A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Stable and Efficient Genetic Modification of Cells in the Adult Mouse V-SVZ for the Analysis of Neural Stem Cell Autonomous and Non-autonomous Effects
In This Article
Summary
Here we describe a procedure based on the use of lentiviral particles for the long-term genetic modification of neural stem cells and/or their adjacent ependymal cells in the adult ventricular-subventricular neurogenic niche which allows the separate analysis of cell autonomous and non-autonomous, niche-dependent effects on neural stem cells.
Abstract
Relatively quiescent somatic stem cells support life-long cell renewal in most adult tissues. Neural stem cells in the adult mammalian brain are restricted to two specific neurogenic niches: the subgranular zone of the dentate gyrus in the hippocampus and the ventricular-subventricular zone (V-SVZ; also called subependymal zone or SEZ) in the walls of the lateral ventricles. The development of in vivo gene transfer strategies for adult stem cell populations (i.e. those of the mammalian brain) resulting in long-term expression of desired transgenes in the stem cells and their derived progeny is a crucial tool in current biomedical and biotechnological research. Here, a direct in vivo method is presented for the stable genetic modification of adult mouse V-SVZ cells that takes advantage of the cell cycle-independent infection by LVs and the highly specialized cytoarchitecture of the V-SVZ niche. Specifically, the current protocol involves the injection of empty LVs (control) or LVs encoding specific transgene expression cassettes into either the V-SVZ itself, for the in vivo targeting of all types of cells in the niche, or into the lateral ventricle lumen, for the targeting of ependymal cells only. Expression cassettes are then integrated into the genome of the transduced cells and fluorescent proteins, also encoded by the LVs, allow the detection of the transduced cells for the analysis of cell autonomous and non-autonomous, niche-dependent effects in the labeled cells and their progeny.
Introduction
The murine ventricular-subventricular zone (V-SVZ), in the walls of the lateral ventricle facing the striatum, is a very active germinal region in which a continual process of progenitor cell replication and differentiation results in the persistent production of olfactory bulb (OB) interneurons and corpus callosum oligodendrocytes1. The lifelong generation of these cells appears to be supported by the presence in this region of neural stem cells (NSCs; also called B1 cells), which express the astrocytic antigen glial fibrillary acidic protein (GFAP) and stem cell markers such as nestin, Id1 and Sox22. GFAP-expressing B1 cells generate transit amplifying progenitor (TAP) cells (C cells), which express transcription factors Dlx2 (distal-less homeobox 2) and Ascl1 (mammalian achaete-schute homolog 1) and divide rapidly a few times before they give rise to migrating neuroblasts (A cells) or oligodendroblasts3. Newly-generated proliferative neuroblasts migrate anteriorly, forming the rostral migratory stream (RMS) to the OB, where they integrate into the granular and glomerular layers as differentiated inhibitory interneurons. Migrating young oligodendroblasts move to the CC, where they become immature NG2-positive cells that continue to divide locally or differentiate into mature myelinating oligodendrocytes1,4.
B1 cells, which derive from fetal radial glial cells, retain the elongated and polarized morphology of their predecessors and exhibit a highly specialized relationship with their niche. They span between the ependyma which lines up the ventricle and the network of blood vessels that irrigate the V-SVZ niche. The small apical process of B1 cells intercalates among multiciliated ependymocytes and ends in a single non-motile primary cilium, whereas their basal process extends long distances to approach the planar vascular plexus that irrigates this niche ending in the basal lamina of the plexus capillaries2,5-8 .
The most reliable way to distinguish B1-NSCs from non-neurogenic astrocytes, which are also GFAP+, in the intact V-SVZ niche is based on whole-mount preparations of the ventricle lateral wall and their analysis by 3-D confocal microscopy after immunostaining for GFAP to label the thin B1-NSC apical process, β-catenin to delineate cell membranes, and either γ-tubulin as a marker of cilial basal bodies or acetylated α-tubulin to label the extent of each cilium5,8. Observations of these whole-mounts from the ventricular surface have indicated that B1 and ependymal cells are arranged in "pinwheels"5, in which the uniciliated apical processes of one or several GFAP+ B1 cells are encircled by a rosette of multiciliated ependymal cells.
The characteristic morphology of B1 cells correlates with experimental evidence indicating that blood vessels/endothelial cells and ventricular cerebrospinal fluid (CSF) constitute regulated sources of soluble signals acting on NSCs2,6,9-11. At the ventricular surface, homotypic and heterotypic apico-lateral interactions involving ependymal and B1 cells include tight junctions and adherens junctions5,12. Moreover, adhesion molecules implicated in the junctional complexes between B1 and ependymal cells, such as N-cadherin and V-CAM, have been shown to regulate not only the highly organized positioning of B1 in the V-SVZ niche, but also their quiescence12,13. The ependymal-B1 cell monolayer appears to act as a diffusion barrier allowing the regulated flux of water and small molecules from the CSF, but restricting the intercellular passage of large proteins10,11. Experimental evidence indicates that the uniquely positioned B1 cell apical cilium could play a role as a sensor of signaling polypeptides present in the CSF2,5-7. Ependymal cells are, per se, also a source of soluble and membrane-bound signals with a role in the regulation of NSC behavior14,15.
Traceable nucleosides, such as bromo-deoxyuridine (BrdU), or retroviruses have been widely used to label progenitor cells, including NSCs, in vivo. However, these methods are not optimal for long-term fate tracing because BrdU signals dilute through repeated cell divisions and retroviruses appear to preferentially target transiently amplifying cells due to their requirement of cell proliferation for transduction16,17. To examine NSC physiology in vivo, including interactions with niche components, it is crucial to establish a method to label and trace rarely dividing cells, as B1-NSCs are largely quiescent and their neighboring ependymal cells never divide under physiological conditions3. Here, we show that lentiviral vectors (LVs) allow for high-efficiency gene marking and long-term modification of adult NSCs and non-dividing ependymal cells, due most reasonably to their ability to transduce and to integrate into the genome of target cells in a cell cycle-independent way. Moreover, we show how the route of delivery and viral titer help to specifically transduce ependymal cells, but not B1 cells thereby allowing the analysis of niche-dependent, ependymal effects on NSCs.
Protocol
ETHICS STATEMENT: This protocol follows the animal care guidelines of the University of Valencia in compliance with European directive 2010/63/EU.
1. Generation of LV for In Vivo Marking Studies (see Figure 1a)
CAUTION: The procedure described herein is biosafety level 2, therefore perform all the following procedures in a biohazard hood. Ensure that research personnel are appropriately qualified and trained in all procedures. Wear personal protective equipment, including gown, double gloves and suitable eye protection. Finally, thoroughly decontaminate all tools and surfaces that could have been in contact with viruses according to approved facility disinfection practices (by wiping with 70% ethanol, 10% bleach and/or autoclaving).
- Production of LV in Human Embryonic Kidney 293T Cells
- Start this protocol by preparing pure DNA for transfection. Prepare and purify each plasmid by double CsCl gradient centrifugation or other commercially available column methods yielding endotoxin-free DNA. In this protocol we have used the transfer vector plasmid pRRL-SIN-PPT.PGK.EGFP.Wpre. Recommended core packaging plasmids are pMDLg/pRRE and pRSV.REV and envelope plasmid pMD2G13,18,19.
- Twenty-four hr before transfection, plate 5 x 106 293T cells in Iscove's Modified Dulbecco's Medium (IMDM) (see Table of Materials) in a 10 cm plastic dish in order to obtain an approximately 1/4 to 1/3 confluent culture for transfection. Incubate at 37 °C in a humidified incubator in an atmosphere of 5-7% CO2.
- Replace the medium with fresh medium 2 hr before transfection.
- In a sterile 1.5 ml microcentrifuge tube mix 10 µg of transfer vector plasmid (containing the cDNA of the transgene or the shRNA to be delivered) with 2.5 µg of the pRSV.REV and 5 µg of the pMDLg/pRRE packaging plasmids, and 3.5 µg of the envelope plasmid pMD2G. Make up the plasmid solution to a final volume of 450 µl with 0.1x TE buffer (see Table of Materials) /dH20 (2:1). Then add 50 µl of 2.5 M CaCl2.
- Form the precipitate by dropwise addition of 500 µl of the 2x Hepes Buffered Saline(HBS, see Table of Materials) solution to the 500 µl DNA-TE-CaCl2 mixture while vortexing at full speed.
- Add the precipitate to the 293T cells immediately. Gently swirl the plate to mix. Return the cells to the incubator and change the medium 14-16 hr after transfection.
- Collect the cell supernatants 30 hr after changing the media. Filter supernatant through a 0.22 µm pore nitrocellulose filter and proceed to concentration.
- Concentration of LVs
- Concentrate the conditioned medium by ultracentrifugation at 50,000 x g (19,000 rpm with SW-28 ultracentrifuge rotor) for 2 hr at room temperature (RT) in a 30 ml polypropylene transparent conical rotor tube.
Note: Use ultracentrifuge adapters for conical rotor tubes (see table of Materials). - Discard the supernatants by decanting and resuspend the pellets in a small volume (200 µl or less if only one centrifugation is performed) of phosphate buffer saline (PBS; see Table of Materials). Then pipette up and down about 20 times.
- Pool the suspensions and concentrate again by ultracentrifugation, also at 50,000 x g (23,000 rpm with SW-55 ultracentrifuge rotor) for 2 hr at room temperature. Use polypropylene transparent rotor tubes with a nominal volume of 5 ml (see Table of Materials).
- Resuspend the final pellet in a very small volume (1/500 or 1/1,000 of the starting volume of medium) of sterile PBS and shake on a rotating wheel for 1 hr at RT. Split into small aliquots (5-20 µl) and freeze them at -80 °C.
- Treat all empty tubes with 10% bleach before discarding.
- Concentrate the conditioned medium by ultracentrifugation at 50,000 x g (19,000 rpm with SW-28 ultracentrifuge rotor) for 2 hr at room temperature (RT) in a 30 ml polypropylene transparent conical rotor tube.
- Lentiviral Titration Using Flow Cytometry
- The day before, plate 5 x 104 HeLa cells per well in 6-well tissue culture plates in 2 ml Dulbecco's Modified Eagle's Medium (DMEM) (see Table of Materials). Incubate at 37 °C in a humidified incubator in an atmosphere of 5-7% CO2 for 24 hr.
- On the day of titration, thaw an aliquot of the viral stock and prepare serial dilutions, from 10-3 to 10-8, in DMEM.
- To do so, take a 24-well plate and add 2 ml of DMEM to the first well and 1.8 ml to the following wells. Then add to the first well 2 µl of the concentrated viral stock (to a final dilution of 1:1,000 or 10-3).
- After pipetting several times to thoroughly mix the solution, change tip and transfer 200 µl of the 10-3 dilution to the second well. Repeat the procedure serially in the following wells until the 10-8 dilution is made.
- Take HeLa cells plated the previous day from the incubator. Carefully remove medium from wells. Add 1 ml of each viral dilution together with 1 µl of 8 mg/ml hexadimethrine bromide to the HeLa cell-containing wells. Gently swirl the plate to mix.
Note: Hexadimethrine bromide is added to increase the virus adsorption to the cells in culture. - Return the cells to the incubator and allow the infection to proceed for 72 hr. After that, remove the medium, wash the cells once with PBS and add 200 µl of trypsin-EDTA (see Table of Materials) to each well.
- After 5 min at 37 °C, add 2 ml of PBS to each well and harvest cells in flow cytometry tubes.
- Centrifuge at 300 x g for 5 min at RT and aspirate the supernatant.
- Resuspend the pellet with 1 ml of fixing solution (1% formaldehyde electron microscopy grade and 2% fetal bovine serum in PBS), then vortex the tubes.
- Analyze the cells in a flow cytometer using a 488 nm argon-ionlaser at 15 mW power.
- Set up the instrument with the standard configuration: forward-scatter (FS), side-scatter (SS), and fluorescence for GFP (525/40 nm). Select cell population gating in a FS vs. SS dot plot to exclude cell aggregates and debris. Collect fluorescence in logarithmic scale. Calculate the number of GFP+ cells in each sample.
- Calculate vector titer using the following formula: % GFP+/100 x number of cells infected x dilution factor (DF) = transducing units (TU)/ml.
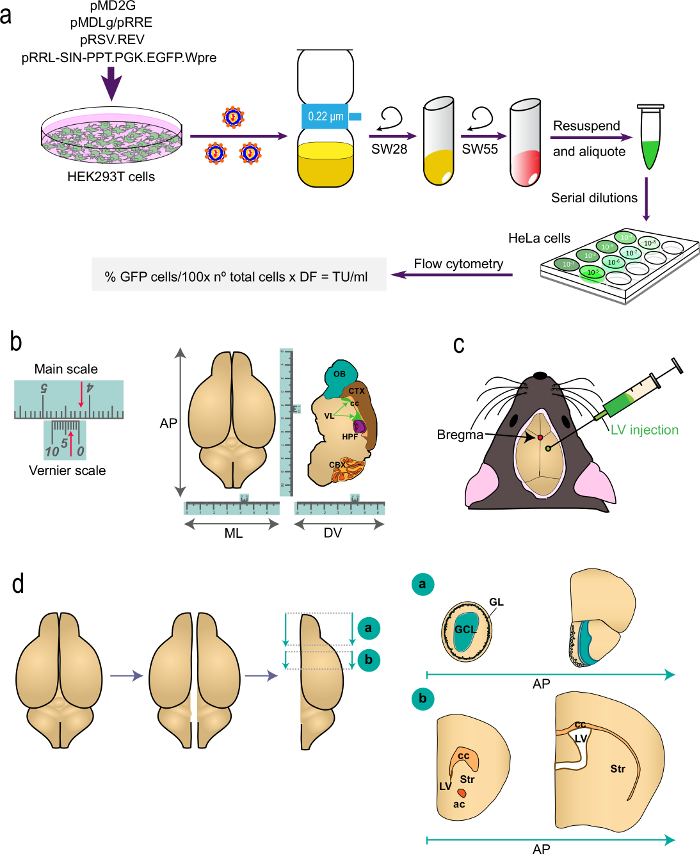
Figure 1: Schematic representation of the different parts of the procedure. (a) Part 1 of the protocol: generation of LVs for in vivo labeling studies, from the transfection of HEK293T cells with appropriate plasmids to generate the LVs to the determination of the virus titer by flow cytometry using the indicated formula. The names of the plasmids and the centrifuge rotors are indicated. (b and c) Part 2 of the protocol: stereotaxic injection of LVs. "b" depicts an example of a Vernier scale, a device that is part of stereotaxic instruments and serves for fine measurements. As an example, the arrows indicate 4.23 cm. A Vernier scale is used to determine the coordinates in the antero-posterior (AP), medio-lateral (ML), and dorso-ventral (DV) axis as shown for a top-view (left) and for a sagittal section (right) of the brain. "c" indicates the position of bregma as the intersection between the sagittal and coronal sutures. LVs are injected using a syringe. (d) Schematic drawings showing how the brain is processed for analysis. The two hemispheres are split and each one is divided into two blocks. Block "a", containing the OBs, is produced by a coronal cut at the AP level immediately posterior to the OB junction with the telencephalon (bregma 2.46 mm; see Paxinos´ Atlas for a reference). Block "b" is produced by two coronal cuts, one at the level just anterior to the most rostral aspect of the corpus callosum (bregma 1.7 mm) and a second one at the level of the junction of the two lateral ventricles (bregma -0.22 mm). GL, glomerular layer; GCL, granule cell layer; st, striatum; cc, corpus callosum; ac, anterior commissure; lv, lateral ventricle.
2. Stereotaxic Injection of LV into the V-SVZ/Striatum Border or into the Lateral Ventricle (see Figure 1b)
- Preparation
- Sterilize a 5 µl capacity syringe with a 33 gauge needle by spraying down the body and needle with 70% ethanol with the plunger pulled out all the way. Repeatedly aspirate ethanol from a 1.5 ml microcentrifuge tube and eject it all the way out several times, and rinse the syringe thoroughly with sterile water afterwards. Place the syringe safely aside in the culture hood and allow it to dry.
- Prepare a biohazard waste container with 10% bleach to a suitable volume for immersion of all waste from this procedure (generally 200 ml in a 500 ml container).
- Prepare and preheat a 37 °C waterbed by filling a sealable plastic storage bag with water and warming it to 37 °C. This will allow mice to recover following injection.
- Remove viral stocks from -80 °C freezer storage 1 hr before starting the injections and place the vial on a rotating wheel at RT. After thawing, maintain the viral stock on ice during the time of the injections. Prior to the stereotaxic injection of LV, dilute the concentrated viral stocks to 106 TU/μl using PBS in the culture hood.
- Sanitize the area selected for performing the surgery with 70% ethanol.
- Microinjection of LV
- Select and sterilize tools needed for surgery (scalpel, drill, and small tweezers).
- Anesthetize a 6-8 week-old mouse by intraperitoneally (ip) injecting a veterinary-supervised mixture of ketamine and medetomidine. Weigh each animal and dose each with 50-75 mg ketamine and 0.5-1 mg medetomidine per kg of mouse body weight (around 100-125 µl of the ketamine/medetomidine working solution per mouse).
- Assess the anesthetic plane by pinching the toes, tail or ear and ensuring that the animal shows no reaction.
- Once the mouse is anesthetized, inject butorphanol subcutaneously at a final dose of 0.4-0.5 mg per kg mouse weight to minimize post-surgical pain.
- Shave the area between the ears and disinfect the skin using an iodophor such as iodopovidone or 70% ethanol. Cleanse using sterile cotton-tipped applicators. Be careful not to excessively wet the animal as this can exacerbate hypothermia.
- Place the animal in prone position on a stereotaxic frame and carefully fix the head using the ear bars and the palate support of the apparatus. Keep the mouse with a heating pad set at 37 °C and apply ophthalmic lubricant to the eyes.
- Make a 1 cm long incision on the head skin longitudinally using a scalpel, and gently retract the skin to expose the skull using fine tweezers.
- Carefully clean the bone surface with a sterile cotton-tipped applicator. Cleanse the exposed skull bone of any remaining tissue.
- Mount the sterilized syringe on the stereotactic device using the syringe holder.
- Move the syringe holder x, y and z axis until the tip of the syringe needle is positioned on the bregma, the conjunction point where the sagittal (longitudinal and medial) suture is perpendicularly intersected by the coronal suture (Figure 1b). Ensure that the "zero" position of the dorso-ventral (DV) axis is at the skull surface at bregma.
- Move the syringe to the x and y destination coordinates (see Table 1 and Figure 1b).
| Region of injection | Coordinates | ||
| Antero-posterior (AP) | Medio-lateral (ML) | Dorso-ventral (DV) | |
| SEZ/striatum border | +0.6 mm | +1.2 mm | -3.0 mm |
| Lateral ventricle | -0.3 mm | +1.0 mm | -2.6 mm |
Table 1: Stereotaxic coordinates for the injections. For the AP and ML axis, x and y coordinates are given as a distance (in mm) from bregma. "-" indicates "towards posterior". For the DV coordinates "zero" is the surface of the skull at the bregma point and DV coordinates indicate the distance (in mm) down from this point.
- Annotate the x, y and z destination coordinates in the Vernier scale in order to be able to come back to the injection site later on. Mark the bone at the x and y coordinates using a surgical marker pen.
- Move the syringe away from the working area.
- Using an electric drill make a hole on the skull carefully not to damage the brain. Do not drill the pial surface as this may damage the brain surface.
- Load the syringe with 1 µl of the 106 TU/µl viral solution. Use a 33 gauge sharp beveled needle whose tip has an angle of 10-12°. Position the syringe needle at a 90° angle with respect to the brain surface.
- Move the syringe back to the site of injection and move it down until the tip touches the pial surface.
- Penetrate the brain with the syringe to the z coordinate in the DV axis.
- Slowly release the viral suspension, at a rate of 0.2 µl/min, in order to minimize damage to the brain tissue due to excessive fluid pressure.
- Wait for 5-10 min to minimize the backflow of viral suspension and then retract the syringe very slowly. Blot any excess of liquid that may appear at the surface as a result of the retraction of the syringe using a laboratory wipe and place it immediately in the bleach-containing biosafety waste container.
- Take the animal out of the stereotaxic set, place it on a warm pad, and close the wound using skin adhesive. Reverse the sedation using 0.1-1.0 mg/kg body weight atipamezole.
- Inject Bupenorphrine subcutaneously at a final dose of 0.1 mg per kg mouse weight every 12 hr, starting 4 hr after the administration of the short lasting Butorphanol analgesic.
- Place the animal in an individualized cage with a warm pad and monitor closely until the mouse recovers from anesthesia. Place one bag of hydrogel in the cage to help the animal hydrate after recovery.
- Dispose of all bio-contaminated waste in the liquid bleach biohazard disposal. Clean the syringe by aspiration and ejection of ethanol and rinse with water. Disinfect the area, the stereotaxic set and the surgical material that has been used with bleach and 70% ethanol.
- Keep injected mice isolated in the biosafety level 2 room for 24-48 hr after which they can be transferred to a conventional housing facility
3. Histological Analysis
- Perfusion, tissue collection, and sectioning
- Deeply anaesthetize the mice using a veterinary-supervised mixture of medetomidine and ketamine (assess the anesthetic plane by pinching the toes, tail or ear), as described before.
- Transcardially perfuse the mice with 25 ml of saline solution followed by 75 ml of 4% PFA in PB at the same rate17.
- Extract the brain and post-fix it by immersing it in at least 10 times its volume of cold 4% PFA in PB for 1-16 hr (increased post-fixation times may decrease the immunoreactivity of some antigens). Wash thoroughly the remaining PFA with PB.
- Cut the brain following indications of Figure 1d and glue the resulting block to the holder of a vibratome using cyanoacrylate.
- Collect 30 µm-thick serial coronal sections using a vibratome. Store the brain slices in 24-multiwell plates with PB at 4 °C. To prevent contamination, 0.05% sodium azide can be added to the PB solution.
- Immunohistochemistry
- Incubate the free-floating sections in blocking buffer (PB with 0.05% sodium azide, 1% glycine, 5% normal goat serum, and 0.1% Triton X-100) for 1h at RT with gentle shaking in a rocking platform.
- Carefully remove the blocking buffer with a pipette, add an appropriate dilution of anti-GFP rabbit primary antibody (see Table of Materials) in blocking buffer and incubate tissue with this dilution for 48 hr at 4 °C with gentle shaking.
- Wash off the primary antibody solution a minimum of 3 times with PB, one wash every 10 min.
- Incubate the free-floating sections with a suitable dilution of fluorophore-conjugated secondary antibodies) in blocking solution (see Table of Materials) for 1 hr at RT and gentle shaking. Protect the sections from direct light during the incubation.
- Wash off the secondary antibody solution with PB, 3 times once every 10 min, and counterstain the tissue by incubating the sections with DAPI (4',6-diamidino-2-phenylindole) at 1 mg/ml in water for 5 min. Wash off the DAPI solution by rinsing twice and quickly with water.
- Gently place the sections on a microscope slide using a fine paint brush. Pour a few drops of mounting medium for fluorescent preparations (see Table of Materials) over the tissue and carefully place a coverslip on top, checking that the mounting solution is correctly distributed over the entire surface and there are no bubbles. Gently squeeze down the coverslip to drain the excess of mounting medium.
- When the mounting solution dries out (2-16 hr), analyze the sample by confocal laser scanning microscopy with the 488 nm laser.
Results
LV-mediated gene delivery system can be used for the long-term in vivo transduction of cells in the adult mouse V-SVZ, allowing their tracking and genetic modification during proliferation, migration and differentiation. The infection and the expression are highly effective and yield numerous cells that can be easily distinguished among other non-infected cells by the expression of the reporter included. We have thus far visualized transduced cells with GFP fluorescent reporters, driven by the ubiquitously expre...
Discussion
LVs offer important advantages over other viral systems for the genetic modification of adult NSCs16,18. Stereotaxic delivery of lentiviruses to the V-SVZ niche represents an efficient method to label and trace infrequently dividing B1-NSCs overcoming the limitations of other commonly used methods such as BrdU, which is diluted after multiple cell divisions, or retrovirus, which only target cells that are proliferating at the moment of application. LVs, together with adenoviruses, can infect cells independentl...
Disclosures
All the manipulations were made in a biosafety level 2 room. Animal protocols were approved by the ethics committee of the University of Valencia and were all in compliance with European directive 2010/63/EU.
Acknowledgements
We acknowledge the help of M.J. Palop and the technical support of the SCSIE of the Universidad de Valencia. We also thank Antonia Follenzi for helpful comments and discussion of the manuscript. I.F is supported by Fundaciòn Botìn, by Banco Santander through its Santander Universities Global Division, and by grants from Generalitat Valenciana (Programa Prometeo, ACOMP, and ISIC) and Ministerio de Economìa y Competitividad (MINECO: SAF2011-23331, CIBERNED and RETIC TerCel). This work was also supported by BFU2010-21823 and RETIC TerCel grants from MINECO and the European Research Council (ERC) 2012-StG (311736- PD-HUMMODEL) to A.C. B.M-P. is the recipient of a Spanish FPI fellowship of the MINECO.
Materials
| Name | Company | Catalog Number | Comments |
| Part 1: Generation of LV for in vivo delivery. | |||
| Equipment: | |||
| Ultracentrifuge | Beckman Coulter | Optima XL-100K | |
| Ultracentrifuge rotor | Beckman Coulter | SW-28 | |
| Ultracentrifuge rotor | Beckman Coulter | SW-55 | |
| Ultracentrifuge tubes | Beckman Coulter | 358126 | 25 mm x 89 mm |
| Ultracentrifuge tubes | Beckman Coulter | 326819 | 13 mm x 51 mm |
| Ultracentrifuge adapters | Beckman Coulter | 358156 | |
| 6-well plate | SPL | PLC-30006 | |
| 24-well plate | SPL | PLC-30024 | |
| 10 cm dish | SPL | PLC-20101 | 100 x 20 style |
| FACS tubes | Afora | DE400800 | 12 mm x 75 mm, 5 ml |
| Cup sterile FACS filter | BD | 340626 | 30 µm |
| Nitrocellulose filter | Millipore | SCGPU05RE | 0.22 μm |
| Flow cytometer | BD | LSR Fortessa | Blue laser 488 nm |
| Steritop filter | Biofil | FPE-204-500 | 0.22 µm |
| Reagents: | |||
| pMDLg/pRRE plasmid | Addgene | #12251 | Core packaging plasmid |
| pRSV.REV plasmid | Addgene | #12253 | Core packaging plasmid |
| pMD2G plasmid | Addgene | #12259 | Envelope plasmid |
| pRRL-SIN-PPT.PGK.EGFP.Wpre plasmid | Addgene | #12252 | Transfer vector plasmid |
| Dulbecco's Modified Eagle's Medium | Biowest | L0101-500 | For HeLa cell culture |
| Iscove's Modified Dulbecco's Medium | Life technologies | 12440-053 | For 293T cell culture |
| Tris-EDTA (TE) | Tris-HCl (sigma, T5941), 0.1 mM EDTA (sigma, E5134), pH 7.6, DNAse/RNAse-free, 0.2 µm sterile-filtered | ||
| 2x HBS | 0.28 M NaCl (Sigma, S7653), 0.05 M HEPES (Sigma, H7523), 1.5 mM anhydrous Na2HPO4 (Sigma, S7907) in dH2O (preferably not MilliQ). Adjust pH to 7.0 with NaOH solution (Calbiochem, 567530). | ||
| Fetal bovine serum (FBS) | Biowest | S181B-500 | Stock solution at 100x, used to prepare HeLa and 293T culture medium at a final concentration of 10x. |
| Glutamine | Sigma-Aldrich | G7513-100 | Stock solution at 200 mM, used to prepare HeLa and 293T culture medium at a final concentration of 6 mM. |
| Sodium pyruvate | Life technologies | 11360-039 | Stock solution at 100 mM, used to prepare HeLa and 293T culture medium at a final concentration of 1 mM. |
| GlutaMAX Supplement | Life technologies | 35050-061 | Used to prepare 293T culture medium at a final concentration of 1%. |
| Penicillin/streptomycin | Sigma-Aldrich | P4458 | Stock solution contains 5,000 units/ml penicillin and 5 mg/ml streptomycin. Used to prepare HeLa and 293T culture medium at a final concentration of 1%. |
| Trypsin-EDTA | Life Technologies | 25200-056 | With phenol red, contains 2.5 g porcine trypsin and 0.2 g EDTA 4Na/L HBSS. |
| Phosphate buffered saline (PBS) | Sigma-Aldrich | D1408 | Without calcium chloride and magnesium chloride, 10x, liquid, sterile-filtered, suitable for cell culture. Stock solution used to prepare 1x PBS in cell culture grade water. |
| Polybrene (hexadimethrine bromide) | Sigma-Aldrich | H9268 | Powder. Prepare a 1,000x stock solution at 8 mg/ml in dHO |
| Paraformaldehyde EM grade 16% | EM Sciences | 15710 | |
| Name | Company | Catalog Number | Comments |
| Part 2: Sterotaxic injection of LV into the SEZ proper or the lateral ventricle. | |||
| Equipment: | |||
| Vernier stereotaxic instrument | NeuroLab, Leica | 39463001 | http://www.leicabiosystems.com/ |
| Cunningham mouse and neonatal rat adaptor | NeuroLab, Leica | 39462950 | |
| Syringe holder | KD Scientific | KDS-311-CE | |
| 33 gauge syringe | Hamilton | P/N 84851/00 | #85RN |
| Electric drill | Fine Science Tool | 98096 | |
| Thermal blanket | Ufesa | AL5512/01 | 230-240 V, 100-110 W, type C_AL01 |
| Shaver | Jata | MP373N | Model: beauty, 3 V, 300 mA, type HT-03. |
| Reagents: | |||
| Medetomidine | Esteve | DOMTOR | Comercial solution at 1 mg/ml. |
| Ketamine | Merial | Imalgene 500 | Comercial solution at 50 mg/ml |
| Medetomidina/ketamine mixture | Prepare a working mixture of medetomidine at a final concentration of 0.2 mg/ml dilution and ketamine at a final concentration of 15 mg/ml in saline solution. Use as anesthesia injecting a volume to get a final concentration of 0.5-1 mg medetomidina per kg body weight and 50-75 mg ketamine per kg body weight | ||
| Butorphanol | Pfizer | Torbugesic | Stock solution at 10 mg/ml. Used as analgesia at 1 mg/ml in saline solution. |
| Atipamezole | Esteve | Antisedan | Stock solution at 5 mg/ml, used in a final concentration of 0.5 mg/ml in saline solution to exit from anesthesia. |
| 0.9% saline solution | Braun | 13465412 | |
| Histoacryl | Braun | 1050052 | Topical skin adhesive |
| HydroGel | Clear H2O | 70-01-5022 | |
| Kimwipes | Kimberly-Clark | 34120 | 11 cm x 21 cm |
| Bleach/Virkon | Dupont | ||
| Surgical marker pen | Staedler | 313-9 | Permanent lumocolor |
| Ophthalmic lubricant | SICCAFLUID | 0.5 g/dosis, carbomer 974P | |
| Povidone-iodine | Betadine | 694109.6 | 10% povidone-iodine |
| Name | Company | Catalog Number | Comments |
| Part 3: Histological analysis. | |||
| Equipment: | |||
| Automatic peristaltic pump | Cole-Parmer Inst. Co. | HV-07524-55 | Masterflex L/S variable-speed economy drive, 1.6-100 rpm, 230 V |
| Pump head | Cole-Parmer Inst. Co. | HV-07518-00 | Masterflex L/S Easy-Load pump head for precision tubing; PSF housing, CRS rotor |
| Silicone tube | Cole-Parmer Inst. Co. | HV-96410-16 | Platinum L/S 16 |
| Scalp vein set | Vygon V-green | 70246.05T | 25 G, 30 cm tube length |
| Vibratome | Leica | VT1000 | |
| Confocal microscope | Olympus | FluoView FV10i | |
| Hot plate | Tehtnica | SHP-10 | |
| Reagents: | |||
| Phosphate buffer (PB) | 0.2 M PB: 0.2 M Na2HPO4 (Sigma, S7907) and 0.2 M NaH2PO4 (Panreac, 141965.1211) in dH2O, adjust pH to 7.2-7.4 | ||
| Paraformaldehyde (PFA) | Panreac | 141451.1211 | Prepare fresh every time. Heat dH2O up to 55–60 °C using a hot plate placed in a fume hood and pour PFA powder while stirring to obtain an 8% solution. The solution is cloudy white as PFA does not dissolve easily. Add 1N NaOH drop by drop just until the solution clears. Cool down, filter through Whatman paper and add an equivalent volume of 0.2 M PB. |
| Saline solution | 0.9% NaCl in dH2O | ||
| Superglue | LOCTITE | 767547 | |
| Sodium azide | Panreac | 122712.1608 | |
| Glycine | Sigma-Aldrich | G7126-100 | |
| Normal goat serum | Millipore | S30-100 | |
| Triton X-100 | Sigma-Aldrich | T9284 | Detergent |
| Anti-GFP rabbit antibody | ROCKLAND | 600-401-215 | Use at a 1:500 dilution |
| Alexa Fluor 488 Donkey Anti-Rabbit IgG (H+L) Antibody | Molecular probes | A-21206 | Use at a 1:750 dilution |
| 6-Diamindino-2-phenylindole dihydrochloride hydrate (DAPI) | Sigma-Aldrich | D9542 | Fluorescent nuclear staining. Use at 2 mg/ml in ddH2O. Keep in the dark at 4 °C. |
| Fluoromount-G | EM Sciences | 17984-25 | Mounting medium for fluorescent preparations |
References
- Fuentealba, L. C., Obernier, K., Alvarez-Buylla, A. Adult neural stem cells bridge their niche. Cell Stem Cell. 10 (6), 698-708 (2012).
- Silva-Vargas, V., Crouch, E. E., Doetsch, F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol. 23 (6), 935-942 (2013).
- Ponti, G., Obernier, K., Alvarez-Buylla, A. Lineage progression from stem cells to new neurons in the adult brain ventricular-subventricular zone. Cell Cycle. 12 (11), 1649-1650 (2013).
- Menn, B., Garcia-Verdugo, J. M., Yaschine, C., Gonzalez-Perez, O., Rowitch, D., Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 26 (30), 7907-7918 (2006).
- Mirzadeh, Z., Merkle, F. T., Soriano-Navarro, M., Garcia-Verdugo, J. M., Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 3 (3), 265-278 (2008).
- Shen, Q., et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 3 (3), 289-300 (2008).
- Tavazoie, M., et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 3 (3), 279-288 (2008).
- Mirzadeh, Z., Doetsch, F., Sawamoto, K., Wichterle, H., Alvarez-Buylla, A. The subventricular zone en-face: wholemount staining and ependymal flow. J Vis Exp. (39), (2010).
- Ramirez-Castillejo, C., et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 9 (3), 331-339 (2006).
- Falcao, A. M., Marques, F., Novais, A., Sousa, N., Palha, J. A., Sousa, J. C. The path from the choroid plexus to the subventricular zone: go with the flow!. Front Cell Neurosci. 6, (2012).
- Delgado, A. C., et al. Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron. 83 (3), 572-585 (2014).
- Kokovay, E., et al. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell. 11 (2), 220-230 (2012).
- Porlan, E., et al. MT5-MMP regulates adult neural stem cell functional quiescence through the cleavage of N-cadherin. Nat Cell Biol. 16 (7), 629-638 (2014).
- Ihrie, R. A., Alvarez-Buylla, A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 70 (4), 674-686 (2011).
- Porlan, E., Perez-Villalba, A., Delgado, A. C., Ferròn, S. R. Paracrine regulation of neural stem cells in the subependymal zone. Arch Biochem Biophys. 1-2 (534), 11-19 (2013).
- Mamber, C., Verhaagen, J., Hol, E. M. In vivo targeting of subventricular zone astrocytes. Prog Neurobiol. 92 (1), 19-32 (2010).
- Ferron, S. R., Andreu-Agullo, C., Mira, H., Sanchez, P., Marques-Torrejon, M. A., Fariñas, I. A combined ex/in vivo assay to detect effects of exogenously added factors in neural stem cells. Nat Protoc. 2 (4), 849-859 (2007).
- Consiglio, A., et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci U S A. 101 (41), 14835-14840 (2004).
- Dull, T., et al. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 72 (11), 8463-8471 (1998).
- Bomsel, M., Alfsen, A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol. 4 (1), 57-68 (2003).
- Castellani, S., Di Gioia, S., Trotta, T., Maffione, A. B., Conese, M. Impact of lentiviral vector-mediated transduction on the tightness of a polarized model of airway epithelium and effect of cationic polymer polyethylenimine. J Biomed Biotechnol. , (2010).
- Bonazzi, M., Cossart, P. Impenetrable barriers or entry portals? The role of cell-cell adhesion during infection. J Cell Biol. 195 (3), 349-358 (2011).
- Padmashali, R., You, H., Karnik, N., Lei, P., Andreadis, S. T. Adherens junction formation inhibits lentivirus entry and gene transfer. PLoS One. 8 (11), (2013).
- Yamashita, T., et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 26 (24), 6627-6636 (2006).
- Platt, R. J., et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell. 159 (2), 440-455 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved