biochemical response of the sample during stretching experiments. This article provides experimental details of the design and usage of the BAXS platform and presents results for single cell and whole tissue studies. The BAXS platform was used to measure the deformation of nuclei in single mouse myoblast cells in response to substrate strain and to measure the stiffness of isolated mouse aortas. The BAXS platform is a versatile tool that can be combined with various optical microscopies in order to provide novel mechanobiological insights at the sub-cellular, cellular and whole tissue levels.
Introduction
The mechanical microenvironment plays an important role in many cell functions such as proliferation, migration, and differentiation, which have a profound impact in the development and homeostasis of tissues and also in diseases1-6. Over the years, a multitude of experimental tools have been used to mechanically stimulate cells or tissues and measure mechanical properties of biological tissues with the goal of increasing our understanding of basic mechanobiology and studying the onset and progression of diseases6-17. However, one must often rely on several different experimental devices in order to achieve the goals of a particular study. This article presents a single, multi-functional, biaxial stretching (BAXS) platform that allows for studies that investigate the role that mechanical properties and mechanical forces play in biology at the sub-cellular to whole tissue length scales. The BAXS platform not only allows for the quantification of the mechanical properties of isolated tissues, but also facilitates the ability to apply simple, complex, and dynamic strain fields to living cells in order to understand their responses to stretching that occurs in vivo. The BAXS platform also maintains the capacity to perform live-cell microscopy during mechanical testing and perturbations on cells and tissues.
The BAXS platform is a custom-built apparatus that can be used to investigate the effect of substrate deformation at the cellular level and perform tensile tests on biological tissues (Figure 1A). An aluminum heater was fabricated to accommodate a standard 10-cm Petri dish and maintain any physiological solutions at 37 °C using a temperature controller and kapton heaters (Figure 1B). This BAXS platform can be integrated onto an inverted phase contrast and/or fluorescence microscope and allows for simultaneous imaging (Figure 1C). In brief, the BAXS platform consists of four linear voice coil motors of which the moving parts are mounted on miniature linear motion ball bearing slides oriented along two perpendicular axes (Figure 1D). A linear positioning stage is mounted to each of the four motors to allow vertical movement of the clamping system that will be used (Figure 1E). The position of each motor is monitored by an optical encoder with a resolution of 500 nm (Figure 1F). All four motors are independently controlled with a motion controller employing optical encoder feedback to execute motion commands (Figure 1G). A LabVIEW interface provides full control over the displacement magnitude, speed, and acceleration of each motor in order to generate completely customizable, static and dynamic, deformation of the cells or tissue samples.
The technique used to induce a deformation in cells is achieved by simply allowing cells to firmly adhere to a flexible and transparent substrate and then stretching this substrate using the four motors of the BAXS platform. The BAXS platform allows mounting of any custom-designed set of clamps to attach the substrate on the voice coil motors. For this purpose, we designed a set of clamps to which a flexible and transparent substrate, made of polydimethylsiloxane (PDMS), can be attached (Figures 2A-C and Figure 3). As the clamps will be exposed to physiological solutions, all parts were machined from stainless steel to allow for sterilization. These clamps have been carefully designed to bring the substrate as close as possible to the microscope objective to enhance image quality while minimizing the stress on the substrate during stretching (Figure 2D).
The same BAXS platform can also be used to quantify the stiffness of small tissue samples, using an appropriate set of clamps with adapted supports for the tissue samples and a load cell to monitor forces. Several approaches can be taken to mount a tissue to the BAXS platform motors; in this case the stainless steel insect minutiens pins can hook through the opening of vascular tissues in order to perform tensile tests (Figures 4A-B). Alternatively, for thick tissues without a natural opening, tissue edges can either be held in position with the clamps attached to the voice coil motors or glued to small glass slides with biological glue and attached to the motors with the clamps. In order to perform tensile tests a miniature load cell is required and can be easily incorporated onto the BAXS platform motors and used to measure the force acting on the tissue during a stretching cycle (Figure 4C). As the BAXS platform is composed of four motors, the introduction of a second load cell allows one to perform tensile testing along two orthogonal directions. This ability allows one to quantify the mechanical stiffness of a single tissue along two perpendicular directions during the same experiment.
Importantly, in all configurations, the cells or tissue samples of interest are always maintained in a temperature-controlled bath that is accessible to the user. This ability allows for the introduction of pharmacological agents during sample stretching in order to examine the temporal response of the sample. Additionally, as the optical axis of the inverted microscope remains unobstructed, all forms of microscopy are still available to the user. Finally, as all four motors of the BAXS platform are independent it is possible to apply highly configurable strain fields to the sample of interest. In vivo cells and tissues are exposed to complex and anisotropic stretching that can be more appropriately mimicked in this platform as opposed to traditional uniaxial stretching platform7,13,15,18,19. Moreover, the physical characteristics of the strain field can be changed on the fly during an experiment. These abilities allow the user to examine the cellular and tissue level response to a wide number of highly complex, anisotropic, temporally, and spatially varying strain fields. This article describes the advantages and limitations of the BAXS platform as well as its design, operating principles, and the experimental details for single cell and whole tissue experiments.

Figure 1. Overview of the BAXS platform. A) Top view of the BAXS platform showing the four voice coil motors. B) Detailed picture of the Petri dish heater used to maintain cells and tissues at 37 °C. C) The platform can be mounted on an inverted microscope to perform live-cell imaging during stretching experiments. D) Detailed picture of the voice coil motor; the moving part of the platform. E) Detailed picture of the linear positioning stage allowing vertical displacement of the clamping systems. F) Detailed picture of the optical encoder that provides real-time position of the motor to the motion controller. G) Detailed picture of the motion controller showing the four optical encoder inputs and power outputs to the four voice coil motors.
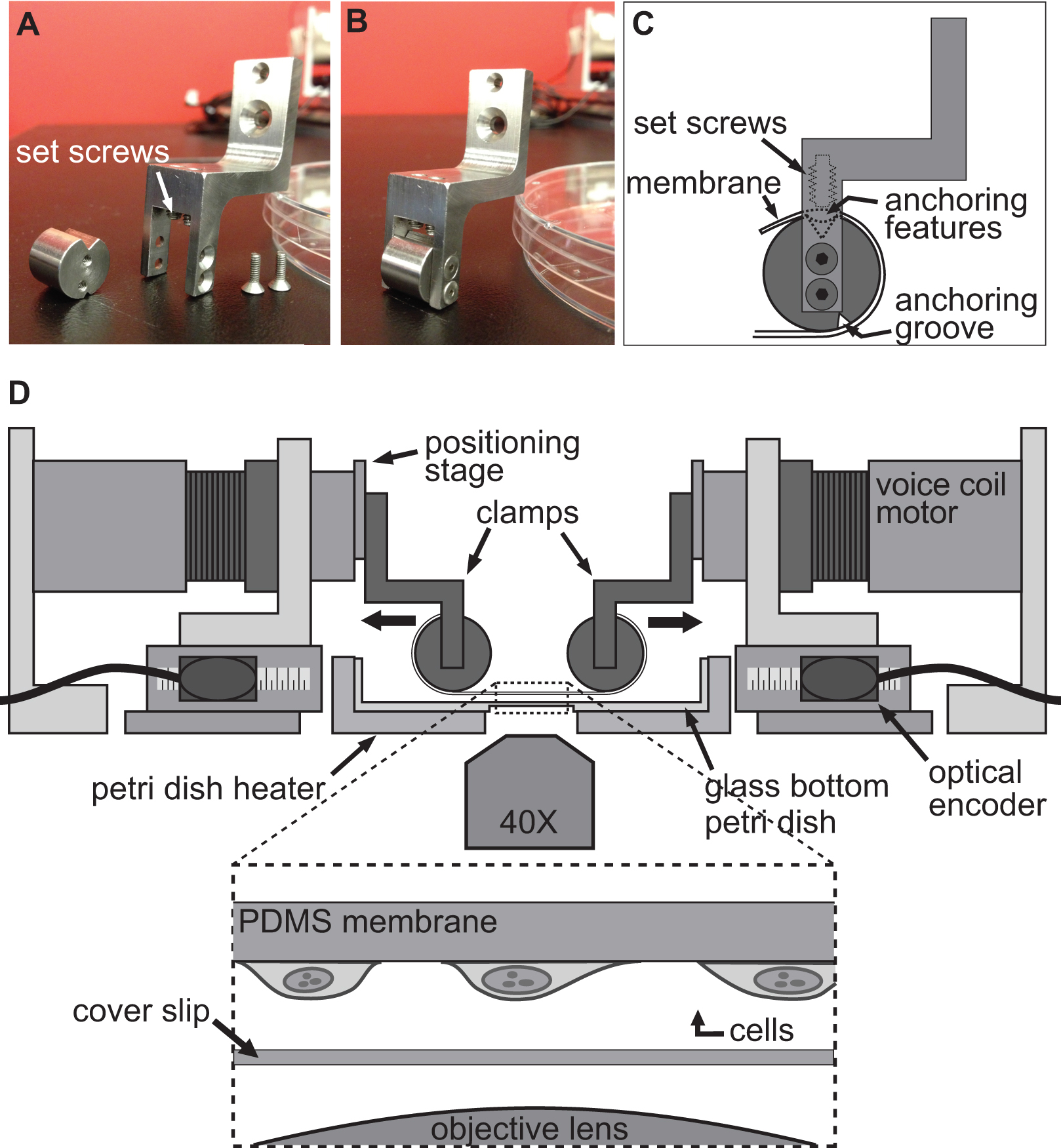
Figure 2. Clamping system for cell stretching experiments. A-B) Pictures showing the details of the clamps used to attach the PDMS substrate to the voice coil motors for stretching. C) The substrate is wrapped around the cylindrical part of the clamp with its anchoring features sitting into the groove at the top. Then the substrate is secured using the setscrews that push the substrate/anchoring features into the top groove. D) Illustration of the BAXS platform with the clamps holding the substrate in place. The inset shows a detailed view of the substrate with cells attached to it sitting just above a cover slip and the microscope objective.

Figure 3. Bill of materials of the membrane and its clamping system. Drawings showing the dimensions of the principal parts integrated to the biaxial platform to perform cell stretching experiments.

Figure 4. Example of a clamping system for stiffness assessment of small caliber vessels. A-B) Detailed pictures of the clamping system used to induce deformation in a 1 mm diameter mouse aorta. Stainless steel pins were carefully shaped into open triangles to allow the vessel to slide on both pins. C) Illustration of the BAXS platform with the clamps holding the vessel and a load cell attached between the fixed motor and the left clamp. The inset shows a detailed top-view of the vessel mounted on the pins.
Protocol
1. Mechanical Deformation of Single Cells
- Fabrication of a PDMS Substrate with Embedded Fluorescent Beads
Prior to fabrication of the substrate, fluorescent microspheres in water solution are resuspended in isopropanol to enhance bead mixing in the PDMS due to its hydrophobic nature.- Pipette 500 μl of fluorescent microspheres into a 1.5 ml microcentrifuge tube and centrifuge at 16,200 x g for 10 min.
- Discard the supernatant and add 500 μl of isopropanol followed with 5 min of vortexing. Put the vial aside overnight in the dark in order to allow any large particles aggregates to sediment.
- The next morning, carefully remove the supernatant to a clean microcentrifuge vial. This bead solution can be used to fabricate more than 5 substrates. NOTE: The bead solution will continue to sediment for the next 3 days. Be careful to avoid resuspending the pellet.
- Pour 0.5 g of the curing agent provided with the PDMS kit in a 1.5 ml microcentrifuge vial using a scientific balance. By successive steps, add a total of 90 μl of beads (in six 15 μl additions), vortexing for 1 min between each addition. Set aside.
- Weigh 10 g of PDMS and mix for at least 12 min with the 0.5 g of the curing agent supplemented with fluorescent beads.
- Fabricate an SU-8 2050 cross-shaped mold using standard photolithography techniques following manufacturer’s instructions. The mold used has a height of 320 μm and an area of 13.4 cm2 (Figure 3). The mold can contain 428 μl or 440 mg of PDMS.
- Pour 400 mg of the PDMS with beads in the cross-shaped mold using a transfer pipette and cure for 2 hr at 80 °C. After curing, peel off the substrate from the mold (Figure 5A). The substrate can be kept in a Petri dish at room temperature for 2 weeks without showing significant changes in its mechanical properties.
- Pour droplets of PDMS (curing agent:PDMS with a ratio of 1:20) in a Petri dish with a final size of approximately 4 mm in diameter and cure them upside down for 2 hr at 80 °C (Figure 5B). These anchoring features can be kept in a Petri dish for weeks. NOTE: Maintain the dish upside down to prevent the drops from flattening during the curing process.
- Air-plasma treat (30 sec at 30 W) the substrate and 8 anchoring features. Bind the features on each end of the substrate at a distance of 4 mm from the square shaped indent present on the substrate (Figure 5C).
- Mounting Membrane on the Clamps
- Wrap each end of the substrate around the grooved cylindrical part of the clamps and secure it in place with the 2 setscrews from the top (Figure 2B and Figures 5D-E).
- Screw the 4 clamps on the clamp holder and pour PDMS (ratio 1:20) using a disposable transfer pipette at the interface between the substrate and the grooved cylindrical part of the clamps. Spread the uncured PDMS around the grooved cylindrical part using a 1.5 mm hex key.
- Pour PDMS (1:20) in the grooves until completely filled by capillary action and cure the assembly at 80 °C for 2 hr (Figure 5F).
- Seeding Cells on the Membrane
- Air-plasma treat (30 sec at 30 W) the whole assembly to sterilize and functionalize the substrate to allow for collagen coating.
- Functionalize the area of the substrate where cells will be seeded with 1 ml of 0.02 M acetic acid supplemented with 16 μg/ml of rat-tail collagen at room temperature for 1 hr. The desired final collagen density is 5 μg/cm2.
- Rinse the substrate 3x with phosphate buffer and let it dry at room temperature for at least for 10 min.
- Add 40 μl of culture medium supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin containing 2,000 cells in the center portion of the substrate to cover an area of 1 cm2 (cell density: 20 cells/mm2). Cell density can be altered according to experimental requirements.
- Put the whole assembly in a standard cell culture incubator with the substrate facing up with the drop of culture medium containing cells on it. NOTE: The assembly must be kept with the substrate facing up for at least 3 hr to allow cells to firmly attach it. To prevent evaporation, 30 μl of warm culture medium is added into the drop on the substrate every 45 min for 3 hr.
- After 3 hr, flip the whole assembly into a Petri dish filled with fresh culture medium to submerge the substrate and incubate overnight to allow cells to proliferate.
- The next day, prepare HEPES-buffered salt solution (HBSS; 20 mM of Hepes, 120 mM of NaCl, 5.3 mM of KCl, 0.8 mM of MgSO4, 1.8 mM of CaCl2, and 11.1 mM of dextrose). Adjust the pH to 7.4. NOTE: The HBSS solution has to be prepared daily and kept at 37 °C during the experiments. This physiological solution is used to maintain the cells on the microscope stage by mimicking the normal tissue/blood environment.
- Mount the set-up on inverted phase contrast or fluorescent microscope Mount the clamp-substrate assembly on the BAXS platform and motors. Fill the Petri dish inside the Petri dish heater with HEPES buffer (Figure 2D).
2. Stiffness Measurement of Small Caliber Vessels
- Preparation
- Krebs physiological solution: Prepare a solution of 118.1 mM NaCl, 11.1 mM D-glucose, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 2.5 mM CaCl2. Adjust the pH to 7.4 and oxygenate the solution with carbogen medical gas (95% O2/5% CO2) for 30 min. NOTE: The Krebs solution has to be prepared daily and kept at 37 °C during the experiments. This physiological solution is used to maintain the tissues alive by mimicking the normal tissue/blood environment.
- Gather instrumentation for the dissection and mechanical assessment of aortic vessels: surgical scissors, bended tweezers, micro-scissors, surgical dissecting microscope, 50 ml polypropylene centrifuge tubes, and 10 ml serological pipettes. The surgical and experiment procedure do not require any sterile conditions. Mount the clamps of the BAXS platform along with the load cell beforehand.
- Tissue Isolation and Dissection
All experimental procedures involving laboratory animals have to be approved by the Animal Care and Use Committee of the users’ institution, which complies with the Health Guide for the Care and Use of Laboratory Animals of the users’ country.- Perform mouse euthanasia with the inhalation of 99% CO2 (7 psi) in a Plexiglas chamber (Figure 6A).
- Open mouse abdomen and cut the thoracic aorta to bleed the mouse.
- Remove the diaphragm, the thoracic cage and the lung lobes (Figure 6B). NOTE: To minimize the risk of damaging the tissue, keep the heart attached to the aorta and avoid touching the vessel directly but manipulate it using the heart.
- Remove the heart, the aortic root and the thoracic aorta by gently cutting between the vessel and the spine. NOTE: Do not induce any elongation in the vessel during the excision to keep the inner structure of the tissue intact (Figure 6C).
- Immediately immerse and keep the heart and aorta in Krebs solution.
- Cut and carefully wash the aorta in Krebs solution to remove any blood clots. Remove connective tissue using micro-scissors, tweezers and surgical dissecting microscope (Figure 6D-E). NOTE: Keep all the vessel length and use the aortic root to determine the vessel orientation.
- Vessel Dimension Determination and Mounting
To determine the stiffness of the vessel, the unloaded vessel dimensions are required and can be determined with a calibrated microscope.- Cut an aortic ring of about 2 mm in length and precisely measure its length using a calibrated microscope setting (Figures 6F-G). Put this segment aside in Krebs solution.
- Cut another aortic ring as small as possible between each of the 2 mm segments (Figure 6F). Put this small segment on a microscope glass slide with the lumen facing up and measure the wall thickness using a calibrated microscope setting (Figure 6H).
- Fill the Petri dish on the BAXS platform with Krebs solution and insert the 2 mm aorta ring segment on the pulling pins (inset in Figure 4C).

Figure 5. PDMS substrate fabrication and mounting. A) After curing, the substrate is carefully peeled off the SU-8 2050 mold and put aside in a Petri dish. B) Anchoring features made out of PDMS and help to secure the substrate on the clamps. C) Substrate with anchoring features ready for mounting. D) The substrate is mounted on the 4 clamps, which are then mounted on the clamp holder (see inset). E) Detailed picture of the substrate mounted on the 4 clamps. F) Procedure of pouring PDMS in the groove underneath the substrate. The arrow shows the PDMS slowly filling the groove by capillarity.

Figure 6. Preparation and isolation of the thoracic aorta. A) Preparing surgical instruments and the euthanized mouse. B) Through a longitudinal abdominal incision, the thoracic cage and lung lobs are removed. C) The aorta is carefully removed using the heart to manipulate the tissue. D) The heart and aorta are put in Krebs physiological solution. The aorta is cleaned by removing all connective tissues. E) Detailed picture showing the heart and aorta. F) Aorta segment used for stiffness assessment along with small segments used for thickness measurement. G-H) The precise length (G) and thickness (H) of each vessel segments are evaluated using an inverse microscope and an analysis program.
Results
Cell Stretching
The BAXS platform was used to investigate the mechanical response of the nucleus in single mouse myoblast cells (C2C12) exposed to a substrate deformation of 25%. Myoblast cells are found in muscle tissue and are constantly exposed to mechanical stretch and compression in vivo. The shape and mechanical properties of the cell nucleus have shown to play a major role in the regulation of gene expression and transcriptional activity20,21 and als...
Discussion
The BAXS platform presented here facilitates numerous experiments in the study of mechanobiology, from investigations of single cells to whole tissues. In addition, the platform is highly flexible and configurable, allowing for numerous mechanical stimulation experiments and multi-axial tensile testing. The platform also enables the maintenance of cells and tissues in physiological conditions and allows for simultaneous microscopy during stretching experiments. The two experiments described in the previous sections demon...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
DT was supported by a postdoctoral studentship from le Fonds de Recherche du Québec-Nature et Technologies (FQRNT) and a MITACS Elevate Strategic Fellowship. CMC was supported by a postdoctoral studentship from le Fonds de Recherche en Santé du Québec (FRSQ) and the Ernest and Margaret Ford cardiology endowed research fellowship from the University of Ottawa Heart Institute. EOB was supported by operating grants MOP80204 from the Canadian Institute for Health Research (CIHR) and T6335 from the Heart and Stroke Foundation of Ontario. The CIHR and Medtronic collectively provide EOB with a peer-reviewed Research Chair (URC #57093). AEP is funded by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant, an NSERC Discovery Accelerator Supplement and gratefully acknowledges the support of the Canada Research Chairs (CRC) program and an Early Researcher Award from the Province of Ontario.
Materials
| Name | Company | Catalog Number | Comments |
| PDMS | Ellsworth Adhesives | 184 SIL ELAST KIT 0.5KG | The ratio base to cross-linker used in this protocol is 20:1. Mix in a laminar hood to keep dust from contamining your |
| FluoSpheres fluorescent microspheres | Invitrogen | F8810 | Keep away from light. |
| Linear voice coil | Moticont | LVCM-051-051-01 | The motro comes in two pieces (magnet and coil). It has to be mounted on a ball bearing sytem to be functional. |
| Ball bearing slide | Edmund Optics | NT37-360 | Miniature and Small Linear Motion Ball Bearing Slides |
| Linear positioning stage | Edmund Optics | 38-960 | Center Drive 1.25" Square Linear Translation Stages |
| Optical encoder | GSI microE systems | Mercury II 1600S - 0.5um resolution | reflective incremental encoder. |
| Motion controller | Galil | DMC-2143(DIN)-DC48 with AMP-20440 | 4 axis controller with a 4 axis amplifier |
| Load cell | Honeywell | 31 low | miniature load cell with a range of 0-150 g |
| Insect minutiens pins (0.20 mm) | Pin Service Austerlitz Insect pins | Stainless steel pins that are bended in an opened triangle shape | |
| SU-8 2050 | Micro Chem | SU-8 2050 | Permanent epoxy negative photoresist. Keep away from heat and light |
| Air-plasma treatment system | Glowresearch | Autoglow Oxygen Plasma System | |
| Rat-tail collagen | Invitrogen | A10483-01 | Collagen I, Rat Tail 5 mg/ml |
| Hoechst 33342 | Invitrogen | R37605 | DNA-specific fluorescent dye. Keep in the fridge. |
| Kapton (Polyimide Film) Insulated Flexible Heaters | omega.ca | KHLV-0504/(10)-P | 28 V flexible heaters; can be supplied with a 24 V |
| 1/16 DIN Autotune Temperature and Process Controllers | omega.ca | CN63200-R1-LV | Temperature controller; supply 24 V. |
| DMEM culture medium | Hyclone | SH3024301 | Dulbecco’s Modified 30 Eagle Medium. Keep at 4 °C |
| Penicillin-Streptomycin | Hyclone | SV30010 | Keep stock frozen. Keep working solution at 4 °C. |
| Fetal bovine serum (FBS) | Hyclone | SH3039603C | Keep frozen. |
| Trypsin 0.05% | Hyclone | SH30236.02 | Keep frozen. Digestion of cell attachement proteins for subcultivation |
| Hepes | Wisent Inc | 330-050-EL | HEPES-buffered salt solution |
| NaCl | Fisher Scientific | BP358-1 | HEPES-buffered salt solution / Krebs physiological solution |
| KCl | Fisher Scientific | BP366-500 | HEPES-buffered salt solution / Krebs physiological solution |
| MgSO4 | Fisher Scientific | M65-500 | HEPES-buffered salt solution / Krebs physiological solution |
| CaCl2 | Fisher Scientific | C614-500 | HEPES-buffered salt solution / Krebs physiological solution |
| Dextrose | Fisher Scientific | BP220-1 | HEPES-buffered salt solution / Krebs physiological solution |
| NaHCO3 | Fisher Scientific | BP328-1 | Krebs physiological solution |
| KH2PO4 | Fisher Scientific | BP362-500 | Krebs physiological solution |
| Carbogen 95% O2/5% CO2 | Lindle | DIN:02154749 | Krebs physiological solution oxygenation |
| Nocodazole | Sigma | M1404 | Microtubules depolymerization agent |
| Cytochalasin-D | Sigma | C8273 | Actin filaments depolymerization agent |
| Anti-α-SMA-FITC | Sigma | F3777 | Used to stain and quantify smooth muscle cells content |
| Picrosirius red stain | Fluka | 43665 | Used to stain and quantify collagen content |
References
- Yim, E. K., Sheetz, M. P. Force-dependent cell signaling in stem cell differentiation. Stem Cell Res Ther. 3, (2012).
- Vogel, V., Sheetz, M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 7, 265-275 (2006).
- Wang, N., Tytell, J. D., Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 10, 75-82 (2009).
- Ingber, D. E. Mechanobiology and diseases of mechanotransduction. Ann Med. 35, 564-577 (2003).
- Janmey, P. A., Miller, R. T. Mechanisms of mechanical signaling in development and disease. J Cell Sci. 124, 9-18 (2011).
- Bukoreshtliev, N. V., Haase, K., Pelling, A. E. Mechanical cues in cellular signalling and communication. Cell Tissue Res. 352, 77-94 (2013).
- Chen, Y., Pasapera, A. M., Koretsky, A. P., Waterman, C. M. Orientation-specific responses to sustained uniaxial stretching in focal adhesion growth and turnover. Proc Natl Acad Sci USA. 110, (2013).
- Rosenzweig, D. H., Matmati, M., Khayat, G., Chaudhry, S., Hinz, B., Quinn, T. M. Culture of Primary Bovine Chondrocytes on a Continuously Expanding Surface Inhibits Dedifferentiation. Tissue Eng Part A. 18, 2466-2476 (2012).
- Balachandran, K., et al. Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci USA. 108, 19943-19948 (1994).
- Steward, R., Cheng, C. M., Ye, J., Bellin, R., LeDuc, P. Mechanical stretch and shear flow induced reorganization and recruitment of fibronectin in fibroblasts. Sci Rep. 1, (2011).
- Wang, D., Xie, Y., Yuan, B., Xu, J., Gong, P., Jiang, X. A stretching device for imaging real-time molecular dynamics of live cells adhering to elastic membranes on inverted microscopes during the entire process of the stretch. Integr Biol (Camb). 2, 288-293 (2010).
- Haskett, D., Johnson, G., Zhou, A., Utzinger, U., Van de Geest, J. Microstructural and biomechanical alterations of the human aorta as a function of age and location. Biomech Model Mechanobiol. 9, 725-736 (2010).
- Duprey, A., Khanafer, K., Schlicht, M., Avril, S., Williams, D., Berguer, R. In vitro characterisation of physiological and maximum elastic modulus of ascending thoracic aortic aneurysms using uniaxial tensile testing. Eur J Vasc Endovasc Surg. 39, 700-707 (2010).
- Tremblay, D., et al. A comparison of mechanical properties of materials used in aortic arch reconstruction. Ann Thorac Surg. 88, 1484-1491 (2009).
- Khanafer, K., Duprey, A., Zainal, M., Schlicht, M., Williams, D., Berguer, R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. The Journal of thoracic and cardiovascular surgery. 142, 682-686 (2011).
- Van de Geest, J. P., Sacks, M. S., Vorp, D. A. The effects of aneurysm on the biaxial mechanical behavior of human abdominal aorta. J Biomech. 39, 1324-1334 (2006).
- Guolla, L., Bertrand, M., Haase, K., Pelling, A. E. Force transduction and strain dynamics in actin stress fibres in response to nanonewton forces. J Cell Sci. 125, 603-613 (2012).
- Wang, J. H., Goldschmidt-Clermont, P., Wille, J., Yin, F. C. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J Biomech. 34, 1563-1572 (2001).
- Jungbauer, S., Gao, H., Spatz, J. P., Kemkemer, R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J. 95, 3470-3478 (2008).
- Dahl, K. N., Ribeiro, A. J. S., Lammerding, J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 102, 1307-1318 (2008).
- Shivashankar, G. V. Mechanosignaling to the cell nucleus and gene regulation. Annu Rev Biophys. 40, 361-378 (2011).
- Chiquet, M., Gelman, L., Lutz, R., Maier, S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 1793, 911-920 (2009).
- Sullivan, T., et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol. 147, 913-920 (1999).
- Lammerding, J., et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest. 113, 370-378 (2004).
- Tremblay, D., Andrzejewski, L., Leclerc, A., Pelling, A. E. Actin and microtubules play distinct roles in governing the anisotropic deformation of cell nuclei in response to substrate strain. Cytoskeleton. , (2013).
- Cuerrier, C. M., et al. Chronic over-expression of heat shock protein 27 attenuates atherogenesis and enhances plaque remodeling: a combined histological and mechanical assessment of aortic lesions. PLoS ONE. 8, (2013).
- Tremblay, D., Cartier, R., Mongrain, R., Leask, R. L. Regional dependency of the vascular smooth muscle cell contribution to the mechanical properties of the pig ascending aortic tissue. J Biomech. 43, 2448-2451 (2010).
- Barker, A. J., Lanning, C., Shandas, R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng. 38, 788-800 (2010).
- Haga, J. H., Li, Y. S. J., Chien, S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 40, 947-960 (2007).
- Frydrychowicz, A., et al. Time-resolved magnetic resonance angiography and flow-sensitive 4-dimensional magnetic resonance imaging at 3 Tesla for blood flow and wall shear stress analysis. The Journal of thoracic and cardiovascular surgery. 136, 400-407 (2008).
- Boccafoschi, F., Mosca, C., Bosetti, M., Cannas, M. The role of mechanical stretching in the activation and localization of adhesion proteins and related intracellular molecules. J Cell Biochem. 112, 1403-1409 (2011).
- Yang, G., Crawford, R. C., Wang, J. H. C. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum-free conditions. J Biomech. 37, 1543-1550 (2004).
- Goldyn, A. M., Rioja, B. A., Spatz, J. P., Ballestrem, C., Kemkemer, R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J Cell Sci. 122, 3644-3651 (2009).
- Heo, S. J., et al. Fiber stretch and reorientation modulates mesenchymal stem cell morphology and fibrous gene expression on oriented nanofibrous microenvironments. Ann Biomed Eng. 39, 2780-2790 (2011).
- Zdero, R., et al. Linear and torsional mechanical characteristics of intact and reconstructed scapholunate ligaments. J Biomech Eng. 131, (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved