A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Investigating the Spreading and Toxicity of Prion-like Proteins Using the Metazoan Model Organism C. elegans
In This Article
Summary
Prion-like propagation of protein aggregates has recently emerged as being implicated in many neurodegenerative diseases. The goal of this protocol is to describe, how to use the nematode C. elegans as a model system to monitor protein spreading and to investigate prion-like phenomena.
Abstract
Prions are unconventional self-propagating proteinaceous particles, devoid of any coding nucleic acid. These proteinaceous seeds serve as templates for the conversion and replication of their benign cellular isoform. Accumulating evidence suggests that many protein aggregates can act as self-propagating templates and corrupt the folding of cognate proteins. Although aggregates can be functional under certain circumstances, this process often leads to the disruption of the cellular protein homeostasis (proteostasis), eventually leading to devastating diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic lateral sclerosis (ALS), or transmissible spongiform encephalopathies (TSEs). The exact mechanisms of prion propagation and cell-to-cell spreading of protein aggregates are still subjects of intense investigation. To further this knowledge, recently a new metazoan model in Caenorhabditis elegans, for expression of the prion domain of the cytosolic yeast prion protein Sup35 has been established. This prion model offers several advantages, as it allows direct monitoring of the fluorescently tagged prion domain in living animals and ease of genetic approaches. Described here are methods to study prion-like behavior of protein aggregates and to identify modifiers of prion-induced toxicity using C. elegans.
Introduction
Many neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Amyotrophic lateral sclerosis (ALS), and transmissible spongiform encephalopathies (TSEs), are associated with aggregation-prone proteins and are hence collectively known as protein misfolding disorders (PMDs). TSEs or prion diseases constitute a unique class of PMDs in that they can be infectious in both humans and animals1. At the molecular level, prions replicate by recruiting and converting monomeric α-helix-rich host-encoded cellular PrP (PrPC) into the pathological β-sheet-rich PrPSc conformation2,3. Self-propagating protein aggregates have been also identified in fungi, which share important characteristics with mammalian prions4,5. Additionally, mammalian prions are capable of moving from cell-to-cell and infect naïve cells6,7.
While PMDs other than TSEs are not infectious, they share a common pathogenic principle with prion diseases8,9. Although the proteins linked to each of the PMDs are not related in structure or function, they all form aggregates via a crystallization-like process called nucleated or seeded polymerization; moreover proteinaceous seeds grow by recruiting their soluble isoforms2,10,11. The efficiency to self-propagate varies in vivo, depending on the intrinsic properties of the protein, which together with additional cellular factors such as molecular chaperones ultimately determine rates of aggregate nucleation, seeding, fragmentation and spreading12-15. Hence, there must exist a fine balance among these factors that allows efficient propagation of protein aggregation. This might also explain why only some amyloidogenic aggregates harbor the characteristics of a prion, and thus not all PMDs are infectious. Prions seem to represent ‘top-performers’ of a wide spectrum of self-replicating proteinaceous aggregates, which makes them a powerful tool to study PMDs8,13.
Intriguingly, the toxicity associated with disease-related aggregates often has a non cell autonomous component16,17. This means that they affect neighboring cells that do not express the corresponding gene, in contrast to a strictly cell autonomous effect, which implies that only the cells expressing the gene exhibit the specific phenotype. This was compellingly demonstrated by tissue-specific expression or knock down of the respective proteins in numerous models of neurodegenerative diseases18-26. Various mechanisms have been suggested as a basis for this non-cell autonomous toxicity in PMDs, including diminished nutrient supply, imbalance in neuronal signaling, glutamate excitotoxicity, and neuroinflammation16,27,28. In addition, a prion-like movement of disease-linked aggregates between cells might contribute to this aspect29,30. Increasing evidence suggests that protein inclusions other than prions can transmit from cell-to-cell, which may explain the characteristic spreading of pathology observed in many PMDs30-36. However, it has yet to be determined whether there is a clear causal link between intercellular movement of disease proteins and the toxic effect on neighboring cells. Therefore, a better understanding of the cellular pathways that underlie cell-to-cell transmission and non cell autonomous toxicity is necessary and essential for the development of novel therapeutics. However, many aspects of prion-like spreading and cellular factors that influence cell-to-cell transmission of misfolded proteins in metazoans are not well understood, in particular at the organismal level.
The nematode Caenorhabditis elegans has several advantages that provide the potential to discover new facets of prion-like spreading in metazoans17. It is transparent, allowing for in vivo tracking of fluorescently tagged proteins in the living organism. Furthermore, many cellular and physiological processes affected by disease are conserved from worms to human, and C. elegans is also amenable to a wide variety of genetic manipulations and molecular and biochemical analyses37-39. Exactly 959 somatic cells make up the adult hermaphrodite with a simple body plan that still has several distinct tissue types, including muscle, neurons and intestine.
To establish a new prion model in C. elegans, we chose to exogenously express the well characterized glutamine/asparagine (Q/N)-rich prion domain NM of the cytosolic yeast prion protein Sup35, since there are no known endogenous prion proteins in worms4,40. Yeast prions have been invaluable in elucidating basic mechanisms of prion replication41-44. Furthermore, NM is the first cytosolic prion-like protein that has been shown to recapitulate the full life cycle of a prion in mammalian cell culture45,46. Likewise, when expressed in C. elegans, the Sup35 prion domain adopted remarkably well to the different requirements for propagation in metazoan cells compared to yeast cells and exhibited key features of prion biology40. NM aggregation was associated with a profound toxic phenotype, including the disruption of mitochondrial integrity and appearance of various autophagy related vesicles on the cellular level, as well as embryonic and larval arrest, developmental delay, and a widespread disturbance of the protein folding environment on the organismal level. Strikingly, the prion domain exhibits cell autonomous and non cell autonomous toxicity, affecting neighboring tissues in which the transgene was not expressed. Furthermore, the vesicular transport of the prion domain within and between cells is monitored real time in vivo40.
Here we describe how to examine prion-like dissemination in C. elegans. We will explain how to monitor the intra- and intercellular transport of vesicles containing the prion domain using time-lapse fluorescence microscopy. We will emphasize the use of tissue-specific folding sensors and ubiquitously expressed stress reporters to evaluate cell autonomous and non cell autonomous effects on cellular fitness. Finally, we will describe the procedure of a recently performed genome wide RNA interference (RNAi) screen to identify new modifiers of prion-induced toxicity. In combination, these methods can help to tease apart genetic pathways involved in the intercellular movement of proteins and their non cell autonomous toxicity.
Protocol
1. Monitoring Transcellular Spreading of Prion-like Proteins By In Vivo Time-lapse Imaging
NOTE: Grow C. elegans wild-type (WT) (N2) and transgenic lines according to standard methods and carefully control the cultivation temperature47.
- Generate transgenic lines of C. elegans expressing the prion-like protein, tagged with monomeric red fluorescent protein (mRFP). Watch this video that demonstrates how to use microinjection48. For further details and methods describing how to integrate these extrachromosomal lines, see49.
- Prepare synchronized populations by egg laying or bleaching according to standard methods50.
- Synchronization by egg laying
- Transfer 10 - 20 gravid adults on a plate and let them lay eggs for 1 - 2 hr. Remove adults from the plate and let the progeny grow until the desired age.
- Synchronization by bleaching
- Collect an unsynchronized population of gravid adults and bleach them with alkaline hypochlorite solution (250 mM NaOH and 1:4 (v/v) dilution of commercial bleach in H2O). Wash the eggs twice (218 x g for 1 min) with M9 buffer47 (21 mMNa2HPO4·7H2O, 22 mMKH2PO4, 86 mMNaCl, 1 mMMgSO4·7H2O, add dH2O up to 1 L).
- Allow them to hatch in M9 buffer with gentle agitation O/N at 20 °C. Worm development will arrest at the L1 stage in the absence of a food source, leaving a synchronized population. Transfer L1s onto fresh Nematode Growth Media (NGM) plates seeded with OP50 E. coli bacteria and let the progeny develop until the desired age47.
- Synchronization by egg laying
- Prepare 2% agarose pads (in H2O) on a microscope slide as described50.
- Prepare two microscope slides with labeling tape placed over their entire length to be used as spacers. Place a third microscope slide between them.
- Dissolve 2% agarose in H2O and pipette one drop onto the clean slide.
- Place a fourth slide perpendicular to the three other slides on top of the agar drop. Gently press it down to flatten the pad to the same thickness as the labeling tape.
- Let it dry for 1 min before removing the spacers and gently pulling the slides apart. The agar pad will stick to one of them.
- Pipette ~10 µl anesthetic (2 mM levamisole in M9 buffer) to the pad and transfer ~10 animals using a platinum wire pick. Cover with a cover slip (~22 x 22 mm) and take images within 1 hr.
- Alternatively, to acquire movies over a longer period of time or to further reduce the possibility of any movement of the animals, use a combination of anesthetic and bead immobilization51.
- Prepare 10% agarose pads (in M9 buffer) as described51 and add worms to 3 µl nanosphere size standards solution (polystyrene beads, 100 nm) plus 3 µl anesthetic (4 mM levamisole in M9 buffer). Cover gently with a cover slip. To avoid desiccation, seal the cover slip with VALAP (mixture of equal amounts of Vaseline, lanolin, and paraffin wax).
- Image immobilized worms using a confocal microscope.
NOTE: Results are obtained using a Spinning Disc AF Confocal Microscope equipped with an EM-CCD camera and a Microscopy Automation & Image Analysis Software such as MetaMorph and outline the specifics below, but other comparable confocal imaging systems can also be used.- Use the 63X or 100X/1.4NA oil objective and place the microscope slide containing worms into the microscope slide holder.
- Open the software. Adjust laser power and filters for mRFP imaging. Use the 561 nm laser at 10% power and emission filter >600 nm.
- Open “MultiDimensional Acquisition”. Under the “Main” tab, check the boxes for “Timelapse” and “Run Journals” (Hardware Auto Focus: off).
- Under the “Saving” tab select or create the directory folder where the files should be saved. Assign a name to the file.
- Under the “Wave Length” tab select the appropriate illumination and adjust exposure and gain. Use “YokoQuad Red” (or an equivalent illumination setting for mRFP imaging) with an exposure between 100 and 300 msec and a camera gain between 100 and 300, depending on the individual sample (assessed by using the live image).
- Under the “Timelapse” tab select “Number of time points” = 301, “Duration” = 5 min and “Time/Interval” = 1 sec.
- Under the “Journals” tab select Journal: “AFC SET Z HOLD” and “AFC Return to Z HOLD”, Type: “Special” (twice), Initial Point: “Start of time point” and “End of time point”. This autofocus option is important to image the same section over a longer period of time.
- When the setup is done, press “Acquire”. The timelapse video is safed as separate TIFF files.
- Open all .tiff files of a given time series in ImageJ. Go to “Image” →“Stacks”→“Images to Stacks”. Optionally, adjust brightness and contrast, add size bar, etc. Export the movie under “File”→“Save as”→“AVI…” (for an example, see Video 1 and 2 corresponding to Figure 1).
2. Using Folding Sensors and Stress Reporters to Investigate Cell Autonomous and Non cell Autonomous Affects on Proteostasis and Toxicity
- Generate transgenic lines that coexpress a folding sensor or stress reporter together with the prion-like protein. For methods on how to establish crosses or generate transgenic lines, see48,49,52. See Table 1 for a list of C. elegans strains that can be used.
- Prepare synchronized populations of transgenic animals and grow them until the desired age as described above (section 1.2)50.
- Using a stereomicroscope, examine the respective phenotype of the folding sensor or stress reporter.
- For the folding sensor, determine the number of animals harboring aggregates on each day after synchronization (for an example, see Figure 2E and F).
- For the stress reporter, test if the coexpression of the prion-like protein results in an increased fluorescence of the reporter (for an example, see Figure 3).
3. Genome-wide Screen for Suppression of Prion-induced Toxicity in C. elegans
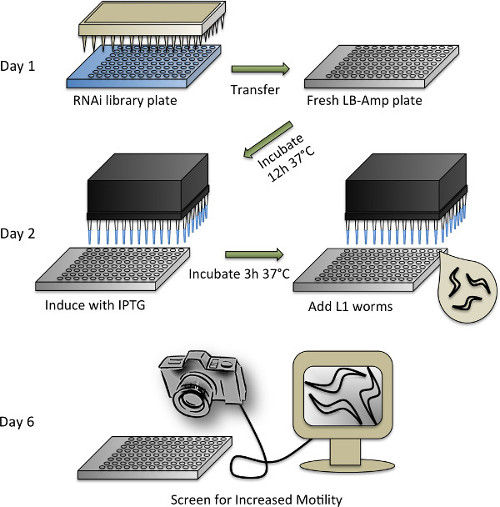
Figure 4. Schematic representation of the RNAi screening protocol. See protocol section 3 for a detailed description of the individual steps.
- Synchronization of C. elegans worms and duplication of RNAi library
- For the RNAi screen, use the Ahringer RNAi library (or the Vidal RNAi library)53,54. Rearray the RNAi library from the original 384 well format into 96 well plates by filling the plates with 100 µl LB-amp media (50 μg/ml Ampicillin in LB) supplemented with 10% glycerol and inoculate using a 96-pin replicator. Grow at 37 °C O/N with agitation and store at -80 °C.
- Maintain C. elegans WT and transgenic lines at 20 °C on NGM plates seeded with OP50 E. coli bacteria according to standard methods47.
- Synchronize prion domain transgenic and WT (control) nematodes by bleaching (see section 1.2).
- On the same day that the worms are bleached, prepare LB media supplemented with 50 μg/ml ampicillin. Use an automated reagent dispenser to dispense (or pipette manually) 65 μl of the LB-amp media into each well of a 96 well plate.
- Remove 96 well Ahringer RNAi library plates from -80 °C and bring them to a sterile hood lined with paper towels. Immediately remove the seal-tape by holding the plate upside down while plates are still frozen, being careful to avoid contamination from ice on the outside of the plates. Reinvert the plates and let them thaw for about 30 min.
- Dip one sterile 96 pin replicator into one RNAi library plate, and then into one fresh LB-amp plate. Use a fresh sterile replicator for each plate. Seal all plates with adhesive foil tape and return the library plates to -80 °C. The replicators can be soaked in bleach, rinsed, and autoclaved to be used again.
- Allow duplicated RNAi plates to grow O/N in an incubator set to 37 °C and 300 rpm. To use HT115 E. coli bacteria harboring the empty vector (L4440) as a control, grow it separately in a culture tube and then pipette 65 μl of the culture with a multichannel pipette into one quarter of two 96 well plates.
- Induction of bacterial dsRNA production and addition of worms
- Dilute Isopropyl-β-D-thiogalactopyranosid (IPTG) to a 5 mM concentration in ddH2O. Use an automated workstation with a multichannel head to dispense (or pipet manually) 10 μl of diluted IPTG into each well of the RNAi bacteria and the control plates. Reseal and place the plates back into the incubator. Let them shake for 3 hr at 37 °C.
- While bacteria are shaking, prepare the worms. Mix the M9/worm suspension well, and pipette a small sample (~5 - 10 µl) to a glass microscope slide. Count the number of worms under a stereomicroscope and calculate the number of worms per μl.
- Using sterile technique, prepare a solution of supplemented M9 (M9+) with the following final concentrations: 0.20 mg/ml IPTG, 8.0 μg/ml Cholesterol, 50 μg/ml Ampicillin, 9.6 μg/ml Tetracycline, 0.0835 μg/ml Fungizone, and 15 worms per 50 μl.
- Make separate solutions for prion domain transgenic and WT worms. The final volume of M9+ worm solution needed will depend on the number of RNAi plates copied (see below), plus ~30 ml of dead volume that will remain in the reservoir, if an automated dispenser is used.
- After the 3 hr induction of bacterial dsRNA production, take the plates out of the incubator and let them cool to RT (~30 min) to avoid heat stressing the animals.
- Dispense 50 μl M9+ prion domain transgenic worm solution to each well of the RNAi plates and one of the empty vector control plates. Make sure to mix the worm solution before each step as the animals tend to sediment to the bottom of the reservoir.
- Dispense WT worms into the second control plate. Leave the plates unsealed to allow aeration. To keep the liquid culture from evaporating, stack 4 - 5 plates together and wrap with a damp paper towel and aluminum foil. Incubate at 200 rpm at 20 °C for 4 days.
- Scoring
NOTE: After 4 days in the incubator, the worms will be at the second day of adulthood and are ready to screen. Let the animals adjust to non shaking conditions for 30 min before screening to ensure undisturbed thrashing.- Using a Falcon 4M60 camera connected to a computer with a monitor, screen visually for increased thrashing as compared with control treated prion domain transgenic animals.
- Compile a list of preliminary hits to confirm using wrMTrck (see next section).
4. Confirmation of Preliminary Screen Hits
- Motility assay on solid plates
- Prepare plates with NGM supplemented with 100 µg/ml ampicillin, 12.5 µg/ml tetracycline and 1 mM IPTG, according to standard methods47. If possible, use a plate-pouring machine to assure all plates have the same height of media, which will allow for a more streamlined video acquisition process.
- Grow the different RNAi clones in ~1 ml LB + 50 µg/ml ampicillin, O/N at 37 °C and 250 rpm.
- The next day, induce the expression of the dsRNA with 1 mM IPTG for 3 hr. Seed the plates with 150 µl of each RNAi bacterial clone, spread into a thin layer. Let the bacteria dry for 2 days at RT in the dark. Prepare 3 plates per RNAi clone.
- Synchronize the worm population by bleaching according to standard methods50 (see section 1.2), and let the eggs hatch O/N in M9 media.
- Take a sample of the worm suspension and determine the amount of nematodes per µl at the stereomicroscope. Then, pipette the proper volume of M9 plus worms into each experimental plate so that it contains 25 - 30 L1 worms. Grow the nematodes at 20 °C for 4 days until the worms reach day 2 of adulthood.
- Quantitative analysis of worm motility with the wrMTrck plugin for ImageJ
NOTE: The videos were recorded using a stereomicroscope at 10X magnification with a Hamamatsu Orca-R2 digital camera C10600-10B and the Hamamatsu Simple PCI imaging software.- Turn on the camera and the Simple PCI imaging software. Click on “Live” to allow for adjustment of the imaging conditions.
- Set up the video conditions as follows: Gain = 0; Light Mode = High; Speed Index = 1; Binning = 2. Click “Auto Expose” and then adjust the lighting conditions, moving the microscope mirrors and the brightness and contrast dials.
NOTE: The video needs to have a high contrast without being overexposed, so that the animals appear as black shapes on a bright background. - Click on “Time Scan” and choose a folder and a file name. Set “Field Delay” to 20 msec and “Stop at Time” to 30 sec. Press “Live Review” in order to choose the area of the plate to record (where most worms are). Tap the plate 3 or 4 times on the stage, quickly confirm its position in the field of view and press “Start”.
- After the movie finishes recording, right click on the image and choose “Export Montage Sequence” to export the movie from a .cxd format to an .avi.
- Analyzing the motility videos
- Open the ImageJ software, go to the “Plugins” tab, then “wrmtrck” and select “wrMTrck Batch”. Select the directory containing all the files to be analyzed.
- In the main input window of wrMTrck_Batch, load the input values as detailed in Figure 5C. Explanation for each of the parameters can be found in the instructions that accompany the plugin. Click “OK” and let the movement analysis run.
- To curate the results and confirm that all detected tracks are from actual C. elegans and eliminate artifacts, open each of the .txt files created for each movie and copy the information to a data analysis software file. Open the “*_labels.zip” files created and run the resulting “*_labels.tif” to manually check and eliminate false worm tracks.
Results
Monitoring intercellular spreading of prion-like proteins by in vivo time-lapse imaging
Transgenic C. elegans lines expressing the prion domain are particularly well suited for the analysis of certain aspects of prion-like proteins, e.g., cell-to-cell transmission and non cell autonomous toxicity. The transparency of the animals enables tracking of fluorescently tagged proteins from within the living organism at ever...
Discussion
The methods described here help to illustrate spreading and the complex cell autonomous and non cell autonomous toxicity of prion-like proteins. We recently discovered that an aggregation-prone cytosolic prion domain is taken up into membrane-bound vesicles in an autophagy related process. A specific subset of these vesicles transports the prion domain within and between cells and tissues40. The key to monitor their movement in the living animal is that the protein has to be tagged with mRFP, because only mRFP...
Disclosures
The authors declare no competing financial interests.
Acknowledgements
We thank Cindy Voisine and Yoko Shibata for helpful discussion and critical comments on the manuscript. We acknowledge the High Throughput Analysis Laboratory (HTAL) and the Biological Imaging Facility (BIF) at Northwestern University for their assistance. This work was funded by grants from the National Institutes of Health (NIGMS, NIA, NINDS), the Ellison Medical Foundation, and the Daniel F. and Ada L. Rice Foundation (to R.I.M.). C.I.N.-K. was supported by the Deutsche Forschungsgemeinschaft (KR 3726/1-1).
Materials
| Name | Company | Catalog Number | Comments |
| Reagent | |||
| Nanosphere size standards 100 nm | ThermoScientific | 3100A | |
| Levamisole | Sigma | L-9756 | |
| IPTG | Sigma | 15502-10G | |
| Ahringer RNAi library | Source BioScience LifeSciences | http://www.lifesciences.sourcebioscience .com/clone-products/non-mammalian/c-elegans/c-elegans-rnai-library/ | |
| Equipment | |||
| Sorvall Legend XTR Refrigerated Centrifuge, 120VAC | ThermoScientific | 75004521 | http://www.coleparmer.com/Product/Thermo_Scientific_Sorvall_Legend_ XTR_Refrigerated_Centrifuge_120 VAC/EW-17707-60 |
| 96 pin replicator | Scionomix | http://www.scinomix.com/all-products/96-pin-replicator/ | |
| HiGro high-capacity, incubating shaker | Digilab | http://www.digilabglobal.com/higro | |
| Multidrop Combi Reagent Dispenser | Titertrek | http://groups.molbiosci.northwestern.edu/hta/titertek.htm | |
| Biomek FX AP96 Automated Workstation | Beckman Coulter | http://groups.molbiosci.northwestern.edu/hta/biomek_multi.htm | |
| Innova44 shaker | New Brunswick | http://www.eppendorf.com/int///index.php?sitemap=2.3&pb=d78efbc05310ec 04&action=products&contentid=1& catalognode=83389 | |
| M205 FA | Leica | http://www.leica-microsystems.com/de/produkte/stereomikroskope-makroskope/fluoreszenz/details/product/leica-m205-fa/ | |
| ORCA-R2 C10600-10BDigital CCD camera | Hamamatsu | http://www.hamamatsu.com/jp/en/community/life_science_camera/product/search/C10600-10B/index.html | |
| Spinning Disc AF Confocal Microscope | Leica | http://www.leica-microsystems.com/products/light-microscopes/life-science-research/fluorescence-microscopes/details/product/leica-sd-af/ | |
| Falcon 4M60 camera | Teledyne Dalsa | http://www.teledynedalsa.com/imaging/products/cameras/area-scan/falcon/PT-41-04M60/ | |
| Software | |||
| MetaMorph Microscopy Automation & Image Analysis Software | Molecular Devices | http://www.moleculardevices.com/products/software/meta-imaging-series/metamorph.html | |
| Hamamatsu SimplePCI Image Analysis Software | Meyer Instruments | http://meyerinst.com/imaging-software/hamamatsu/index.htm | |
| ImageJ | NIH | http://rsbweb.nih.gov/ij/download.html | |
| wrMTrck plugin for ImageJ | http://www.phage.dk/plugins/wrmtrck.html | ||
| C. elegans strains | |||
| N2 (WT) | Caenorhabditis Genetics Center (CGC) | http://www.cgc.cbs.umn.edu/strain.php?id=10570 | |
| AM815 rmIs323[myo-3p::sup35(r2e2)::rfp] | Morimoto lab | available from our laboratory | |
| See table 1 for a source for folding sensor and stress reporter strains |
References
- Prusiner, S. B. Novel proteinaceous infectious particles cause scrapie. Science. 216 (4542), 136-144 (1982).
- Jarrett, J. T., Lansbury, P. T. Seeding 'one-dimensional crystallization' of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie. Cell. 73 (6), 1055-1058 (1993).
- Caughey, B., Kocisko, D. A., Raymond, G. J., Lansbury, P. T. Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistant state. Chem Biol. 2 (12), 807-817 (1995).
- Wickner, R. B. [URE3] as an altered URE2 protein: evidence for a prion analog in Saccharomyces cerevisiae. Science. 264 (5158), 566-569 (1994).
- Chien, P., Weissman, J. S., DePace, A. H. Emerging principles of conformation-based prion inheritance. Annu Rev Biochem. 73, 617-656 (2004).
- Kimberlin, R. H., Walker, C. A. Pathogenesis of mouse scrapie: patterns of agent replication in different parts of the CNS following intraperitoneal infection. J R Soc Med. 75 (8), 618-624 (1982).
- Beekes, M., McBride, P. A., Baldauf, E. Cerebral targeting indicates vagal spread of infection in hamsters fed with scrapie. J Gen Virol. 79 (3), 601-607 (1998).
- Jucker, M., Walker, L. C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 501 (7465), 45-51 (2013).
- Aguzzi, A. Cell biology: Beyond the prion principle. Nature. 459 (7249), 924-925 (2009).
- Scherzinger, E., et al. Self-assembly of polyglutamine-containing huntingtin fragments into amyloid-like fibrils: implications for Huntington's disease pathology. Proc Natl Acad Sci U S A. 96 (8), 4604-4609 (1999).
- Wood, S. J., et al. alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J Biol Chem. 274 (28), 19509-19512 (1999).
- Wang, Y. Q., et al. Relationship between prion propensity and the rates of individual molecular steps of fibril assembly. J Biol Chem. 286 (14), 12101-12107 (2011).
- Cushman, M., Johnson, B. S., King, O. D., Gitler, A. D., Shorter, J. Prion-like disorders: blurring the divide between transmissibility and infectivity. J Cell Sci. 123 (8), 1191-1201 (2010).
- Tanaka, M., Collins, S. R., Toyama, B. H., Weissman, J. S. The physical basis of how prion conformations determine strain phenotypes. Nature. 442 (7102), 585-589 (2006).
- Winkler, J., Tyedmers, J., Bukau, B., Mogk, A. Chaperone networks in protein disaggregation and prion propagation. J Struct Biol. 179 (2), 152-160 (2012).
- Ilieva, H., Polymenidou, M., Cleveland, D. W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 187 (6), 761-772 (2009).
- Nussbaum-Krammer, C. I., Morimoto, R. I. Caenorhabditis elegans as a model system for studying non-cell-autonomous mechanisms in protein-misfolding diseases. Dis Model Mech. 7 (1), 31-39 (2014).
- Lino, M. M., Schneider, C., Caroni, P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 22 (12), 4825-4832 (2002).
- Li, J. Y., et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 14 (5), 501-503 (2008).
- Desplats, P., et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 106 (31), 13010-13015 (2009).
- Clement, A. M., et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 302 (5642), 113-117 (2003).
- Gu, X., et al. Pathological cell-cell interactions elicited by a neuropathogenic form of mutant Huntingtin contribute to cortical pathogenesis in HD mice. Neuron. 46 (3), 433-444 (2005).
- Yamanaka, K., et al. Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice. Proc Natl Acad Sci U S A. 105 (21), 7594-7599 (2008).
- Garden, G. A., et al. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J Neurosci. 22 (12), 4897-4905 (2002).
- Raeber, A. J., et al. Astrocyte-specific expression of hamster prion protein (PrP) renders PrP knockout mice susceptible to hamster scrapie. EMBO J. 16 (20), 6057-6065 (1997).
- Yazawa, I., et al. Mouse model of multiple system atrophy alpha-synuclein expression in oligodendrocytes causes glial and neuronal degeneration. Neuron. 45 (6), 847-859 (2005).
- Lobsiger, C. S., Cleveland, D. W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 10 (11), 1355-1360 (2007).
- Sambataro, F., Pennuto, M. Cell-autonomous and non-cell-autonomous toxicity in polyglutamine diseases. Prog Neurobiol. 97 (2), 152-172 (2012).
- Polymenidou, M., Cleveland, D. W. Prion-like spread of protein aggregates in neurodegeneration. J Exp Med. 209 (5), 889-893 (2012).
- Brundin, P., Melki, R., Kopito, R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 11 (4), 301-307 (2010).
- Braak, H., Braak, E., Bohl, J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 33 (6), 403-408 (1993).
- Meyer-Luehmann, M., et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 313 (5794), 1781-1784 (2006).
- Luk, K. C., et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 338 (6109), 949-953 (2012).
- Clavaguera, F., et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 11 (7), 909-913 (2009).
- Nonaka, T., et al. Prion-like Properties of Pathological TDP-43 Aggregates from Diseased Brains. Cell Rep. 4 (1), 124-134 (2013).
- Lundmark, K., et al. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci U S A. 99 (10), 6979-6984 (2002).
- Lai, C. H., Chou, C. Y., Ch'ang, L. Y., Liu, C. S., Lin, W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 10 (5), 703-713 (2000).
- Xu, X., Kim, S. K. The early bird catches the worm: new technologies for the Caenorhabditis elegans toolkit. Nat Rev Genet. 12 (11), 793-801 (2011).
- Boulin, T., Hobert, O. From genes to function: the C. elegans genetic toolbox. Wiley Interdiscip Rev Dev Biol. 1 (1), 114-137 (2012).
- Nussbaum-Krammer, C. I., Park, K. W., Li, L., Melki, R., Morimoto, R. I. Spreading of a prion domain from cell-to-cell by vesicular transport in Caenorhabditis elegans. PLoS Genet. 9 (3), e1003351 (2013).
- Chernoff, Y. O., Lindquist, S. L., Ono, B., Inge-Vechtomov, S. G., Liebman, S. W. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi. Science. 268 (5212), 880-884 (1995).
- Liu, J. J., Lindquist, S. Oligopeptide-repeat expansions modulate 'protein-only' inheritance in yeast. Nature. 400 (6744), 573-576 (1999).
- Halfmann, R., et al. Prions are a common mechanism for phenotypic inheritance in wild yeasts. Nature. 482 (7385), 363-368 (2012).
- Tyedmers, J., Madariaga, M. L., Lindquist, S. Prion switching in response to environmental stress. PLoS Biol. 6 (11), e294 (2008).
- Krammer, C., et al. The yeast Sup35NM domain propagates as a prion in mammalian cells. Proc Natl Acad Sci U S A. 106 (2), 462-467 (2009).
- Hofmann, J. P., et al. Cell-to-cell propagation of infectious cytosolic protein aggregates. Proc Natl Acad Sci U S A. 110 (15), 5951-5956 (2013).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , (2006).
- Berkowitz, L. A., Knight, A. L., Caldwell, G. A., Caldwell, K. A. Generation of Stable Transgenic C. elegans Using Microinjection. J. Vis. Exp. (18), e833 (2008).
- Evans, T. C. Transformation and microinjection. WormBook. , (2006).
- Shaham, S. Methods in cell biology. Wormbooks. , (2006).
- Kim, E., Sun, L., Gabel, C. V., Fang-Yen, C. Long-term imaging of Caenorhabditis elegans using nanoparticle-mediated immobilization). PLoS One. 8 (1), e53419 (2013).
- Fay, D. Genetic mapping and manipulation: Chapter 1-Introduction and basics. WormBook. , (2006).
- Kamath, R. S., Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 30 (4), 313-321 (2003).
- Rual, J. F., et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 14 (10B), 2162-2168 (2004).
- Shaner, N. C., Steinbach, P. A., Tsien, R. Y. A guide to choosing fluorescent proteins. Nat Methods. 2 (12), 905-909 (2005).
- Kern, A., Ackermann, B., Clement, A. M., Duerk, H., Behl, C. HSF1-controlled and age-associated chaperone capacity in neurons and muscle cells of C. elegans. PLoS One. 5 (1), e8568 (2010).
- Becker, J., Walter, W., Yan, W., Craig, E. A. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol Cell Biol. 16 (8), 4378-4386 (1996).
- Salvaterra, P. M., McCaman, R. E. Choline acetyltransferase and acetylcholine levels in Drosophila melanogaster: a study using two temperature-sensitive mutants. J Neurosci. 5 (4), 903-910 (1985).
- Goloubinoff, P., Mogk, A., Zvi, A. P., Tomoyasu, T., Bukau, B. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci U S A. 96 (24), 13732-13737 (1999).
- Schroder, H., Langer, T., Hartl, F. U., Bukau, B. D. n. a. K. DnaJ and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 12 (11), 4137-4144 (1993).
- Rampelt, H., et al. Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 31 (21), 4221-4235 (2012).
- Gupta, R., et al. Firefly luciferase mutants as sensors of proteome stress. Nat Methods. 8 (10), 879-884 (2011).
- Gidalevitz, T., Ben-Zvi, A., Ho, K. H., Brignull, H. R., Morimoto, R. I. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 311 (5766), 1471-1474 (2006).
- Ben-Zvi, A., Miller, E. A., Morimoto, R. I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci U S A. 106 (35), 14914-14919 (2009).
- Karady, I., et al. Using Caenorhabditis elegans as a model system to study protein homeostasis in a multicellular organism. J Vis Exp. (82), e50840 (2013).
- Gidalevitz, T., Krupinski, T., Garcia, S., Morimoto, R. I. Destabilizing protein polymorphisms in the genetic background direct phenotypic expression of mutant SOD1 toxicity. PLoS Genet. 5 (3), e1000399 (2009).
- Morley, J. F., Brignull, H. R., Weyers, J. J., Morimoto, R. I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 99 (16), 10417-10422 (2002).
- Brignull, H. R., Moore, F. E., Tang, S. J., Morimoto, R. I. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. J Neurosci. 26 (29), 7597-7606 (2006).
- Mohri-Shiomi, A., Garsin, D. A. Insulin signaling and the heat shock response modulate protein homeostasis in the Caenorhabditis elegans intestine during infection. J Biol Chem. 283 (1), 194-201 (2008).
- Libina, N., Berman, J. R., Kenyon, C. Tissue-specific activities of C. elegans DAF-16 in the regulation of lifespan. Cell. 115 (4), 489-502 (2003).
- Schatzl, H. M., et al. A hypothalamic neuronal cell line persistently infected with scrapie prions exhibits apoptosis. J Virol. 71 (11), 8821-8831 (1997).
- Keith, S. A., Amrit, F. R., Ratnappan, R., Ghazi, A. The C. elegans healthspan and stress-resistance assay toolkit. Methods. , (2014).
- Pierce-Shimomura, J. T., et al. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A. 105 (52), 20982-20987 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved