A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
DNA-affinity-purified Chip (DAP-chip) Method to Determine Gene Targets for Bacterial Two component Regulatory Systems
In This Article
Summary
This video article describes an in vitro microarray based method to determine the gene targets and binding sites for two component system response regulators.
Abstract
In vivo methods such as ChIP-chip are well-established techniques used to determine global gene targets for transcription factors. However, they are of limited use in exploring bacterial two component regulatory systems with uncharacterized activation conditions. Such systems regulate transcription only when activated in the presence of unique signals. Since these signals are often unknown, the in vitro microarray based method described in this video article can be used to determine gene targets and binding sites for response regulators. This DNA-affinity-purified-chip method may be used for any purified regulator in any organism with a sequenced genome. The protocol involves allowing the purified tagged protein to bind to sheared genomic DNA and then affinity purifying the protein-bound DNA, followed by fluorescent labeling of the DNA and hybridization to a custom tiling array. Preceding steps that may be used to optimize the assay for specific regulators are also described. The peaks generated by the array data analysis are used to predict binding site motifs, which are then experimentally validated. The motif predictions can be further used to determine gene targets of orthologous response regulators in closely related species. We demonstrate the applicability of this method by determining the gene targets and binding site motifs and thus predicting the function for a sigma54-dependent response regulator DVU3023 in the environmental bacterium Desulfovibrio vulgaris Hildenborough.
Introduction
The ability of bacteria to survive and thrive is critically dependent on how well they are able to perceive and respond to perturbations in their environments, and this in turn is dependent on their signal transduction systems. The number of signaling systems a bacterium encodes has been called its “microbial IQ” and can be an indication of both variability of its environment and its ability to sense multiple signals and fine tune its response1. Two component signal transduction systems (TCS) are the most prevalent signaling systems used by bacteria, and they consist of a histidine kinase (HK) that senses the external signal and transmits via phosphorylation to an effector response regulator (RR)2. RRs can have a variety of output domains and thus different effector modes, but the most common response is transcriptional regulation via a DNA binding domain1. The signals sensed and the corresponding functions of the vast majority of TCSs remain unknown.
Although in vivo methods such as ChIP-chip are routinely used for determination of genomic binding sites of transcription factors3, they can only be used for bacterial two component system RRs if the activating conditions or signals are known. Often the environmental cues that activate a TCS are harder to determine than their gene targets. The in vitro microarray based assay described here can be used to effectively and rapidly determine the gene targets and predict functions of TCSs. This assay takes advantage of the fact that RRs can be phosphorylated and thus activated in vitro using small molecule donors like acetyl phosphate4.
In this method, named DAP-chip for DNA-affinity-purified-chip (Figure 1), the RR gene of interest is cloned with a His-tag in E. coli, and a subsequently purified tagged protein is allowed to bind to sheared genomic DNA. The protein-bound DNA is then enriched by affinity-purification, the enriched and input DNA are amplified, fluorescently labeled, pooled together and hybridized to a tiling array that is custom made to the organism of interest (Figure 1). Microarray experiments are subject to artifacts and therefore additional steps are employed to optimize the assay. One such step is to attempt to determine one target for the RR under study using electrophoretic mobility shift assays (EMSA) (see workflow in Figure 2). Then, following binding to genomic DNA and the DAP steps, the protein-bound and input DNA are examined by qPCR to see if the positive target is enriched in the protein-bound fraction relative to the input fraction, thus confirming optimal binding conditions for the RR (Figure 2). After array hybridization, the data are analyzed to find peaks of higher intensity signal indicating genomic loci where the protein had bound. Functions may be predicted for the RR based on the gene targets obtained. The target genomic loci are used to predict binding site motifs, which are then experimentally validated using EMSAs (Figure 2). The functional predictions and gene targets for the RR may then be extended to closely related species that encode orthologous RRs by scanning those genomes for similar binding motifs (Figure 2). The DAP-chip method can provide a wealth of information for a TCS where previously there was none. The method can also be used for any transcriptional regulator if the protein can be purified and DNA binding conditions can be determined, and for any organism of interest with a genome sequence available.
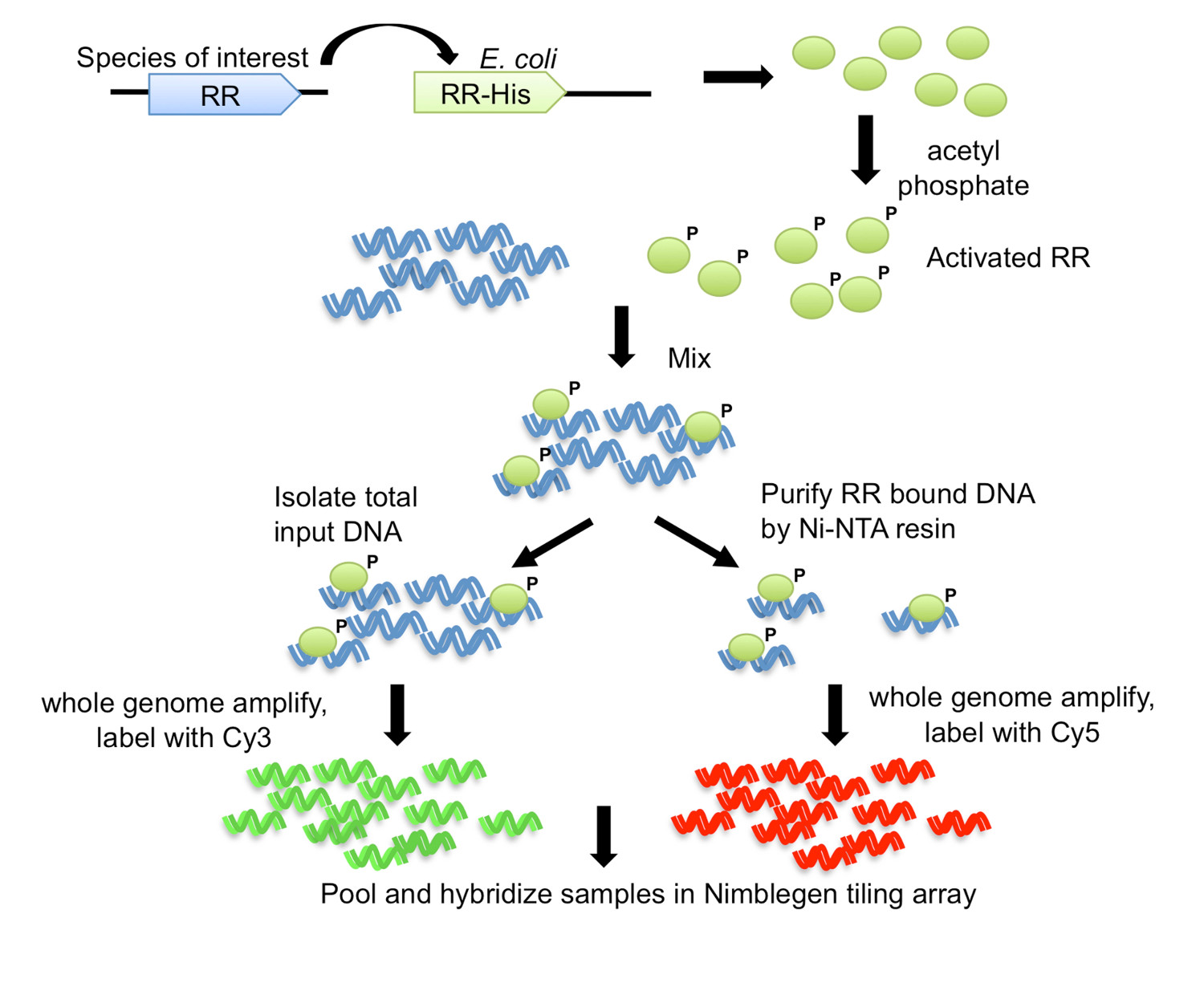
Figure 1. The DNA-affinity-purified-chip (DAP-chip) strategy7. The RR gene from the organism of interest is cloned with a carboxy-terminal His-tag into an E. coli expression strain. Purified His-tagged protein is activated by phosphorylation with acetyl phosphate, and mixed with sheared genomic DNA. An aliquot of the binding reaction is saved as input DNA, while the rest is subjected to affinity purification using Ni-NTA resin. The input and the RR-bound DNA are whole genome amplified, and labeled with Cy3 and Cy5, respectively. The labeled DNA is pooled together and hybridized to a tiling array, which is then analyzed to determine the gene targets. Figure modified and reprinted using the creative commons license from7.
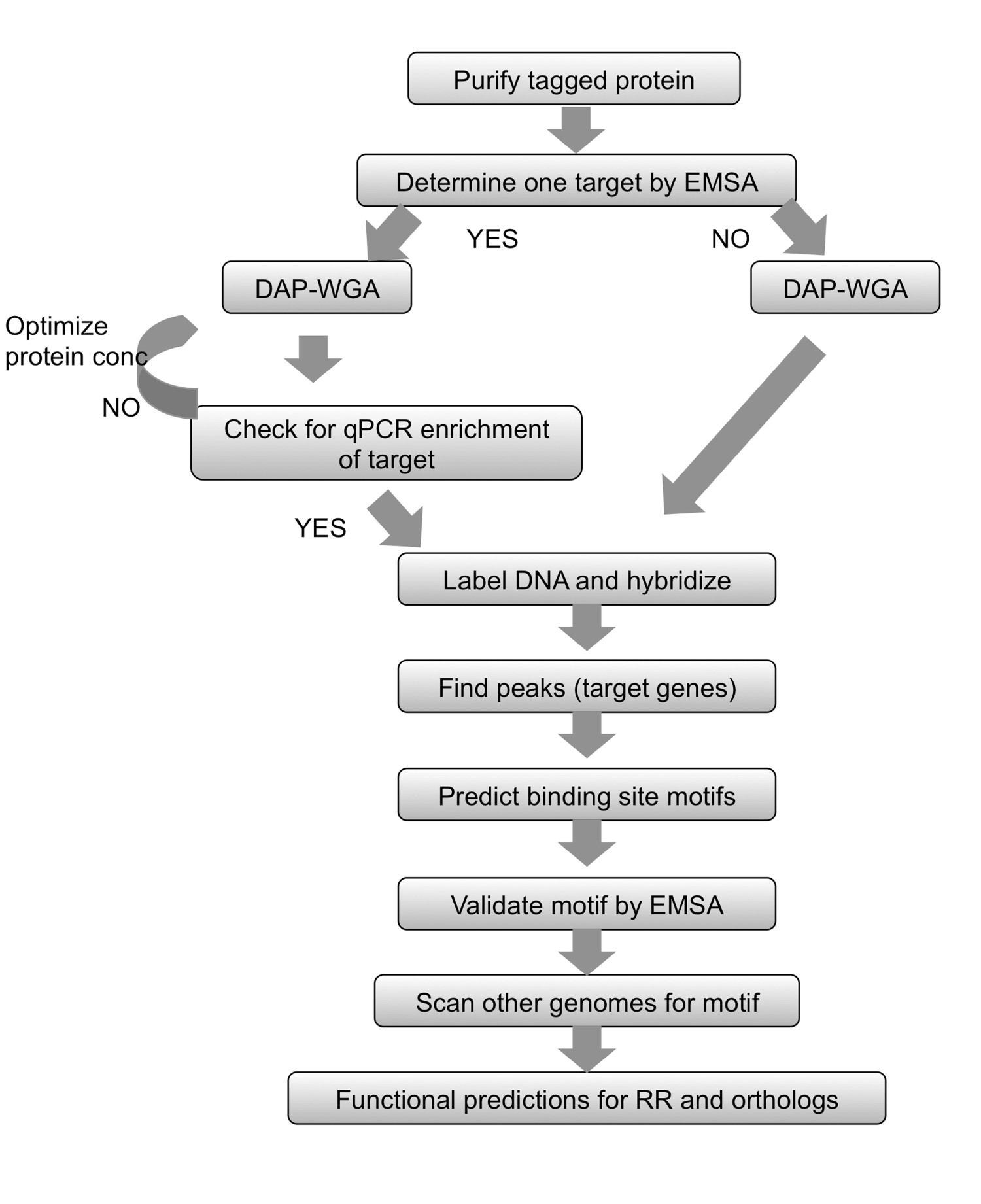
Figure 2. Summary of workflow. For any purified tagged protein, begin by determining a target using EMSA. Allow protein to bind genomic DNA and then DNA-affinity-purify (DAP) and whole genome amplify (WGA) the enriched and input DNA. If a gene target is known, use qPCR to ensure that the known target is enriched in the protein-bound fraction. If no target could be determined, proceed directly to DNA labeling and array hybridization. If enrichment by qPCR could not be observed, then repeat the protein-gDNA binding and DAP-WGA steps using different protein amounts. Use array analysis to find peaks and map them to target genes. Use the upstream regions of target genes to predict binding site motifs. Validate the motifs experimentally using EMSAs. Use the motif to scan the genomes of related species encoding orthologs of the RR under study, and predict genes targeted in those species as well. Based on the gene targets obtained, the physiological function of the RR and its orthologs may be predicted. Figure modified and reprinted using the creative commons license from7.
Protocol
Note: The protocol below is tailored for determination of gene targets of the RR DVU3023 from the bacterium Desulfovibrio vulgaris Hildenborough. It can be adapted to any other transcriptional regulator of interest.
1. Clone and Purify RR
- Clone the RR gene, specifically DVU3023, from D. vulgaris Hildenborough into an Escherichia coli expression vector such that the gene is C-terminally His-tagged and expression is under the control of a T7 promoter.
Note: Several cloning methods may be used and is determined by the researcher. Alternate affinity tags may also be used. - Transform the expression construct into E. coli BL21 (DE3) expression strain.
- Grow up 1 L of the BL21 expression strain at 37 °C. At mid-log phase, add IPTG to 0.5 mM to induce protein expression. Continue growth at room temperature for 24 hr.
- Centrifuge cells at 5,000 x g for 10 min at 4 °C. Resuspend cells in lysis buffer (20 mM sodium phosphate, pH 7.4, 500 mM NaCl, 40 mM imidazole, 1 mg/ml lysozyme, 1x benzonase nuclease). Lyse cells using a French press at 4 °C.
- Centrifuge lysate at 15,000 x g for 30 min at 4 °C. Filter using a 0.45 µm syringe filter.
- Wash a 1 ml Ni Sepharose column on an FPLC instrument using 10 ml of wash buffer (20 mM sodium phosphate, pH 7.4, 500 mM NaCl, 40 mM imidazole).
- Load lysate on the column. Wash column with 20 ml of wash buffer.
- Elute DVU3023 using a gradient of 0-100% elution buffer (20 mM sodium phosphate, pH 7.4, 500 mM NaCl, 500 mM imidazole).
- Load eluted fractions on a desalting column, and wash with a desalting buffer (20 mM sodium phosphate pH 7.4, 100 mM NaCl).
- Centrifuge protein sample in a high molecular weight cutoff centrifuge filter to concentrate the protein. Add DTT to 0.1 mM and glycerol to 50% and store protein at -20 °C.
Note: Purification methods will need to be optimized individually for each protein under study.
2. Determine Gene Target for RR Using Electrophoretic Mobility Shift Assay (EMSA)
- PCR amplify 400 bp region upstream of the candidate target gene DVU3025, using D. vulgaris Hildenborough genomic DNA as the template, and a forward unlabeled primer and a reverse 5’-biotin-labeled primer.
Note: Tips to select a candidate target gene: Often RRs bind the upstream regions of their own gene/operon, or they may regulate proximally encoded genes. If the RR has orthologs in other species, look for genes that are conserved in the neighborhood. Alternate methods include choosing candidate genes based on regulon predictions (e.g., RegPrecise5), or predicting sigma54-dependent promoters6 for RRs that are themselves sigma54-dependent. - Run the PCR product on a 1% agarose gel, cut out the 400 bp sized product, and purify the DNA using a gel extraction clean up kit.
- Mix DVU3023 protein (0.5 pmol) with 100 fmol of biotinylated DNA substrate in 10 mM Tris HCl, pH 7.5, 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 25% glycerol and 1 µg/ml poly dI.dC (non-specific competitor DNA) in a total volume of 20 µl. Also set up a reaction without any protein as a control. Incubate the reactions at room temperature on the bench for 20 min.
- Note: These are standard conditions listed and other reaction components may be added depending on the RR of interest. If activation is desired, add 50 mM acetyl phosphate to the reaction (often RRs will bind DNA in vitro without activation).
- Pre-run a precast mini 6% polyacrylamide-0.5x TBE gel in 0.5x TBE buffer at 100 V for 30 min. Add 5 µl of 5x loading buffer (0.1% bromophenol blue, 0.1% xylene cyanol, 30% glycerol in 1x TBE) to the binding reactions and load 18 µl of the reactions on to the gel. Run at 100 V for 2 hr.
Note: In order to avoid overheating, which can lead to disassociation of the protein-DNA complexes, fill the outer buffer chamber in the electrophoresis system with running buffer such that the majority of the gel is insulated by buffer. Alternately, the gel may be run at 4 °C. - Cut a charged nylon membrane to the size of the gel and soak it in 0.5x TBE for at least ten minutes. Remove the gel from the cassette. Cut any ridges off the gel and sandwich the gel and membrane between two thick filter papers soaked in 0.5x TBE, and place inside a semi-dry blotting apparatus and run at 20 V for 30 min.
- Place the membrane inside a commercial UV light crosslinker instrument and set the time to 3 min.
Note: The membrane may now be stored dry at room temperature for several days before proceeding to the next step. - Use a commercially available chemiluminescent detection kit that employs a streptavidin-horse radish peroxidase conjugate to develop the blot as per manufacturer’s instructions.
- Image the blot using a computer hooked up to a CCD equipped camera and look for a shift in the DNA substrate mobility in the presence of the RR, which indicates that the RR binds the DNA being tested.
Note: The specificity of the protein-DNA shift can be tested by including in the reaction an excess of unlabeled competitor DNA, which should eliminate or decrease the shift seen.
3. Verify Target Enrichment after Genomic DNA-protein Binding
- Genomic DNA-protein binding reaction
- Shear genomic DNA to an average size of 500 bp by sonicating 100 µl genomic DNA (at concentrations 100-200 ng/µl) in a 1.5 ml microfuge tube using a microtip with 9 low amplitude pulses of 1 sec, with 2 sec gap in between each.
Note: If the DNA splatters to the sides of the tube, spin down the contents after every 3 pulses. The exact conditions used will vary with the sonicator instrument used. The sheared DNA fragments may range from 100-1000 bp, but the average size should be within 400-600 bp. Too much shearing may result in fewer intact binding sites, and either too much or too little shearing will affect library preparation during the downstream whole genome amplification steps. - Mix 2-3 µg of sheared genomic DNA with RR protein (0.5 pmol of DVU3023) in 10 mM Tris-HCl pH 7.5, 1 mM DTT, 50 mM KCl, 5 mM MgCl2, 25% glycerol, and 50 mM acetyl phosphate. Incubate reactions at 25 °C for 30 min. Transfer 10 µl of this reaction to a 1.5 ml tube and label as input DNA.
Note: Acetyl phosphate is added to activate the protein by in vitro phosphorylation. Phosphorylation stimulates DNA binding, but many RRs also bind DNA in vitro without activation7. The amount of protein added to the reaction will depend on activity of the protein prep. The EMSA may be used to optimize this amount.
- Shear genomic DNA to an average size of 500 bp by sonicating 100 µl genomic DNA (at concentrations 100-200 ng/µl) in a 1.5 ml microfuge tube using a microtip with 9 low amplitude pulses of 1 sec, with 2 sec gap in between each.
- Affinity purify the protein-bound DNA
- Add 30 µl of Ni-NTA agarose resin to a 0.6 ml microfuge tube. Centrifuge at 100 x g for 1 min to collect the resin at the bottom, and remove the supernatant. Add 100 µl of wash buffer (10 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 50 mM KCl, 25% glycerol), flick the tube to mix, and centrifuge at 100 x g for 2 min. Remove supernatant.
- Add the remaining 90 µl of the binding reaction to the washed Ni-NTA resin and incubate in a rotary shaker for 30 min.
- Centrifuge at 100 x g for 2 min, and remove the supernatant (unbound DNA). Add 100 µl of wash buffer, flick the tube to mix contents, and centrifuge at 100 x g for 2 min. Remove supernatant. Repeat the wash step twice more.
- Add 35 µl of elution buffer (20 mM sodium phosphate buffer pH 8, 500 mM NaCl, 500 mM imidazole) to the resin and mix by vortexing. Incubate on the bench at RT for 5 min. Centrifuge at 100 x g for 2 min. Transfer the supernatant to a new 1.5 ml tube and label as the protein-bound DNA fraction.
- Also add 35 µl of the elution buffer to the input DNA.
- Purify the input and the protein-bound DNA fractions using a PCR purification kit.
- Whole genome amplify the input and protein-bound DNA samples.
- Add 10 µl of the input and protein-bound DNA to separate PCR tubes. Add 1 µl of 10x Fragmentation buffer, 2 µl of Library Preparation buffer, and 1 µl of Library Stabilization solution to each sample. Mix well by vortexing. Heat in a thermocycler at 95 °C for 2 min, and chill on ice.
Note: The starting amount of DNA should be at least 10 ng in order to avoid introducing amplification bias. - Add 1 µl of Library Preparation Enzyme, mix by pipetting, and incubate in thermal cycler at 16 °C/20 min, 24 °C/20 min, 37 °C for 20 min, 75 °C/5 min, and hold at 4 °C.
- To each tube, add 47.5 µl of water, 7.5 µl of 10x amplification master mix, and 5 µl of polymerase. Mix well and heat at 95 °C/3 min, followed by 20 cycles of 94 °C/15 sec, 65 °C/5 min. Hold reactions at 4 °C.
- Purify the amplified DNA samples using a PCR purification kit and measure DNA concentration spectrophotometrically.
- Add 10 µl of the input and protein-bound DNA to separate PCR tubes. Add 1 µl of 10x Fragmentation buffer, 2 µl of Library Preparation buffer, and 1 µl of Library Stabilization solution to each sample. Mix well by vortexing. Heat in a thermocycler at 95 °C for 2 min, and chill on ice.
- Verify target enrichment in the protein-bound DNA using qPCR
- Design qPCR primers to amplify 200 bp of the upstream region of the EMSA-verified target gene (DVU3025) using any freely available primer design software.
- Set up triplicate qPCR reactions for each DNA template with each primer set. Prepare a master mix for each primer set. 1x master mix contains 10 µl of 2x Sybr Green qPCR mix, 0.5 µM of each primer and water to a total of 18 µl. Aliquot 18 µl of the master mix per well of a 96-well PCR plate.
- Dilute the amplified and purified input and protein-bound DNA samples to 5 ng/µl with water and use as DNA template. Add 2 µl of DNA template to the wells.
- Seal the plate with an ultra clear qPCR sealing film, spin down plate in a centrifuge at 200 x g/1 min. Place plate in a real time qPCR machine. Cycle as follows using the associated qPCR software: 95 °C/1 min, and 40 cycles of 95 °C/10 sec, 59 °C/15 sec, and 70 °C/35 sec.
- Also set up triplicate qPCR reactions with primers to amplify upstream regions of an unrelated gene (negative control).
- Calculate ΔCT by subtracting CT value of protein-bound DNA from that of the input DNA. Calculate fold enrichment of target gene in the protein-bound DNA as 2ΔCT.
Note: If the target upstream region was enriched in the protein-bound DNA vs. the input DNA, it indicates that the binding reactions and affinity purification were successful. Proceed to step 4. If enrichment of the target upstream region was not observed, repeat the binding reactions (step 3.1) with different amount of protein.
4. DNA Labeling and Array Hybridization
- Label Input DNA with Cy3 and Enriched DNA with Cy5
ote: Cy3 and Cy5 dyes are light sensitive and care should be taken to keep light exposure to a minimum.- Mix 1 µg DNA with 40 µl Cy3/Cy5-labeled 9-mers and adjust volume to 80 µl with water.
- Heat denature at 98 °C for 10 min in the dark (in a thermocycler). Quick chill on ice for 2 min.
- Add 2 µl Klenow polymerase (3’-5’-exo-, 50,000 U/ml), 5 mM dNTPs, and 8 µl water to each reaction, mix well, and incubate at 37 °C for 2 hr in the dark (in a thermocycler).
- Add EDTA to 50 mM to stop the reaction and NaCl solution to 0.5 M.
- Transfer samples to 1.5 ml tubes containing 0.9 volume of isopropanol, incubate in dark for 10 min, and centrifuge at 12,000 x g for 10 min. The pellet should be pink for Cy3-labeled DNA and blue for Cy5-labeled DNA.
- Wash pellet with 80% ethanol (500 µl) and centrifuge at 12,000 x g for 2 min. Air-dry pellets for 5-10 min in dark.
Note: Pellets may be stored at -20 °C. - Resuspend pellet in 25 µl water. Measure DNA concentration spectrophotometrically.
- Pool together 6 µg each of the Cy3- and Cy5-labeled DNA in a 1.5 ml tube and vacuum dry in a centrifuge on low heat in the dark (cover the centrifuge lid if it is transparent).
Note: Pellets may be stored at -20 °C until ready for hybridization.
- Microarray Hybridization
- Turn the hybridization system on 3-4 hr prior to use and set the temperature to 42 °C.
- Prepare a hybridization solution master mix such that 1x solution contains 11.8 µl 2x Hybridization buffer, 4.7 µl Hybridization Component A, and 0.5 µl alignment oligo.
- Resuspend the pellets in 5 µl water. Add 13 µl of this mix to the sample. Vortex for 15 sec, incubate at 95 °C in a dry bath for 5 min. Keep samples at 42 °C in the hybridization system until ready for loading.
- Prepare the microarray slide-mixer assembly, according to manufacturer’s protocol.
- Place the mixer-slide assembly within the hybridization system. Load 16 µl of sample into the fill port, seal the ports with an adhesive film, turn mixing on in the system, and hybridize for 16-20 hr at 42 °C.
- Prepare 1x wash buffers I (250 ml), II (50 ml) and III (50 ml) by diluting the 10x commercially available buffers in water, and to each add DTT to 1 mM. Warm Buffer I to 42 °C.
- Slide the mixer-slide into a disassembly tool, and place inside a dish containing warm Buffer I. Peel the mixer off, while vigorously shaking the disassembly tool by hand.
- Place the slide into a container with 50 ml of Wash buffer I and shake vigorously for 2 min by hand.
- Transfer slide into a second container with 50 ml Wash buffer II and shake vigorously for 1 min by hand.
- Transfer slide into a third container with 50 ml Wash buffer III, and shake vigorously for 15 sec by hand.
- Quickly blot the edges of the slide on a paper towel, and place in a slide rack. Centrifuge at 200 x g for 2 min to dry the slide. Place within slide case, wrap it with foil, and store in a desiccator.
- Scanning the Array
- Place the slide within the microarray scanner according to the instrument’s instructions.
- Use a scanner software to set the wavelengths as 532 nm = Cy3 and 635 nm = Cy5, and the initial photomultiplier gains between 350 and 400.
- Preview the slide to locate the array on the slide. Select the array region for scanning.
- Scan the array and adjust the photomultiplier settings such that the array features are mostly yellow. The histogram should show the red and green curves superimposed or as close to each other as possible. The curves should end above the 10-5 normalized counts at saturation.
- Save both 532 and 635 nm images separately.
- Array Data Analysis
- Import the images into an array analysis software. Using the software, create two pair reports (.pair) for each array (for Cy3 and Cy5 images). Create scaled log2ratio files using the pair files. Use log2ratio files to search for peaks using a sliding window of 500 bp. Map the peak loci to the upstream regions of gene targets.
- Check if the positive target (determined using EMSA and confirmed by qPCR, DVU3025 in this example) appears within the top peaks.
Note: If the positive target is not among the top hits, it is possible that the RR under consideration has several gene targets and replicate DAP-chips can be conducted and peaks common to all replicates can be used to generate a target list.
5. Binding Site Motif Prediction and Validation
- Retrieve sequences for upstream regions (400 bp) for the top gene targets as generated by DAP-chip analysis. Apply MEME on these sequences through Microbes Online (meme.nbcr.net) to predict motifs.
Note: Enhancer binding proteins such as sigma54-dependent regulators can bind several hundred base pairs upstream of the start site. For other transcriptional regulators, a shorter region such as 200 bp upstream will be sufficient. - Design top and bottom strand DNA oligomers that contain the predicted binding site motif flanked by 10 bases on either end. Order the top strand 5’ biotinylated.
- Mix the top and bottom strand oligos in 1:1.5 ratio in 10 mM Tris HCl, pH 8.0, 1 mM EDTA, and 50 mM NaCl in a total reaction volume of 20 µl in PCR tubes. Heat to 95 °C for 5 min in a thermal cycler, followed by slow cooling to 25 °C. Dilute ten fold and use as wild type dsDNA substrate for EMSA.
- Prepare modified substrates using the same steps as above, but design oligomers to carry 4-6 substitutions in the conserved bases of the binding motif.
- Set up binding reactions with the RR and the wild type or modified substrates and examine using EMSA as described in step 2.
- Note: The predicted motif is validated if the RR binds the wild type substrate but not the modified one.
6. Conservation of Motif in Other Related Bacterial Species
- Select genomes of interest that contain orthologs of DVU3023. Obtain sequence and annotation files for the genomes from the NCBI site.
- Use a programming language such as Perl to write scripts that will use the motif sequences from the DAP-chip targets to build a position weighted matrix and use the matrix to score similar motifs present in other sequenced genomes.
Results
The above method was applied to determine the global gene targets of the RRs in the model sulfate reducing bacterium Desulfovibrio vulgaris Hildenborough7. This organism has a large number of TCSs represented by over 70 RRs, indicating the wide variety of possible signals that it senses and responds to. In vivo analyses on the functions of these signaling systems are hard to perform since their signals and thus their activating conditions are unknown. Here the DAP-chip method was used to dete...
Discussion
The DAP-chip method described here was successfully used to determine the gene targets for several RRs in Desulfovibrio vulgaris Hildenborough7 of which one is shown here as a representative result. For RR DVU3023, choosing a candidate gene target was straightforward. DVU3025 is located immediately downstream of the RR gene, and the RR and target genes are conserved in several Desulfovibrio species, and additionally DVU3025 has a predicted sigma54-dependent promoter. The EMSA provides a simpl...
Disclosures
The authors have no conflict of interest to disclose.
Acknowledgements
We thank Amy Chen for her help in preparing for the video shoot and for demonstrating the technique. This work conducted by ENIGMA: Ecosystems and Networks Integrated with Genes and Molecular Assemblies (http://enigma.lbl.gov), a Scientific Focus Area Program at Lawrence Berkeley National Laboratory, was supported by the Office of Science, Office of Biological and Environmental Research, of the U. S. Department of Energy under Contract No. DE-AC02-05CH11231.
Materials
| Name | Company | Catalog Number | Comments |
| Name of Material/Equipment | Company | Catalog number | Comments |
| HisTrapFF column (Ni-Sepharose column) | GE Lfe Sciences, Pittsburgh, PA, USA | 17-5255-01 | |
| Akta explorer (FPLC instrument) | GE Lfe Sciences, Pittsburgh, PA, USA | ||
| HiPrep 26/10 Desalting column | GE Lfe Sciences, Pittsburgh, PA, USA | 17-5087-01 | |
| Qiaquick Gel extraction kit | Qiagen Inc, Valencia, CA, USA | 28704 | |
| Biotin-labeled oligonucleotides | Integrated DNA Technologies | N/A | |
| 6% polyacrylamide-0.5X TBE precast mini DNA retardation gel | Life Technologies, Grand Island, NY, USA | EC63652BOX | Alternately, you can pour your own gel. |
| Nylon membrane | EMD Millipore, Billerica, MA, USA | INYC00010 | |
| Trans-Blot SD Semi-dry electrophoretic transfer cell | Biorad, Hercules, CA, USA | 170-3940 | |
| Extra thick blot paper, 8 x 13.5 cm | Biorad, Hercules, CA, USA | 170-3967 | |
| UV crosslinker Model XL-1000 | Fisher Scientific | 11-992-89 | |
| Nucleic Acid chemiluminescent detection kit (Pierce) | Thermo fisher Scientific, Rockford, IL, USA | 89880 | |
| Ni-NTA agarose resin | Qiagen Inc, Valencia, CA, USA | 30210 | |
| GenomePlex Whole genome amplification kit (Fragmentation buffer, library preparation buffer, library stabilization solution, library preparation enzyme, 10X amplification master mix, WGA polymerase ) | Sigma-Aldrich, St. Louis, MO, USA | WGA2-50RXN | |
| Nanodrop ND-1000 | Thermo Scientific, Wilmington, DE, USA | For quantitation of DNA | |
| Perfecta Sybr Green SuperMix, with ROX | Quanta biosciences | 95055-500 | Any Sybr Green PCR mix may be used |
| PlateMax Ultra clear heat sealing film for qPCR | Axygen | ||
| 96 well clear low profile PCR microplate | Life Technologies, Grand Island, NY, USA | PCR-96-LP-AB-C | |
| Applied Biosystems StepOne Plus Real time PCR system | Life Technologies, Grand Island, NY, USA | 4376600 | Any real time PCR system may be used |
| Qiaquick PCR purification kit | Qiagen Inc, Valencia, CA, USA | 28104 | Any PCR clean up kit may be used |
| Cy3/Cy5-labeled nonamers | Trilink biotechnologies, San Diego, CA, USA | N46-0001, N46-0002 | |
| Klenow polymerase 50,000U/ml, 3'-5' exo- | New England Biolabs, Ipswich, MA | M0212M | |
| Hybridization system | Roche-Nimblegen, Madison, WI, USA | N/A | This company no longer makes arrays or related items, so alternate sources such as Agilent or Affymetrix will need to be used, |
| Custom printed microarrays and mixers | Roche-Nimblegen, Madison, WI, USA | N/A | |
| Hybridization kit (2X Hybridization buffer, Hybridization component A, Alignment oligo) | Roche-Nimblegen, Madison, WI, USA | N/A | |
| Wash buffer kit (10X Wash buffer I, II, III, 1 M DTT) | Roche-Nimblegen, Madison, WI, USA | N/A | |
| GenePix 4200A microarray scanner | Molecular Devices, Sunnyvale CA, USA | This model has been replaced by superior ones | |
| GenePix Pro microarray software | Molecular Devices, Sunnyvale CA, USA | ||
| Nimblescan v.2.4, ChIP-chip analysis software | Roche-Nimblegen, Madison, WI, USA | N/A | |
References
- Galperin, M. Y. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 13, 150-159 (2010).
- Casino, P., Rubio, V., Marina, A. The mechanism of signal transduction by two-component systems. Curr Opin Struct Biol. 20, 763-771 (2010).
- Aparicio, O., Geisberg, J. V., Struhl, K. Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Cell Biol. 17 (17), (2004).
- McCleary, W. R., Stock, J. B. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem. 269, 31567-31572 (1994).
- Novichkov, P. S., et al. RegPrecise web services interface: programmatic access to the transcriptional regulatory interactions in bacteria reconstructed by comparative genomics. Nucleic Acids Res. 40, 604-608 (2012).
- Studholme, D. J., Buck, M., Nixon, T. Identification of potential sigma(N)-dependent promoters in bacterial genomes. Microbiology. 146 (12), 3021-3023 (2000).
- Rajeev, L., et al. Systematic mapping of two component response regulators to gene targets in a model sulfate reducing bacterium. Genome Biol. 12, 99 (2011).
- Crooks, G. E., Hon, G., Chandonia, J. M., Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188-1190 (2004).
- Barbieri, C. M., Mack, T. R., Robinson, V. L., Miller, M. T., Stock, A. M. Regulation of response regulator autophosphorylation through interdomain contacts. J Biol Chem. 285, 32325-32335 (2010).
- Bourret, R. B. Receiver domain structure and function in response regulator proteins. Curr Opin Microbiol. 13, 142-149 (2010).
- Gao, R., Stock, A. M. Molecular strategies for phosphorylation-mediated regulation of response regulator activity. Curr Opin Microbiol. 13, 160-167 (2010).
- Hung, D. C., et al. Oligomerization of the response regulator ComE from Streptococcus mutans is affected by phosphorylation. J Bacteriol. 194, 1127-1135 (2012).
- Ladds, J. C., et al. The response regulator Spo0A from Bacillus subtilis is efficiently phosphorylated in Escherichia coli. FEMS Microbiol Lett. 223, 153-157 (2003).
- Wen, Y., Feng, J., Scott, D. R., Marcus, E. A., Sachs, G. Involvement of the HP0165-HP0166 two-component system in expression of some acidic-pH-upregulated genes of Helicobacter pylori. J Bacteriol. 188, 1750-1761 (2006).
- Liu, X., Noll, D. M., Lieb, J. D., Clarke, N. D. DIP-chip: rapid and accurate determination of DNA-binding specificity. Genome Res. 15, 421-427 (2005).
- Gossett, A. J., Lieb, J. D. DNA Immunoprecipitation (DIP) for the Determination of DNA-Binding Specificity. CSH Protoc. 2008, (2008).
- Mascher, T., et al. The Streptococcus pneumoniae cia regulon: CiaR target sites and transcription profile analysis. J Bacteriol. 185, 60-70 (2003).
- Park, P. J. ChIP-seq: advantages and challenges of a maturing technology. Nat Rev Genet. 10, 669-680 (2009).
- Lefrancois, P., Zheng, W., Snyder, M. ChIP-Seq using high-throughput DNA sequencing for genome-wide identification of transcription factor binding sites. Methods Enzymol. 470, 77-104 (2010).
- Ho, J. W., et al. ChIP-chip versus ChIP-seq: lessons for experimental design and data analysis. BMC Genomics. 12, 134 (2011).
- Liu, X., Lee, C. K., Granek, J. A., Clarke, N. D., Lieb, J. D. Whole-genome comparison of Leu3 binding in vitro and in vivo reveals the importance of nucleosome occupancy in target site selection. Genome Res. 16, 1517-1528 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved