A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Dynamic Contrast Enhanced Magnetic Resonance Imaging of an Orthotopic Pancreatic Cancer Mouse Model
In This Article
Summary
The goal of this protocol is to apply dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) for orthotopic pancreatic tumor xenografts in mice. DCE-MRI is a non-invasive method to analyze microvasculature in a target tissue, and useful to assess vascular response in a tumor following a novel therapy.
Abstract
Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) has been limitedly used for orthotopic pancreatic tumor xenografts due to severe respiratory motion artifact in the abdominal area. Orthotopic tumor models offer advantages over subcutaneous ones, because those can reflect the primary tumor microenvironment affecting blood supply, neovascularization, and tumor cell invasion. We have recently established a protocol of DCE-MRI of orthotopic pancreatic tumor xenografts in mouse models by securing tumors with an orthogonally bent plastic board to prevent motion transfer from the chest region during imaging. The pressure by this board was localized on the abdominal area, and has not resulted in respiratory difficulty of the animals. This article demonstrates the detailed procedure of orthotopic pancreatic tumor modeling using small animals and DCE-MRI of the tumor xenografts. Quantification method of pharmacokinetic parameters in DCE-MRI is also introduced. The procedure described in this article will assist investigators to apply DCE-MRI for orthotopic gastrointestinal cancer mouse models.
Introduction
The overall goal of this method is to apply dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) for orthotopic pancreatic tumor xenografts in mice. DCE-MRI is a non-invasive method to assess microvasculature in a target tissue by monitoring the change of MR contrast over a certain period of time after injection. DCE-MRI has been utilized to diagnose malignant tumors and to assess tumor response to various therapies1-4. Quantitative DCE-MRI has presented high reproducibility5. To quantitate pharmacokinetic parameters of an MR contrast agent in a target tissue, all DCE-MR images acquired at different time points and T1 map obtained before contrast injection must be coregistered6. However, due to respiratory and peristaltic motions in the abdominal area, quantitative DCE-MRI has had limited application for gastrointestinal tumors.
Orthotopic pancreatic tumor models have been utilized to assess pancreatic-tumor response following biological therapies and chemotherapies7,8. Orthotopic tumor models are considered superior to conventional subcutaneous models, since the microenvironment in the original tumor site is reflected and thereby human tumor response to therapy can be more accurately predicted. However, the mouse pancreas is located in the left upper quadrant of the abdomen, so quantitative DCE-MRI of orthotopic pancreatic tumor xenografts in mice has not been readily implemented.
We have established a protocol of DCE-MRI of abdominal tumors in mice by fixing the tumors using an orthogonally bent plastic board to prevent motion transfer from the chest region9. The pressure applied by this board was localized on the abdominal area, and has not resulted in respiratory difficulty. An automated image coregistration technique has been validated for DCE-MRI of abdominal organs in a free-breathing mode, but it performs effectively only when the target regions move slowly and regularly10. Respiratory rate of animals is variable during imaging, so physical restraint in the abdominal area will be necessary to retrieve reliable pharmacokinetic parameters in orthotopic pancreatic tumor mouse models. We have successfully quantitated the pharmacokinetic parameters of an MR contrast agent in orthotopic pancreatic tumor xenografts using the orthogonally bent plastic board in DCE-MRI11-13. Here we present the detailed procedure of orthotopic pancreatic tumor modeling, DCE-MRI of the tumor xenografts in mice, and quantification of pharmacokinetic parameters.
Protocol
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
1. Orthotopic Pancreatic Tumor Mouse Modeling
- Culture standard human pancreatic-cancer cell lines in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. Maintain all cultures at 37 °C in humidified atmosphere with 5% CO2.
- Use 8-10 week-old female severe combined immunodeficient mice. Place animal cages at 12 hr light and 12 hr darkness cycle at RT (21 ± 2 °C) and 60% humidity.
- Anesthetize all animals using ventilation with 2% of isoflurane mixed with oxygen (2 L/min) throughout surgery. Confirm the depth of anesthesia by toe pinch reflex. Place animals on a heating pad (37 °C) to maintain the body temperature. Apply veterinary ophthalmic ointment on eyes to prevent dryness while under anesthesia.
- Remove the hair in the left upper quadrant of the abdomen of each mouse, and give an analgesic drug (carpofen, 5 mg/kg of body weight subcutaneously) into the area. Apply a betadine solution to the exposed skin. Prepare autoclaved surgical instruments.
- Make a 1 cm incision in the skin and peritoneum using iris straight scissors. Gently remove the pancreas from the abdomen using surgical tweezers.
- Insert 28 G needle of a 0.5 ml insulin syringe into the tail of pancreas and then slowly infuse a solution of 2.5 million human pancreatic cancer cells in 30 µl of DMEM. Confirm that a small bleb is created in the head of pancreas by the solution.
- Gently place the pancreas back into the abdomen using surgical tweezers. Close the peritoneum and the skin in 1 layer with 2 interrupted 5-0 Prolene sutures, and then terminate the anesthesia. Do not return an animal that has undergone surgery to the company of other animals until fully recovered. Remove sutures at 7~10 days post surgery.
- Give another dose of the analgesic drug (carpofen, 5 mg/kg of body weight subcutaneously) at 24 hr after surgery.
- Check tumor size by palpating the surgery area using two fingers. Tumors typically feel denser and bumpier than the surrounding tissues and organs. Usually it takes ~1 - 2 weeks to start feeling a tumor.
- Monitor animals daily for signs of illness. When animals appear ill (lack normal grooming and avoidance behaviors), we terminated them using cervical dislocation while under anesthesia.
2. Magnetic Resonance Imaging
- Apply MRI when tumor size is about 5 - 7 mm in diameter at usually 2~4 weeks after cell implantation. Use an MR scanner dedicated to small animal imaging or a clinical MR scanner equipped with a specialized coil for small animal imaging.
NOTE: We used a 9.4T small animal MR scanner with a combination of a 1H volume resonator/transmitter and a surface coil receiver (30 mm in diameter)(Bruker BioSpin Corp., Billerica, MA). A surface coil provides better signal-to-noise ratio (SNR)14. - Prepare a gadolinium-based MRI contrast agent to inject ~0.1 - 0.2 mmol/kg to each animal in ~0.1 - 0.2 ml PBS (phosphate buffered saline).
NOTE: We used gadoteridol, and injected 0.2 mmol/kg in 0.15 ml PBS over a period of 15 sec (0.1 ml/sec). - Prepare a micro-polyethelene tube (length: 7.62 mm, inner diameter: 0.28 mm, outer diameter: 0.64 mm). Insert a 30 G needle (12.7 mm length) into one end of the tube, and a 30 G blunt tip needle (9.5 mm length) into the other end. Connect a 1 ml syringe containing MR contrast agent to the blunt tip needle, and slowly push the syringe to fill up the entire tube with the MR contrast agent.
- Anesthetize animals using ventilation with ~1 - 2% of isoflurane mixed with oxygen (2 L/min) throughout preparation and imaging. Confirm the depth of anesthesia by toe pinch reflex. Apply veterinary ophthalmic ointment on eyes to prevent dryness while under anesthesia. Dilate the tail vein using a heat lamp before needle insertion. Grab the middle of the 30 G needle using Kelly forceps, and insert it into the tail vein. Tape both the tail and tube onto a piece of plastic or cardboard paper (10 mm width x 100 mm length) to keep the tail straight.
- Place the animal in supine position in an animal bed equipped with circulating warm water (or warm air) to regulate body temperature during imaging. Set the temperature on the bed to 37 °C. Insert a rectal temperature probe to monitor the body temperature during imaging.
- Apply an orthogonally bent plastic board into the abdominal area. Make sure the tumor is located behind the upper end of the board, and then pull down the board slightly (~2 mm) to ensure the tumor is caught by the board. Tape the board to animal bed firmly.
- Tape a respiration pad transducer (SA Instrument, Inc., Stony Brook, NY) on the chest area to monitor animal respiration during imaging. Place a surface coil on the top of the tumor region, and tape it to animal bed firmly. Push the animal bed into the MR scanner to place the tumor region at the center of the volume coil (inner diameter: 72 mm).
- Perform matching and tuning for both the receiver and transmitter, followed by shimming.
- Begin with an anatomical MR sequence to locate the tumor. Use a T2-weighted (T2W) turbo spin-echo sequence to obtain axial images with the following acquisition parameters. Repetition time (TR)/echo time (TE) = 3,000/34 msec, 128 x 128 matrix, 30 x 30 mm field of view, number of averages = 1, echo train length = 4, and 20 contiguous 1 mm thick slices in an interlaced mode to cover the entire tumor region (total scanning time: 1.6 min).
NOTE: Since orthotopic pancreatic tumors are more difficult to be located than subcutaneous ones, conventional localizer images having lower resolution may not be useful. - Acquire T1-weighted (T1W) images with various flip angles to retrieve T1 map. For this purpose, use a gradient echo multiflip angle approach with the following parameters: repetition time (TR)/echo time (TE) = 115/3 msec, 128 x 128 matrix, a 30 x 30 mm field of view, number of averages = 4, ~5 - 7 contiguous 1 mm thick slices in an interlaced mode to cover the tumor region, and seven flip angles of 10, 20, 30, 40, 50, 60, and 70 (total scanning time per flip angle: 1 min).
NOTE: However, multiflip angle approach is efficient only when B1 field homogeneity is high. If not, T1 maps can be obtained with multiple TR approach instead15. - Acquire T1W images before and after gadolinium based MR contrast injection. Use the same acquisition parameters and geometry for T1 mapping but with the fixed flip angle of 30. Use linear encoding to ensure steady state when the center of k-space is obtained, especially when a short TR and a low degree flip angle are used. Acquire 5 baseline images before contrast injection. Then acquire 40 images after contrast injection (total scanning time: 45 min). Use a syringe pump to inject contrast agent at a constant rate (0.01 ml/sec).
- Monitor animal breathing continuously, and adjust isoflurane concentration to keep the respiratory rate to 50 - 100 breaths per min. Monitor animal body temperature throughout imaging.
- After completing DCE-MRI, take off the needle and other probes, and place the animal in an empty cage bedded with paper towels. Massage softly the lower abdominal area. The cage should be placed half under a heat lamp to allow the animal to move in and out of the heat gradient as it recovers. Do not leave an animal unattended until it has regained sufficient consciousness to maintain sternal recumbency.
3. Image Processing and Analysis
- Segment tumor region in T2W images. In T2W images, the signal intensity in tumor region is brighter than that of surrounding tissues, so the tumor boundary can be manually delineated.
NOTE: Semi-automatic segmentation techniques such as global thresholding or active contouring can be used16,17, but uneven background intensity should be corrected especially when a surface coil is used. - Create T1 and proton density maps. In T1W images acquired with a gradient echo sequence, assuming that echo time (TE) is much less than T2* value, the pixel value is determined by
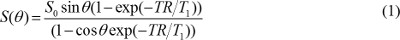
where S0 is proton density, T1 is T1 relaxation time constant, TR is repetition time, and θ is a flip angle. Equation (1) can be rewritten to
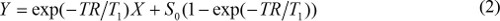
when S(θ)/sinθ is replaced with Y, and S(θ)/tanθ is replaced with X. Equation (2) is a linear equation, and its slope and intercept can be used to retrieve T1 and S0 values, respectively. - Calculate MR contrast concentration in DCE-MR images. When gadolinium based MR contrast agent is injected, T1 relaxation time constant is changed over time. So, equation (1) can be rewritten to
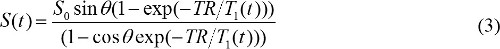
T1(t) is related with MR contrast concentration, C(t), as follows,

where r1 is longitudinal relaxivity of MR contrast agent. So, by combining equations (3) and (4), MR contrast concentration is determined by
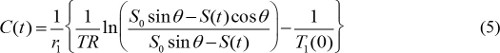
- Quantitate the pharmacokinetic parameters of MR contrast agent. Cp(t) presents MR contrast concentration in blood plasma at time t after initiating contrast injection. Cp(t) is called arterial input function (AIF). If AIF is available, the pharmacokinetic parameters of MR contrast agent can be calculated by
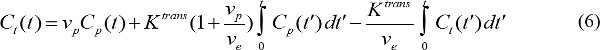
where Ct(t) is MR contrast concentration in a target tissue, vp is fractional blood plasma volume, ve is fractional extravascular extracellular volume, and Ktrans is volume transfer constant. Flux rate constant, kep, is equal to Ktrans divided by ve. If AIF is not available, then the reference region model can be used instead18,19. The reference region model is based on the flow-limited Kety model20 and uses contrast concentration in a reference region to remove the need for AIF as follows,
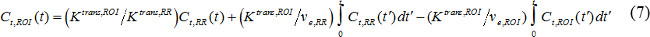
where Ct,ROI(t), Ktrans,ROI, and ve,ROI are contrast concentration, volume transfer constant, and fractional extravascular-extracellular volume, respectively, in the region of interest (ROI), while Ct,RR(t), Ktrans,RR, and ve,RR are those in the reference region. Paravertebral muscle is often selected as the reference region, and ve,RR in murine paravertebral muscle is assumed to be constant at 0.08 21. We used the reference region model.
Results
Human pancreatic tumor cells grow successfully in mouse pancreas creating a solid tumor. Figure 1 shows photographs of (A) a normal pancreas where tumor cell solution is injected, and (B) a representative mouse bearing an orthotopic pancreatic tumor xenograft (MIA PaCa-2). Tumor is located in the left upper quadrant of abdomen, next to the spleen. It usually takes 2 - 4 weeks for the tumors to grow up to 5 - 7 mm in diameter after cell implantation.
Motion of orthotopic pancre...
Discussion
We have introduced the detailed methods of orthotopic pancreatic tumor modeling using immunodeficient mice, DCE-MRI of abdominal tumors in mice, and quantification of its kinetic parameters. In orthotopic pancreatic tumor modeling, care must be taken when inserting a needle into the tail of pancreas. If successful, cells will be transferred to the head of pancreas creating a small bleb. When applying an orthogonally bent plastic board, it is critical to confirm that the tumor is located below the upper end of the board. ...
Disclosures
Authors do not have any conflicts of interest to declare.
Acknowledgements
Authors thank Jeffrey Sellers to assist orthotopic pancreatic cancer mouse modeling. This work was supported by Research Initiative Pilot Awards from the Department of Radiology at UAB and NIH grants 2P30CA013148 and P50CA101955.
Materials
| Name | Company | Catalog Number | Comments |
| DMEM | Invitrogen | 11965-118 | |
| Fetal bovine serum | Harlan Laboratories | BT-9501 | |
| Betadine | Purdue products | 67618-153-01 | |
| 5-0 Prolene sutures | Ethicon | 8720H | |
| 9.4T MR scanner | Bruker Biospin Corporation | BioSpec 94/20 USR | |
| Gadoteridol | Bracco Diagnostics Inc | NDC 0270-1111-03 | |
| Micro-polyethelene tube | Strategic Applications, Inc | #PE-10-25 | |
| 30 G blunt tip needle | Strategic Applications, Inc | 89134-194 | |
| Monitoring and gating system | SA instruments, Inc | Model 1030 | This is an MR compatiable system to measure resiratory rating and body temperature of small animals at the same time. |
| Syringe pump | New Era Pump Systems, Inc. | NE-1600 |
References
- Ergul, N., et al. Assessment of multifocality and axillary nodal involvement in early-stage breast cancer patients using 18F-FDG PET/CT compared to contrast-enhanced and diffusion-weighted magnetic resonance imaging and sentinel node biopsy. Acta Radiol. , (2014).
- Park, J. J., et al. Assessment of early response to concurrent chemoradiotherapy in cervical cancer: value of diffusion-weighted and dynamic contrast-enhanced MR imaging. Magn Reson Imaging. , (2014).
- Nguyen, H. T., et al. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging. 10, (2014).
- Teo, Q. Q., Thng, C. H., Koh, T. S., Ng, Q. S. Dynamic Contrast-enhanced Magnetic Resonance Imaging: Applications in Oncology. Clin Oncol (R Coll Radiol). , (2014).
- Zhang, X., Pagel, M. D., Baker, A. F., Gillies, R. J. Reproducibility of magnetic resonance perfusion imaging. PLoS One. 9 (2), e89797 (2014).
- Kim, H., et al. Pancreatic adenocarcinoma: a pilot study of quantitative perfusion and diffusion-weighted breath-hold magnetic resonance imaging. Abdominal imaging. , (2014).
- Derosier, L. C., et al. Combination Treatment with TRA-8 Anti Death Receptor 5 Antibody and CPT-11 Induces Tumor Regression in an Orthotopic Model of Pancreatic Cancer. Clin Cancer Res. 13 (18), 5535s-5543s (2007).
- Derosier, L. C., et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 6 (12), 3198-3207 (2007).
- Kim, H., et al. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging. Cancer Res. 68 (20), 8369-8376 (2008).
- Klein, S., Staring, M., Murphy, K., Viergever, M. A., Pluim, J. P. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 29 (1), 196-205 (2010).
- Kim, H., et al. Early therapy evaluation of combined cetuximab and irinotecan in orthotopic pancreatic tumor xenografts by dynamic contrast-enhanced magnetic resonance imaging. Mol Imaging. 10 (3), 153-167 (2011).
- Kim, H., et al. Antagonistic effects of anti-EMMPRIN antibody when combined with chemotherapy against hypovascular pancreatic cancers. M Mol Imaging Biol. 16 (1), 85-94 (2014).
- Kim, H., et al. Dual combination therapy targeting DR5 and EMMPRIN in pancreatic adenocarcinoma. Mol Cancer Ther. 11 (2), 405-415 (2012).
- Moyher, S. E., Vigneron, D. B., Nelson, S. J. Surface coil MR imaging of the human brain with an analytic reception profile correction. J Magn Reson Imaging. 5 (2), 139-144 (1995).
- Voigt, T., Nehrke, K., Doessel, O., Katscher, U. T1 corrected B1 mapping using multi-TR gradient echo sequences. Magn Reson Med. 64 (3), 725-733 (2010).
- Liu, H., Liu, Y., Zhao, Z., Zhang, L., Qiu, T. A new background distribution-based active contour model for three-dimensional lesion segmentation in breast DCE-MRI. Medical physics. 41 (8), 082303 (2014).
- Sarkar, S., Das, S. Multilevel image thresholding based on 2D histogram and maximum Tsallis entropy--a differential evolution approach. IEEE Trans Image Process. 22 (12), 4788-4797 (2013).
- Yankeelov, T. E., et al. Quantitative pharmacokinetic analysis of DCE-MRI data without an arterial input function: a reference region model. Magn Reson Imaging. 23 (4), 519-529 (2005).
- Cardenas-Rodriguez, J., Howison, C. M., Pagel, M. D. A linear algorithm of the reference region model for DCE-MRI is robust and relaxes requirements for temporal resolution. Magn Reson Imaging. 31 (4), 497-507 (2013).
- Tofts, P. S., et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 10 (3), 223-232 (1999).
- Yankeelov, T. E., et al. Comparison of a reference region model with direct measurement of an AIF in the analysis of DCE-MRI data. Magn Reson Med. 57 (2), 353-361 (2007).
- Cao, R. Y., Amand, T., Ford, M. D., Piomelli, U., Funk, C. D. The Murine Angiotensin II-Induced Abdominal Aortic Aneurysm Model: Rupture Risk and Inflammatory Progression Patterns. Front Pharmacol. 1 (9), (2010).
- Parker, G. J., et al. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med. 56 (5), 993-1000 (2006).
- Tseng, W., Leong, X., Engleman, E. Orthotopic mouse model of colorectal cancer. J Vis Exp. (10), 484 (2007).
- Bhullar, J. S., et al. A true orthotopic gastric cancer murine model using electrocoagulation. J Am Coll Surg. 217 (1), 64-70 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved