A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Isolation of Primary Human Decidual Cells from the Fetal Membranes of Term Placentae
In This Article
Summary
This protocol demonstrates a method for the isolation of primary human decidual cells collected from the fetal membranes of term placentae which can be used for a variety of applications (i.e. immunocytochemistry, flow cytometry, etc.) aiming to study the role of different cell populations in pregnancy complications.
Abstract
The decidua, also known as the pregnant endometrium, is a critically important reproductive tissue. Decidual cells, comprised mainly of decidualized stromal cells and immune cells, are responsible for the secretion of hormonal and inflammatory factors which are critical for successful blastocyst implantation, placental development and play a role in the initiation of labor at term and preterm. Many pregnancy complications can arise from perturbations of a fine balance of different cell populations comprising decidua. Alterations in the proportion of specific decidual cell types may disrupt these crucial processes and increase the risk of developing serious complications of pregnancy, such as embryo implantation failure, intrauterine growth restriction, preeclampsia and preterm labor. The protocol outlined here demonstrates a cost and time effective method for the isolation of primary human decidual cells collected from the fetal membranes of term placentae. By combining enzymatic digestion and gentle mechanical disruption of the decidual tissue, a high yield of decidual cells was obtained with virtually no chorion contamination. Importantly, isolated decidual cells were characterized (stromal cells (55-60%), leukocytes (35%), epithelial (1%) or trophoblast (0.01%) cells) and maintained high viability (80%) which was confirmed by multicolor imaging flow cytometry assay. This protocol is specific to the decidua parietalis and can be adapted to first and second trimester placentae. Once isolated, decidual cells can be used for a multitude of experimental applications aiming to understand the role of different decidual cell sub-populations in pregnancy complications.
Introduction
The endometrium, one of the most active adult female tissues, undergoes dramatic remodeling each menstrual cycle in response to stimulation by ovarian hormones, estrogen (E2) and progesterone (P4). The decidua, also known as the pregnant endometrium, is a critically important reproductive tissue that is formed by the end of the postovulatory phase as a result of P4-driven differentiation following the E2-dominant proliferative phase. Decidual cells are responsible for the secretion of hormonal factors for successful blastocyst implantation and for development of the utero-placental interface for maintaining maternal tolerance to the fetal allograft.
Decidualization is required for implantation and subsequent remodeling of the decidual spiral arteries. Endometrial stromal cells undergo decidualization, under the control of P4 and cAMP, during the late luteal phase of the menstrual cycle1. This process is initiated around the blood vessels and spreads throughout the stroma, suggesting its role in vasculature remodeling and leukocyte trafficking regulation. This cellular transformation is characterized by a circular morphology, increased nuclear size, and expansion of the rough endoplasmic reticulum and Golgi apparatus2. Decidualized stromal cells are capable of producing paracrine factors supporting blastocyst implantation and characterized by the secretion of numerous hormones (i.e. prolactin), angiogenic growth factors, insulin growth factor binding protein-1 (IGFBP-1), prostaglandin (PG) E (stimulator of intracellular cAMP), cytokines, extracellular matrix components and nutrients essential for placental implantation and development3,4,5,6.
The decidual cell population is not solely comprised of decidualized stromal cells but also contains large, pregnancy-specific decidual leukocyte populations. Decidualization involves transient localized oedema and influx of Natural killer (NK) cells, T-cells, dendritic cells, and macrophages. The largest leukocyte subpopulation is the uterine NK cells, comprising approximately 50-70% of all maternal leukocytes infiltrating the decidua which are a source of cytokines and angiogenic factors which may aid in the decidualization process and increase in number throughout pregnancy7. Macrophages, being the second largest subpopulation of immune cells, are found around the implantation site and increase during pregnancy8. They are a source of cytokines and growth factors such as colony stimulating factor (CSF-1)9, tumor necrosis factor α (TNFα)10 and prostaglandin (PG) E11.
Throughout pregnancy, and before term labor, the decidua is a major source of cytokines and chemokines responsible for maternal peripheral leukocyte activation and subsequent migration into the uterine tissues to initiate labor. Animal studies showed that numerous pro-inflammatory cytokines are up-regulated in the mouse decidua during labor, such as TNF-a, IL-6, IL-12, and IL-1b12. In the human decidua, pro-inflammatory cytokines IL-1b, IL-6 and IL-8 (major neutrophil chemoattractant) exhibit higher expression during labor compared to not in labor13. These secreted cytokines result in an activation and influx of leukocytes into the decidual tissues14; an increase in decidual macrophage and neutrophil infiltration in both the human and rat is seen during term labor, with decidual infiltration preceding myometrial 4-fold greater, indicating a cascade of activation between this two-adjacent uterine tissues15. These infiltrating leukocytes produce PGs capable of activating synchronous contractions of the myometrium16, matrix metalloproteinases (MMPs) to initiate membrane rupture17,18, as well as pro-inflammatory cytokines to amplify the uterine activation process ('cytokine storm').
Due to many important functions of decidual cells, such as playing a critical role in the implantation process, maintaining maternal-fetal tolerance in early gestation and participating in the activation of labor at term, different pathologies can arise during pregnancy. For instance, (1) infertility due to recurrent implantation failure and recurrent pregnancy loss can result from a failure of decidual maturation; (2) intrauterine growth restriction (IUGR) and preeclampsia due to improper development and dysfunction of the decidua/placenta or compromised vascular transformation at the decidual-myometrial junction; as well as (3) preterm birth which can result from premature decidual activation.
In light of these major disorders, coupled with the ethical and practical limitations of human in vivo studies, establishing primary human decidual cell lines is essential for in vitro analysis with the purpose of better understanding and improving clinical management of pregnancy complications. Therefore, the objective of our research was to develop a protocol which allows for the isolation of human primary decidual cells with high cell yield and viability collected from the fetal membranes of term placentae. This current protocol clearly describes a time- and cost-effective method for isolating of specific subtypes of decidual cells which be used for a variety of in vitro analyses. Characterization of the abundance and phenotype of decidual sub-populations at term and comparison to first or second trimester is crucial to defining their roles throughout human gestation.
Access restricted. Please log in or start a trial to view this content.
Protocol
Placentae are collected from healthy term, not in labor women undergoing elective cesarean sections. The Collection, processing, and disposable of human samples adhere to the guidelines of the Mount Sinai Hospital Ethics board. A written consent is obtained from each patient. This study is approved by the Research Ethics Board at Mount Sinai Hospital.
1. Preparations
NOTE: All steps must be conducted under a fume hood and all surgical equipment must be sterilized via autoclave prior to placement in the fume hood. All other materials (bottles, 50 mL tubes, etc.) must be sterilized with 70% ethanol solution. Always wear personal protective equipment at all times when working with biohazardous waste (lab coat, gloves, long hair tied back, etc.).
- Enzymatic digestion solution (Final solution: 200 mL)
- Pipette 180 mL of HBSS-/- into a 500 mL beaker, add sterile stirring magnet and place on stirring plate at room temperature.
- Weigh out and add the following to the HBSS-/- solution slowly and sequentially: 20 mL of FBS, 400 mg Collagenase 2 ([final] = 2 mg/mL), 20 mg Soy bean trypsin inhibitor ([final] = 0.1mg/mL), 30 mg DNase 1 ([final] = 0.15 mg/mL), and 200 mg BSA ([final] = 1 mg/mL)
- Set the stirring to medium speed, and allow the mixture to stir for 10-20 min (cover with tin foil or paraffin film while stirring).
- Pour the mixed solution into a 250 mL glass bottle using a plastic funnel and place in the fume hood.
- Pass the solution through a plastic top filtration unit (0.22 μm membrane filter, 500 mL), aliquot 20 mL into 50 mL tubes (10 tubes total) and store at -20 °C until use.
- RPMI 1640 Media (2% washing solution and 10% complete growth medium)
- Combine RPMI 1640 and FBS in a glass bottle in the fume hood.
- For 2% FBS solution, combine 490 mL of RPMI 1640 and 10 mL FBS.
- For 10% FBS solution, combine 450 mL RPMI 1640 and 50 mL FBS.
- Pipette 500 μL of normocin into the 500 mL glass bottle from the previous step containing RPMI media and FBS (50 mg/mL stock, 0.05 mg/mL working concentration).
- Pass media through a 0.22 μm membrane filter and store in a 500 mL glass bottle at 4 °C until use.
- 30 min prior to starting the experiment, place a 20 mL aliquot of frozen enzymatic digestion solution in a 37 °C bead bath, place the 2% FBS and the 10% FBS RPMI 1640 media in a 37 °C bead bath, turn on the rocking water bath and set to 37 °C, 2 g, and set a temperature controlled centrifuge to 4 °C.
2. Collection of Decidual Tissue from Term Placental Membranes
- Place bottles with HBSS+/+, HBSS-/- and five 50 mL tubes in a rack under the fume hood. Pipette 25 mL of HBSS+/+ in one tube.
- Using gloved hands, take the term placenta out from the container used for transporting it from the operating room theatre. Place the placenta on the diaper pad (maternal side up) and spread the fetal membranes out using scissors and forceps.
- Lay overlapping diaper pads on the fume hood.
- Use a separate diaper to flip the placenta so the maternal side is face up.
- Find the point of membrane rupture and make incisions to allow the membrane to unfold and lay flat on the fumehood surface.
NOTE: Once the membrane is pulled back from the placenta, the decidua will be face up resting on the chorion layer, with the amnion layer at the back.
- Using a plastic cell scraper, carefully scrape the decidual tissue off the chorion and place in the 50 mL tube containing 25 mL HBSS+/+.
- Scrape the membrane with moderate pressure. Do not apply too much pressure as it may result in chorion contamination. Collect small blood clots as well, as these contain some decidual cells.
Caution: After decidual collection, placenta must be packaged in a biohazard plastic bin and frozen in a -20 °C freezer prior to appropriate disposal (according to your institutional rules). Bloody diaper pads must be wrapped in a seal-tight plastic bag and disposed of in a biohazard safety box.
3. Washing and Enzymatic Digestion of the Decidual Tissue
- Wash the collected tissues by gently shaking the 50 mL tube by hand and passing it through a 250 μm metal sieve (250 μm, size 60 mesh) resting over a sterile specimen (urine) container.
- Repeat the wash twice with HBSS+/+ and twice with HBSS-/- in fresh tubes for each wash. (final washes with HBSS-/- allows for the removal of calcium and magnesium present in HBSS+/+, which would otherwise interfere with the following enzymatic digestion process).
NOTE. During the washing steps, chorion tissue contamination will be apparent as the decidual tissue is light pink in color and amorphous, while the chorion is white, dense, and stringy. Therefore, chorion contamination can be easily removed with forceps. - Optionally, if the decidual tissue is thick, transfer it to a sterile 10 cm diameter petri dish and proceed to mince of the tissue with two opposing scalpels in the dish.
- Place the washed tissue in sterile 50 mL tube containing 100 mg of tissue/mL of enzymatic digestion solution (see Preparations).
- As a reference point, ensure that the level of decidual tissue reaches the 5-10 mL mark on the 50 mL tube.
- Use approximately 20 mL of enzymatic digestion solution (Prepared in step 1.1) to digest the total decidua collected from a whole term fetal membrane.
- Seal the cap of the tube with the paraffin film (seal the tube tightly and wrap paraffin film around the cap and top of the tube) under the culture hood and incubate decidual tissue at 37 °C for 20 min in a shaking water bath (145 rpm, 2 g).
- After incubation, remove the paraffin film and sterilize the surface of the tube containing digested tissue with 70% ethanol and bring under the fume hood. Shake the tube briefly by hand.
- Collect cell suspension through the metal sieve (250 μm, size 60 mesh) into a new sterile specimen container. Dilute with an equal volume (20 mL) of RPMI + 10% FBS containing 0.1% normocin to stop enzymatic reaction. Proceed directly to centrifugation step 4.1.
- If necessary, place the remaining undigested tissue back into a new 50 mL tube with 20 mL of fresh enzymatic digestion solution and repeat digestion (20 min, 37 °C, shaking water bath).
- If a second digestion is necessary, place the first tube with cell suspension on ice (cover with paraffin film to keep sterile). Repeat steps 3.5-3.6 and combine the two cells suspensions.
4. Generating a Single Cell Suspension
- Centrifuge the cell suspension (420 g, 4 °C, 11 min).
- Remove the supernatant and resuspend the cells in 40 mL of RPMI + 2% FBS containing 0.1% normocin ("wash buffer").
NOTE: Cell pellet will be loose and gelatinous due to red blood cell contamination, aspirate supernatant with caution. Removal of the upper phase with a manual pipette may be required. - Repeat the centrifugation at 420 g at 4 °C for 11 min.
- Carefully remove the supernatant (As mentioned in Note Step 4.2) and resuspend the cell pellet in 5 mL of wash buffer and add 35 mL of erythrocyte lysis buffer in the same tube.
NOTE: If the pellet is large or very bloody, it can be split into two tubes for the erythrocyte lysis step by adding 10 mL of wash buffer and dividing equally into two tubes and then adding 35 mL of Erythrocyte lysis buffer to each tube. - Incubate on ice for 20 min. Briefly vortex tube(s) at the beginning and the end of the incubation to lyse the red blood cells.
- Centrifuge at 420 g for 11 mins at 4 °C.
- Carefully, remove the supernatant and resuspend the pellet in 40 mL of wash buffer.
- Pass the cells through a 70 μm nylon filter to remove cell clumps.
- Centrifuge at 420 g for 11 min at 4 °C.
- Remove the supernatant and resuspend the cell pellet in 10 mL of complete medium (RPMI 10% + FBS containing 0.1% normocin).
- Count cells using the trypan blue dye-exclusion hemocytometer procedure as described below:
- Under the culture hood gently pipette cell suspension up and down 3x in order to thoroughly mix before combining with trypan blue. In a 1.5 mL tube prepare cell suspension, diluted 1:2 (combine 20 μL of trypan blue solution and 20 μL of decidual cell suspension). Carefully mix trypan blue-cell solution by pipetting up and down a couple of times (this solution is not sterile).
- Place the hemocytometer on the stage of the microscope with glass coverslip on top.
NOTE: Hemocytometer is a microscope slide with grids on it to give nine large squares divided by triple lines. Each large square has an area of 1 mm2, and the depth of fluid in the chamber is 0.1 mm. Therefore, the volume of fluid that can fill each large square is 1 mm*1 mm*0.1 mm= 0.1 mm3= 10-4 mL. - Slowly add 10 μL of the trypan blue-cell mixture into the groove of the hemocytometer, allowing capillary action to disperse the cell mixture over the entire slide (stop before mixture fills well).
- View the cells under a microscope (at 10X magnification) and count all white/green cells that exclude trypan blue (these are the viable cells) in the four large outer squares of the hemocytometer. When counting cells that touch the line, count only those that touch the right and upper lines but not those touching the left and bottom lines. Do not count dark blue cells; blue color indicates that the cell is dead as trypan blue dye can easily penetrate through the plasma membrane into the cytoplasm.
- To calculate the number of viable cells in 1 mL of cell suspension (X):
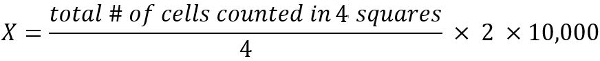
2 = dilution factor
10,000 = conversion factor (1 mL = 1 cm3 = 10,000*0.1 mm3)
- Dilute decidual cells to a desirable final concentration in RPMI + 10% FBS (growth medium).
NOTE: For tissue culture plating, pipette 2*106 cells/well into a plastic 6-well plate, 10*106 cells into a 10-cm plastic plate or 75,000 cells to a glass coverslip.
Access restricted. Please log in or start a trial to view this content.
Results
To validate the efficiency and viability of the isolated cells, they were characterized by two methods: flow cytometry and immunocytochemistry (ICC). 4 cell populations were targeted; decidualized stromal cells were detected by the anti-vimentin antibody, pan-leukocyte marker CD45 was used to identify decidual immune cells, cytokeratin was used to detect epithelial/endothelial cells and finally, cytokeratin 7 was used to detect any potential trophoblast (chorion or placental) contaminatio...
Access restricted. Please log in or start a trial to view this content.
Discussion
The protocol described here demonstrates a cost and time effective method for isolating primary decidual cells collected from the fetal membranes of whole human term placentae that is highly accessible and straightforward. The success of this protocol is dependent on two critical factors, (1) efficiency of decidual scraping from the chorion layer of the fetal membranes and (2) the care with which the decidual cells are handled throughout the protocol. It is important that chorion tissue contamination is controlled by ens...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The authors have nothing to disclose
Acknowledgements
The authors would like to thank the donors, the RCWIH BioBank, and the Mount Sinai Hospital/UHN Department of Obstetrics and Gynaecology for the human specimens used in this study. We would like to thank the members of the Lye lab, particularly Dr. Caroline Dunk for her help with the method development. This work is supported by the Burroughs Welcome Fund (grant #1013759).
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| Hank’s balanced salt solution with calcium and magnesium | Prepared in facility (LTRI) | ||
| Hank’s balanced salt solution without calcium and magnesium | Prepared in facility (LTRI) | ||
| Diaper pads | Sigma-Aldrich | D9542 | |
| Large surgical scissors | AL Medical | 2018-12-20. | |
| Large surgical forceps | Fine Science Tools | 11000-18 | |
| Plastic disposable cell scraper (25 cm) | Sarstedt | 83.183 | |
| 250 mm (size 60 mesh) metal sieve | Sigma-Aldrich | S1020-5EA | |
| Disposable scalpel with plastic handle (#21) | Fisher Scientific | 08-927-5D | |
| Sterile plastic petri dish (diameter 10 cm) | Sarstedt | 82.1473.001 | |
| Sterile specimen container (urine cup, 4.5 oz) | VWR | 25384-146 | |
| Nylon filter (70 mm) | VWR/Corning | 21008-952 | |
| Erythrocyte lysis buffer | Qiagen | 79217 | |
| Trypan blue, 0.4% solution | Lonza | 17-942E | |
| Parafilm | Fisher Scientific | 13-374-10 | |
| Hemocytometer | Reichert | 1490 | |
| Roswell Park Memorial Institute (RPMI) 1640 culture media | Invitrogen | 11835-055 | |
| Fetal bovine serum | Wisent | 080-150 | |
| Normocin (50mg/ mL) | Invivogen | ant-nr-1 | |
| Plastic top filtration unit (0.22 mm membrane, 500 mL) | Millipore | SCGPT05RE | |
| Collagenase 2, lyophilized powder | Sigma-Aldrich | C6885 | |
| Soy bean trypsin inhibitor, powder | Sigma-Aldrich | T9003-250mg | |
| DNase powder | Roche | 10104159001 | |
| Bovine serum albumin (BSA powder) | Fisher Scientific | BP1600-100 | |
| Spinning disc confocal microscope - Leica DMI 6000B | Leica | ||
| Imaging Flow cytometer - Image Stream MK2 | Amnis | ||
| IDEA software | Millipore Sigma | ||
| APC-conjugated Vimentin antibody | R&D Systems | IC2105A | |
| APC H7-conjugated CD45 antibody | BD | 641399 | |
| FITC-conjugated Cytokeratin antibody | MACs Miltenyi Biotec | 130-080-101 | |
| PerCP -conjugated Cytokeratin 7 antibody | Novus | NBP2-47941PCP | |
| eFluor450 Fixable Viability dye | Thermo Fisher Scientific | 65-0863-14 | |
| Vimentin primary antibody | Santa Cruz | sc-7558 | |
| CD45 primary antibody | Dako | M0701 | |
| Cytokeratin primary antibody | Dako | M0821 | |
| Cytokeratin 7 primary antibody | Dako | M7018 | |
| Mouse IgG | Santa Cruz | sc-2025 | |
| Goat IgG | Santa Cruz | sc-2028 | |
| Alexa Fluor 546 secondary antibody | Invitrogen | A10036 | |
| Alexa Fluor 594 secondary antibody | Fisher Scientific | A-11058 | |
| DAPI | Sigma-Aldrich | D9542 |
References
- Brosens, N., Hayashi, N., White, J. O. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 140, 4809-4820 (1999).
- Bell, S. C., D'Arcangues, C., Frase, I. S., Newton, J. R., Odlind, V. Decidualization and relevance to menstruation. , Cambridge University press. 187-212 (1990).
- Kariya, M. Interleukin-1 inhibits in vitro decidualization of human endometrial stromal cells. Journal of Clinical Endocrinology and Metabolism. 73, 1170-1174 (1991).
- Dimitriadis, E., Robb, L., Salamonsen, L. A. Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Molecular and Human Reproduction. 8, 636-643 (2002).
- Wu, W. -X., Brooks, J., Glasier, A. F., McNeilly, A. S. The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. Society for Endocrinology. 14, 255-261 (1995).
- Bell, S. C. Synthesis and secretion of protein by the endometrium and decidua. Implantation: Biology and Clinical Aspects. , 95-118 (1988).
- Croy, B. A., Chantakru, S., Esadeg, S., Ashkar, A. A., Wei, Q. Decidual natural killer cells: key regulators of placental development. Journal of Reproductive Immunology. 57, 151-168 (2002).
- Smarason, A. K., Gunnarsson, A., Alfredsson, J. H., Valdimarsson, H. Monocytosis and monocytic infiltration of decidua in early pregnancy. Journal of Clinical and Laboratory Immunology. 21, 1-5 (1986).
- Daiter, E., Pampfer, S., Yeung, Y. G., Barad, D., Stanley, E. R., Pollard, J. W. Expression of colony- stimulating factor-1 in the human uterus and placenta. Journal of Clinical Endocrinology and Metabolism. 74, 850-858 (1992).
- Casey, M. L., Cox, S. M., Beutler, B., Milewich, L., MacDonald, P. C. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. Journal of Clinical Investigation. 83, 430-436 (1989).
- Lala, P. K., Kennedy, T. G., Parhar, R. S. Suppression of lymphocyte alloreactivity by early gestational human decidua. II. Characterization of the suppressor mechanisms. Cellular Immunology. 127, 368-381 (1988).
- Shynlova, O., Nedd-Roderique, T., Li, Y., Dorogin, A., Nguyen, T., Lye, S. J. Infiltration of myeloid cells into decidua is a critical early event in the labour cascade and post-partum uterine remodelling. Journal of Cellular and Molecular Medicine. 17, 311-324 (2013).
- Osman, I., Young, A., Ledingham, M. A., Thomson, A. J., Jordan, F., Greer, I. A., Norman, J. E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Molecular Human Reproduction. 9, 41-45 (2003).
- Farine, T., Lye, S. J., Shynlova, O. Peripheral maternal leukocytes are activated in response to cytokines secreted by uterine tissues of pregnant women. Journal of Cellular and Molecular Immunology. 14, 635-638 (2017).
- Hamilton, S., et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biology of Reproduction. 86, 39(2011).
- Casey, M. L., Cox, S. M., Word, A., Macdonald, P. C. Cytokines and infection-induced preterm labour. Reprodution Fertility and Development. 2, 499-510 (1990).
- Yellon, S. M., Mackler, A. M., Kirby, M. A. The role of leukocyte traffic and activation in parturition. Journal of the Society for Gynecologic Investigation. 10, 323-338 (2003).
- Gomez-Lopez, N., StLouis, D., Lehr, M. S., Sanchez-Rodriguez, E. N., Arenas-Hernandez, M. Immune cells in term and preterm labor. Cellular & Molecular Immunology. 11, 571-581 (2014).
- Xu, Y., Plazyo, O., Romero, R., Hassan, S. S., Gomez-Lopez, N. Isolation of leukocytes from the human maternal-fetal interface. Journal of Visualized Experiments. 99, (2015).
- Trundley, T., Gardner, L., Northfield, J., Moffett, A. Methods for isolation of cells from the human fetal-maternal interface. Methods in Molecular Medicine. 122, 109-122 (2006).
- Jividen, K., Movassagh, M. J., Jazaeri, A., Li, H. Two methods for establishing primary human endometrial stromal cells from hysterectomy specimens. Journal of Visualized experiments. 87, (2014).
- Pelekanos, R. A., Sardesai, V. S., Futrega, K., Lott, W. B., Kuhn, M., Doran, M. R. Isolation and expansion of mesenchymal stem/stromal cells derived from human placenta tissue. Journal of Visualized experiments. 112, (2016).
- De Clercq, K., Hennes, A., Vrien, J. Isolation of mouse endometrial epithelial and stromal cells for in vitro decidualization. Journal of Visualized Experiments. 121, (2017).
- Zhang, J., Shynlova, O., Sabras, S., Bang, A., Briollais, L., Lye, S. J. Immunophenotyping and activation status of maternal and peripheral blood leukocytes during pregnancy and labour, both term and preterm. Journal of Cellular and Molecular Medicine. 10, 2386-2402 (2017).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved