A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microscopy of Fission Yeast Sexual Lifecycle
* These authors contributed equally
In This Article
Summary
We provide a reproducible basic method for the long-term microscopy of the fission yeast sexual lifecycle. With minor adjustments described, the presented protocol allows research focus on different steps of the reproductive process.
Abstract
The fission yeast Schizosaccharomyces pombe has been an invaluable model system in studying the regulation of the mitotic cell cycle progression, the mechanics of cell division and cell polarity. Furthermore, classical experiments on its sexual reproduction have yielded results pivotal to current understanding of DNA recombination and meiosis. More recent analysis of fission yeast mating has raised interesting questions on extrinsic stimuli response mechanisms, polarized cell growth and cell-cell fusion. To study these topics in detail we have developed a simple protocol for microscopy of the entire sexual lifecycle. The method described here is easily adjusted to study specific mating stages. Briefly, after being grown to exponential phase in a nitrogen-rich medium, cell cultures are shifted to a nitrogen-deprived medium for periods of time suited to the stage of the sexual lifecycle that will be explored. Cells are then mounted on custom, easily built agarose pad chambers for imaging. This approach allows cells to be monitored from the onset of mating to the final formation of spores.
Introduction
Though genetic exchange between two cells is the central event in sexual reproduction, it relies on a chain of events that promote cell differentiation, allow for partner choice, carry out cell-cell fusion and maintain genomic stability. Thus the sexual lifecycle presents itself as a model system to study a number of biological questions regarding developmental switches, response to extrinsic stimuli, plasma membrane fusion, chromosome segregation, etc. Exploring the fission yeast sexual cycle to study these phenomena brings the benefits of the model system's powerful genetics, well-established high-throughput approaches and sophisticated microscopy. Sex in fission yeast is a heterotypic event between a P-cell and an M-cell of distinct mating types. The two cell types differentially express a number of genes1,2 including those for production of the secreted P- and M-pheromones, pheromone-receptors Map3 and Mam2 as well as pheromone-proteases Sxa1 and Sxa2. Homothallic strains, such as the commonly used h90 strain, carry the genetic information for both mating types in a single genome and cells undergo a complex pattern of mating type switching throughout the mitotic lifecycle (reviewed in Ref.3). Multiple isolates of heterothallic fission yeast that rarely or never switch mating type are also commonly used4, most prominently the h+N (P-type) and h-S (M-type) strains.
In fission yeast, entry into the sexual lifecycle is under strict nutritional regulation. Only nitrogen-starved fission yeast cells arrest mitotic reproduction and produce diffusible pheromones to signal presence of a mating partner and promote further steps of the sexual cycle (reviewed in Ref.5). Nitrogen deprivation de-represses the key transcriptional regulator of mating Ste11 that acts as a developmental switch and promotes expression of mating specific genes including the pheromone receptor and the pheromone production genes6,7. Pheromone-receptor engagement activates the receptor-coupled protein G-alpha and downstream MAPK signaling which further enhances Ste11 transcriptional activity8-10, thus increasing pheromone production in a positive feedback between mating partners. Pheromone levels are crucial to induce different cell polarization states by regulating the master organizer of cell polarity, the Rho-family GTPase Cdc4211. Upon exposure to low pheromone concentrations, active Cdc42 is visualized in dynamic patches exploring the cell periphery, and no cell growth is observed at this stage. Increased pheromone levels promote the stabilization of Cdc42 activity to a single zone and growth of a polarized projection, termed the shmoo, which brings partner cells in contact. Subsequently, the two haploid mating partners fuse to form a diploid zygote. Recent work reveals the existence of a novel actin structure essential for fusion that is assembled by the mating-induced formin Fus112. This fusion focus concentrates type-V myosin dependent processes and positions the cell wall degradation machinery, thus allowing remodeling of the cell wall to permit plasma membrane contact without cell lysis12. Upon cell-cell fusion, the nuclei come in contact and undergo karyogamy. A prominent dynein-dependent back-and-forth movement of the nucleus inside the zygote (the horse-tail movement) then promotes the pairing of chromosome homologs13,14, which is followed by meiosis. Finally, the four products of meiosis are packaged into individual spores during sporulation.
Because of its complexity and the numerous steps involved, detailed monitoring of mating has been challenging. Two notable difficulties are that the entire process takes well over fifteen hours and that cells are difficult to synchronize. These difficulties are circumvented by single-cell microscopy approaches. Here a general protocol to investigate the sexual lifecycle in fission yeast is presented. With minor adjustments, this protocol permits the study of all the different steps of the process, namely the induction of mating gene product, cell polarization and pairing between sister-cells after mating type switching and between non-sister partners, cell-cell fusion, and post-fusion horse-tail movement, meiosis and sporulation. This method allows to 1) easily visualize fluorescently tagged proteins over time pre-, during and post-fusion; 2) discriminate the behavior of cells of opposite mating type; and 3) measure and quantify parameters such as shmooing, mating, fusion or sporulation efficiency.
Protocol
Microscopy analysis of fission yeast sexual reproduction
1. Media Preparation
- Prepare Minimum Sporulation medium (MSL-N)15 by mixing the following components: Glucose: 10 g/L, KH2PO4: 1 g/L, NaCl: 0.1 g/L, MgSO4·7H2O: 0.2 g/L. Add Trace elements (10,000x): 100 µl/L, Vitamins (1,000x): 1 ml/L, and 0.1 M CaCl2 ml/L. Filter-sterilize using a 0.22 μm pore size filter and store at room temperature (RT).
- Use the following Vitamins (1,000x) stock: Pantothenate: 1 g/L, Nicotinic Acid: 10 g/L, Inositol: 10 g/L, Biotin: 10 mg/L. Filter-sterilize using a 0.22 μm pore size filter and store at 4 °C.
- Use the following Trace elements (10,000x) stock: Boric Acid: 5 g/L, MnSO4: 4 g/L, ZnSO4·7H2O: 4 g/L, FeCl2·6H2O: 2 g/L, MoO3: 0.4 g/L, KI: 1 g/L, CuSO4·5H2O: 0.4 g/L, Citric Acid: 10 g/L. Filter-sterilize using a 0.22 μm pore size filter and store at 4 °C.
- Prepare MSL-N Agarose 2% (used to make agarose pad chambers) by combining 10 ml of MSL-N with 0.2 g of agarose. Melt for ~2 min at high power in a microwave oven until agarose dissolves and aliquot 0.5 ml in microcentrifuge tubes. Store at RT.
- Prepare Minimum Sporulation medium with nitrogen (MSL+N) from MSL-N by adding (NH4)2SO4: 2 g/L, Leucine: 0.225 g/L, Adenine: 0.225 g/L, Uracil: 0.225 g/L. Filter-sterilize using a 0.22 µm pore size filter. Store at RT.
Note: Leucine, adenine and uracil are added to this medium to allow the growth of auxotrophic strains. Additional amino-acid supplement should be included if the strain used carries a distinct auxotrophy (for instance his3Δ). Please also note that work with fully prototroph strains is recommended, as only such strains will allow high mating efficiency. - Prepare 200 ml VALAP for chamber sealing. In a beaker add equal weights of Lanolin, Vaseline (or other petroleum jelly), and Paraffin. Heat mixture at low temperature and stir occasionally until thoroughly blended. Aliquot the mix into several small petri dishes. Store at RT.
2. Culturing Fission Yeast Strains for Mating Experiments (Figure 1).
- Day 1, Evening:
Inoculate freshly streaked strains from solid media into culture tubes containing 3 ml of MSL+N. Use flat-bottom tubes of about 2.5 cm diameter. Use other culture tubes or flasks with adapted culture volume. Incubate overnight (O/N) with shaking at 25 °C, 200 rpm. If working with heterothallic strains, inoculate separately.- Dilute cell suspensions in media the following morning to ensure that cultures have optical density measured at 600 nm (O.D.600) of 0.4-0.8 in the evening.
Note: O.D.600 measurements as a proxy of cell concentration for fission yeast are linear in the range of approximately 0.1-1.0. For our spectrophotometer O.D.600 = 0.1 corresponds to 1.4 x 106 cells per ml. Thus, if initial O.D.600 reads are outside this range samples need to be diluted/concentrated for reliable measurements.
- Dilute cell suspensions in media the following morning to ensure that cultures have optical density measured at 600 nm (O.D.600) of 0.4-0.8 in the evening.
- Day 2, Evening:
Dilute cells in 20 ml of MSL+N media to O.D.600 = 0.025 in 100 ml flasks. Incubate strains O/N at 30 °C, 200 rpm.
Note: Wild-type cell cultures should reach O.D.600 ~0.8 the following morning (15 hr later). If working with strains with longer generation time adjust dilution accordingly. - Day 3, Morning:
Measure O.D.600 to verify that cultures have cell density of O.D.600 ~0.8. If working with heterothallic strains, mix equal numbers of partner cells. Pellet cells at 1,000 x g and transfer to a 1.5 ml tube. Wash cells three times in 1 ml of MSL-N medium. Re-suspend cells in 3 ml of MSL-N medium and dilute cells to O.D.600 = 1.5.- To monitor mating between sister cells directly mount cells for imaging (see Protocol Section 2.4). Use homothallic h90 strains, in which mating type switching will take place during the mitotic divisions occurring after nitrogen deprivation. Immediate mounting of cells on the agarose pad ensures that, after cell division, sister-cells remain next to each other.
- To monitor cell polarization (exploratory dynamics and shmooing) incubate 1-3 ml of cell culture at 30 °C, 200 rpm for 3-4 hr prior to mounting cells for imaging. Within these 3-4 hr, the last mitotic division will have occurred. A few cells will have initiated polarization, but most will just be starting their exploratory polarization dynamics.
- To monitor cell-cell fusion incubate 1-3 ml of cell culture at 30 °C, 200 rpm for 4-6 hr prior to mounting cells for imaging.
- To monitor post-fusion events incubate 1-3 ml of cell cultures at 30 °C, 200 rpm for 8 hr prior to mounting cells for imaging.
- To monitor response to a specific pheromone concentration (only for heterothallic strains) incubate 3 ml of cell culture at 30 °C, 200 rpm for 3-4 hr prior to mounting cells for imaging.
- Remove the MSL-N-containing microcentrifuge tube from the 95 °C heat block (see Protocol section 2.4.1. below). Add P-factor or M-factor at the desired concentration directly in the melted MSL-N agarose. Mix well by vortexing before immediate pad preparation.
- After cell mounting, incubate the pad for 15-30 min at 25 °C prior to imaging. P-factor and M-factor pheromones were used from a stock solution of 1 mg/ml in methanol.
Note: If working with mutant strains incubation times may vary. Clumping of cells may occur after prolonged incubation in liquid media and may be particularly strong in some mutant strains. Clumped cells cannot be spotted onto agarose pads in a single layer and thus are difficult to autofocus and image. In case of extensive cell clumping mount cells on agarose pads immediately after washes and re-suspension in nitrogen-lacking media (as in the Protocol Section 2.3.1.) and incubate them at 30 °C prior to imaging.
- Mounting Cells for Imaging:
- Prepare MSL-N agarose pads16 by melting an aliquot in a 95 °C heat-block for 10-15 min and adding 200 μl between two glass slides separated by spacers (Figure 1B). Use spacers with a thickness of ~0.5 mm. To make spacers, use strips cut out of cardboard, such as that delivered with restriction enzymes.
- Pellet 100 μl of cells at 1,000 x g for 1 min, remove the supernatant and re-suspend cells in residual 2-4 μl medium. Do NOT re-suspend in fresh medium as it delays mating.
- After 2-3 min carefully remove spacers and the top glass slide. If several strains are to be mounted, prepare the cell concentrates before preparing the agarose pad.
- Add 1 μl of cells to the pad and wait until the drop starts drying (~1 min) before covering with cover slip and sealing with VALAP.
- Let the pad sit for at least 30 min before imaging at 25 °C or RT.
- To obtain a mixture of cells in various stages of mating, make a pad immediately after washes in MSL-N (see Section 2.3.) and incubate at 18 °C O/N (~15 hr). This approach is useful for imaging cells at distinct mating stages with high temporal resolution or samples with weak signal.
3. Live-cell Imaging of Mating Yeast Cells
- Adjust Image Acquisition Settings.
Note: Image acquisition settings presented here were optimized for the DeltaVision platform composed of a customized Olympus IX-71 inverted microscope with Plan Apo 60X/1.42 NA or U-Plan Apo 100X/1.4 NA objectives, a CoolSNAP HQ2 camera and an Insight SSI 7 color combined unit illuminator. The hardware is controlled by softWoRx v4.1.2 that also enables digital autofocusing.- Ensure that the autofocusing intervals do not exceed 15 min since the Z-drift over longer time can surpass the capacity of the software autofocusing system. If using a hardware-based autofocusing system, use larger intervals.
- Adjust imaging interval. Take images every 10 min for ~15 hr as a starting point when working with a fluorescently tagged protein for the first time. This interval provides a good overview of the entire mating process without extensive bleaching.
- Image with 5 min intervals or shorter to more precisely time events such as fusion. Shorter intervals require strong fluorophores enabling low exposure times and little bleaching. For imaging with intervals below 1 min avoid O/N microscopy and instead prepare a mixture of all mating stages as detailed in Protocol Section 2.4.6.
- Adjust image exposure time. Test exposure times between 50 and 300 msec. Aim to strike a balance between the signal-to-noise ratio and photobleaching as to achieve a large number of time-points with a discernable signal (typically over 100).
- Adjust Z-sectioning. Sections every 0.3 µm covering 5 µm provide a good imaging depth. Note that Z-sectioning further increases photo-damage, which can be minimized by using the OAI (optical axis integration - a single sweep acquisition of the total sample depth). Good auto-focus system reduces the need for many Z-sections and is highly recommended.
- Tips to Study Mating Type-specific Behaviors:
- For heterothallic strains, use h- and h+ cells expressing distinct fluorophores, so that the two cell types can be distinguished. Use any genetically encoded fluorophore expressed as a cytosolic version or fusion to an endogenous protein, though we have had good success with sfGFP, a fast-folding variant of GFP17. Endogenous proteins are usually tagged at their endogenous genomic locus by homologous recombination14.
- For homothallic strains, express a genetically encoded cytosolic fluorescent protein under the control of mating type specific promoters such as those of the pheromone receptor genes map3 and mam2 to drive the expression in P and M cells, respectively. The promoters of map3 and mam2 are inactive during vegetative growth and are induced upon nitrogen starvation18,19. Constructs driving the expression of cytosolic GFP or mCherry under the control of these promoters are typically integrated at a genomic locus (ura4, leu1) that does not interfere with the normal progression of the sexual life cycle12.
- Use mating type specific cytosolic fluorophores (as in Protocol Section 3.2.2) to determine precise timing of cell fusion visualized as the transfer of fluorescent signal from one partner cell to the other.
4. Quantification of Mating and Fusion Efficiencies
- To quantify mating and fusion efficiencies11,12, spot cells directly after MSL-N wash onto MSL-N agarose pads, as in step 2.3.1 above, and leave for 24 hr at 25 °C prior to imaging (Figure 1A). Using this protocol a homothallic wild-type strain mating efficiency reaches ~60% and fusion efficiency 99%. To test whether any reduction in these values observed in mutant strains reflect a terminal phenotype or a delay, repeat the experiment with a 36 hr incubation time.
- Calculate mating efficiency as
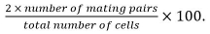 Mating pairs include zygotes, asci, and unfused cell pairs identified from their position and growth towards each others.
Mating pairs include zygotes, asci, and unfused cell pairs identified from their position and growth towards each others. - Calculate fusion efficiency as
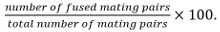
Note: Fused mating pairs are identified by their ability to form spores if it is known that the mutant under study does not influence sporulation. If sporulation cannot be used as read-out, fusion is determined by observing the spreading of a cytosolic marker expressed in only one mating partner into both partners.
Results
Fission Yeast Growth and Mating Dynamics Upon Removal of a Nitrogen Source
As nitrogen starvation is a prerequisite for initiation of sexual reproduction in fission yeast, wild-type homothallic h90 strain was monitored upon shift from nitrogen-rich to nitrogen-deprived medium (Figure 2), following the protocol outlined in Figure 1. Briefly, cells were grown O/N to exponential phase (O.D.600 = 0.5) in MSL+N medium, collected, wash...
Discussion
Environmental conditions, and nutrient availability in particular, strongly affect the fission yeast physiology. Nitrogen starvation is necessary for commitment to the sexual reproduction and initially leads to striking changes in the mitotic cell cycle progression (Ref.21 and Figure 2). Upon nitrogen removal from exponentially growing population, cell size at division rapidly decreases (Figure 2C) and the majority of cells arrest mitotic progression shorter than the length of...
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgements
AV was supported by an EMBO long-term postdoctoral fellowship. Research in the Martin lab is funded by an ERC Starting grant (GeometryCellCycle) and a Swiss National Science Foundation grant (31003A_155944) to SGM.
Materials
| Name | Company | Catalog Number | Comments |
| Glucose | Sigma-Aldrich | G8270-10KG | |
| KH2PO4 | Sigma-Aldrich | 1.05108.0050 | |
| NaCl | Sigma-Aldrich | 71381 | |
| MgSO4•7H2O | Sigma-Aldrich | 63140 | |
| CaCl2 | Sigma-Aldrich | 12095 | |
| Pantothenate | AppliChem | A2088,0025 | |
| Nicotinic Acid | AppliChem | A0963,0100 | |
| Inositol | AppliChem | A1716,0100 | |
| Biotin | AppliChem | A0967,0250 | |
| Boric Acid | Sigma-Aldrich | B6768-1KG | |
| MnSO4 | AppliChem | A1038,0250 | |
| ZnSO4•7H2O | Sigma-Aldrich | Z4750 | |
| FeCl2•6H2O | AppliChem | A3514,0250 | |
| Molybdenum oxide (VI) (MoO3) | Sigma-Aldrich | 69850 | |
| KI | AppliChem | A3872,0100 | |
| CuSO4•5H2O | AppliChem | A1034,0500 | |
| Citric Acid | AppliChem | A2344,0500 | |
| Agarose | Promega | V3125 | |
| (NH4)2SO4 | Merck | 1.01217.1000 | |
| L-Leucine | Sigma-Aldrich | L8000-100G | |
| Adenine Hemisulfat Salt, mini 99% | Sigma-Aldrich | A9126-100G | |
| Uracil | Sigma-Aldrich | U0750 | |
| Lanolin | Sigma-Aldrich | L7387 | |
| Vaseline | Reactolab | 92045-74-4 | |
| Paraffin | Reactolab | 7005600 |
References
- Mata, J., Bahler, J. Global roles of Ste11p, cell type, and pheromone in the control of gene expression during early sexual differentiation in fission yeast. Proc Natl Acad Sci U S A. 103 (42), 15517-15522 (2006).
- Xue-Franzen, Y., Kjaerulff, S., Holmberg, C., Wright, A., Nielsen, O. Genomewide identification of pheromone-targeted transcription in fission yeast. BMC Genomics. 7, 303 (2006).
- Klar, A. J. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet. 41, 213-236 (2007).
- Beach, D. H., Klar, A. J. Rearrangements of the transposable mating-type cassettes of fission yeast. EMBO J. 3 (3), 603-610 (1984).
- Merlini, L., Dudin, O., Martin, S. G. Mate and fuse: how yeast cells do it. Open Biol. 3 (3), 130008 (2013).
- Mochizuki, N., Yamamoto, M. Reduction in the intracellular cAMP level triggers initiation of sexual development in fission yeast. Mol Gen Genet. 233 (1-2), 17-24 (1992).
- Sugimoto, A., Iino, Y., Maeda, T., Watanabe, Y., Yamamoto, M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 5 (11), 1990-1999 (1991).
- Kjaerulff, S., Lautrup-Larsen, I., Truelsen, S., Pedersen, M., Nielsen, O. Constitutive activation of the fission yeast pheromone-responsive pathway induces ectopic meiosis and reveals ste11 as a mitogen-activated protein kinase target. Mol Cell Biol. 25 (5), 2045-2059 (2005).
- Obara, T., Nakafuku, M., Yamamoto, M., Kaziro, Y. Isolation and characterization of a gene encoding a G-protein alpha subunit from Schizosaccharomyces pombe: involvement in mating and sporulation pathways. Proc Natl Acad Sci U S A. 88 (13), 5877-5881 (1991).
- Toda, T., Shimanuki, M., Yanagida, M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 5 (1), 60-73 (1991).
- Bendezu, F. O., Martin, S. G. Cdc42 explores the cell periphery for mate selection in fission yeast. Curr Biol. 23 (1), 42-47 (2013).
- Dudin, O., et al. A formin-nucleated actin aster concentrates cell wall hydrolases for cell fusion in fission yeast. J Cell Biol. 208 (7), 897-911 (2015).
- Chikashige, Y., et al. Telomere-led premeiotic chromosome movement in fission yeast. Science. 264 (5156), 270-273 (1994).
- Bahler, J., et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 14 (10), 943-951 (1998).
- Egel, R., Willer, M., Kjaerulff, S., Davey, J., Nielsen, O. Assessment of pheromone production and response in fission yeast by a halo test of induced sporulation. Yeast. 10 (10), 1347-1354 (1994).
- Tran, P. T., Marsh, L., Doye, V., Inoue, S., Chang, F. A mechanism for nuclear positioning in fission yeast based on microtubule pushing. J Cell Biol. 153 (2), 397-411 (2001).
- Pedelacq, J. D., Cabantous, S., Tran, T., Terwilliger, T. C., Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol. 24 (1), 79-88 (2006).
- Kitamura, K., Shimoda, C. The Schizosaccharomyces pombe mam2 gene encodes a putative pheromone receptor which has a significant homology with the Saccharomyces cerevisiae Ste2 protein. EMBO J. 10 (12), 3743-3751 (1991).
- Tanaka, K., Davey, J., Imai, Y., Yamamoto, M. Schizosaccharomyces pombe map3+ encodes the putative M-factor receptor. Mol Cell Biol. 13 (1), 80-88 (1993).
- Motegi, F., Arai, R., Mabuchi, I. Identification of two type V myosins in fission yeast, one of which functions in polarized cell growth and moves rapidly in the cell. Mol Biol Cell. 12 (5), 1367-1380 (2001).
- Sajiki, K., Pluskal, T., Shimanuki, M., Yanagida, M. Metabolomic analysis of fission yeast at the onset of nitrogen starvation. Metabolites. 3 (4), 1118-1129 (2013).
- Miyawaki, A., Nagai, T., Mizuno, H. Mechanisms of protein fluorophore formation and engineering. Curr Opin Chem Biol. 7 (5), 557-562 (2003).
- Dyer, J. M., et al. Tracking shallow chemical gradients by actin-driven wandering of the polarization site. Curr Biol. 23 (1), 32-41 (2013).
- Beta, C., Bodenschatz, E. Microfluidic tools for quantitative studies of eukaryotic chemotaxis. Eur J Cell Biol. 90 (10), 811-816 (2011).
- Lee, S. S., et al. Quantitative and dynamic assay of single cell chemotaxis. Integr Biol (Camb). 4 (4), 381-390 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved