A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Surface Properties of Synthesized Nanoporous Carbon and Silica Matrices
In This Article
Summary
Here we report the synthesis and characterization of ordered nanoporous carbon (with a 4.6 nm pore size) and SBA-15 (with a 5.3 nm pore size). The work describes the surface and textural properties of nanoporous molecular sieves, their wettability, and the melting behavior of D2O confined in the materials.
Abstract
In this work, we report the synthesis and characterization of ordered nanoporous carbon material (also called ordered mesoporous carbon material [OMC]) with a 4.6 nm pore size, and ordered silica porous matrix, SBA-15, with a 5.3 nm pore size. This work describes the surface properties of nanoporous molecular sieves, their wettability, and the melting behavior of D2O confined in the differently ordered porous materials with similar pore sizes. For this purpose, OMC and SBA-15 with highly ordered nanoporous structures are synthesized via impregnation of the silica matrix by applying a carbon precursor and by the sol-gel method, respectively. The porous structure of investigated systems is characterized by an N2 adsorption-desorption analysis at 77 K. To determine the electrochemical character of the surface of synthesized materials, potentiometric titration measurements are conducted; the obtained results for OMC shows a significant pHpzc shift toward the higher values of pH, relative to SBA-15. This suggests that investigated OMC has surface properties related to oxygen-based functional groups. To describe the surface properties of the materials, the contact angles of liquids penetrating the studied porous beds are also determined. The capillary rise method has confirmed the increased wettability of the silica walls relative to the carbon walls and an influence of the pore roughness on the fluid/wall interactions, which is much more pronounced for silica than for carbon mesopores. We have also studied the melting behavior of D2O confined in OMC and SBA-15 by applying the dielectric method. The results show that the depression of the melting temperature of D2O in the pores of OMC is about 15 K higher relative to the depression of the melting temperature in SBA-15 pores with a comparable 5 nm size. This is caused by the influence of adsorbate/adsorbent interactions of the studied matrices.
Introduction
In 1992, ordered nanoporous silica materials were obtained for the first time, using an organic template; since then, a large number of publications related to different aspects of these structures, synthetic methods, the investigation of their properties, their modifications, and different applications have appeared in the literature1,2,3. The interest in SBA-15 nanoporous silica matrix4 is due to their unique quality: a high surface area, wide pores with a uniform pore size distribution, and good chemical and mechanical properties. Nanoporous silica materials with cylindrical pores, such as SBA-155, are often used as a porous matrix for catalysts as they are efficient catalysts in organic reactions6,7. The material can be synthesized with a wide range of methods that can influence their characteristics8,9,10. Therefore, it is crucial to optimize these methods for potential applications in many fields: electrochemical devices, nanotechnology, biology and medicine, drug delivery systems, or in adhesion and tribology. In the present study, two different types of nanoporous structures are presented, namely silica and carbon porous matrices. To compare their properties, the SBA-15 matrix is synthesized using the sol-gel method, and the ordered nanoporous carbon material is prepared by the impregnation of the resulting silica matrix with a carbon precursor.
Porous carbon materials are important in many appliances due to their high surface area and their unique and well-defined physicochemical properties6,11,12. Typical preparation results in materials with randomly distributed porosity and a disordered structure; there is also a limited possibility for the change of the general pore parameters, and thus, structures with relatively broad pore size distributions are obtained13. This possibility is broadened for nanoporous carbon materials with high surface areas and ordered systems of nanopores. More predicted geometry and more control of the physicochemical processes inside the pore space are important in many applications: as catalysts, separation media systems, advanced electronic materials, and nanoreactors in many scientific fields14,15.
To obtain the porous carbon replicas, the ordered silicates can act as a solid matrix to which carbon precursors are directly introduced. The method can be divided into several stages: the selection of ordered silica material; the deposition of a carbon precursor in a silica matrix; carbonization; then, the removal of the silica matrix. Many different types of carbonaceous materials can be obtained by this method, but not all nonporous materials have an ordered structure. An important element of the process is the selection of a suitable matrix whose nanopores must form a stable, three-dimensional structure16.
In this work, the influence of the type of pore walls on the surface properties of synthesized nanoporous matrices is investigated. The surface properties of OMC material are reflected by the surface properties of silica analog (SBA-15) of OMC. The textural and structural properties of both types of materials (OMC and SBA-15) are characterized by low-temperature N2 adsorption/desorption measurements (at 77 K), transmission electron microscopy (TEM), and energy dispersive X-ray analysis (EDX).
Low-temperature gas adsorption/desorption measurement is one of the most important techniques during the characterization of porous materials. Nitrogen gas is used as an adsorbate due to its high purity and the possibility to create a strong interaction with solid adsorbents. Important advantages of this technique are the user-friendly commercial equipment and relatively easy data-processing procedures. The determination of nitrogen adsorption/desorption isotherms is based on the accumulation of the adsorbate molecules on the surface of solid adsorbent at 77 K in a wide range of pressure (P/P0). The Barrett, Joyner, and Halenda (BJH) procedure for calculating the pore size distribution from experimental adsorption or desorption isotherms is applied. The most important assumptions of the BJH method include a planar surface and an even distribution of the adsorbate on the investigated surface. However, this theory is based on the Kelvin equation and it remains the most widely used manner for calculating the pore size distribution in the mesoporous range.
To evaluate the electrochemical character of the samples, a potentiometric titration method is applied. The surface chemistry of the material depends on the surface charge related to the presence of heteroatoms or functional groups on the surface. The surface properties are also investigated by contact angle analysis. The wettability inside the pores provides information about the adsorbate-adsorbent interactions. The influence of the wall roughness on the melting temperature of the water confined in both samples is studied with the dielectric relaxation spectroscopy (DRS) technique. Measurements of the dielectric constant allow the investigation of melting phenomena as the polarizability of the liquid and solid phases are different from each other. A change in the slope of the temperature dependence of the capacitance shows that melting occurs in the system.
Protocol
1. Preparation of the OMC Materials
- Synthesis of a silica matrix as OMC precursor
- Prepare 360 mL of 1.6 M HCl by adding 50 mL of HCl (36% - 38%) in a 500 mL round-bottom flask and, then, adding 310 mL of ultrapure water (resistivity of 18.2 MΩ·cm).
- To that, add 10 g of PE 10500 polymer (6.500 g/mol).
- Place the flask in an ultrasonic bath. Heat the solution to 35 °C and stir it until the solid polymer is completely dissolved, making a homogeneous mixture.
- Add 10 g of 1,3,5-trimethylbenzene to the flask and stir the content (at a stirring rate of 220 rpm) by maintaining it at 35 °C in the water bath.
- After stirring for 30 min, add 34 g of tetraethyl orthosilicate (TEOS) to the flask. Add the TEOS slowly and dropwise with constant stirring. Ensure that it takes 10 min to add 34 g of TEOS.
- Stir the solution mixture again for 20 h at the same temperature (35 °C).
- Transfer the contents of the flask into a polytetrafluoroethylene cartridge and place it in an autoclave. Leave the solution for 24 h at 90 °C.
- Filter the resulting precipitate, using a Büchner funnel, and wash it with distilled water, using at least 1 L.
- Dry the obtained solid at room temperature and apply a thermal treatment to the sample at 500 °C, using a muffle furnace in an air atmosphere for 6 h.
- Impregnation of the resulting silica matrix, using a carbon precursor
- Prepare impregnation solutions (IS1 and IS2) with appropriate proportions of water, 3 M sulfuric acid (VI), and sugar (glucose), where glucose plays the role of carbon precursor and sulfuric acid acts as catalyst.
CAUTION: Sulfuric acid is very toxic, it causes severe skin burns and eye damage.- Prepare IS1. For each gram of silica, mix 5 g of water, 0.14 g of 3 M sulfuric acid (VI), and 1.25 g of sugar.
- Prepare IS2. For each gram of silica, mix 5 g of water, 0.08 g of 3 M sulfuric acid (VI), and 0.75 g of sugar.
- Place the silica material (1 g) and the prepared solution IS1 of the carbon precursor and the catalyst in a 500 mL flask. Heat the mixture in a vacuum dryer at 100 °C for 6 h.
NOTE: In this step, use only IS1. IS2 should be applied in the next step. - Add the IS2 to the mixture in the vacuum dryer (to the solution with the partially carbonized carbon precursor). Heat the mixture again in the vacuum dryer at 160 °C for 12 h.
- Prepare impregnation solutions (IS1 and IS2) with appropriate proportions of water, 3 M sulfuric acid (VI), and sugar (glucose), where glucose plays the role of carbon precursor and sulfuric acid acts as catalyst.
- Tempering/carbonization
- Transfer the obtained composite to a mortar for the fragmentation of the larger particles and a homogenization of the material.
- Place the obtained product into the flow furnace and heat it to 700 °C (at a heating rate of 2.5 °C/min) and heat for 6 h at this temperature. Heat the material in a nitrogen atmosphere.
- Allow the solution to cool before opening the furnace.
- Removal of the silica matrix by etching
- Prepare 100 mL of etching solution (ES). Mix 50 mL of 95% ethyl alcohol and 50 mL of water. Add 7 g of potassium hydroxide and stir until it is dissolved.
- Place all obtained carbonized material (at least 1 g) in a 250 mL round-bottom flask and add 100 mL of ES.
- Supply the system with a reflux condenser and magnetic stirrer and heat to a boil while stirring constantly. Boil the mixture for 1 h.
- Transfer the obtained material to the Büchner funnel, wash it with at least 4 L of distilled water, and dry it.
2. Preparation of the Silica SBA-15 Matrix
- Synthesize a silica matrix.
- Prepare 150 mL of 1.6 M HCl.
- Dissolve 4 g of PE 6400 polymer (EO13PO70EO13) in 150 mL of acid solution in a round-bottom flask.
- Place the flask in an ultrasonic bath. Heat the solution to 40 °C and stir it so that the polymer can dissolve (at least for 30 min).
- Slowly add 8.5 g of TEOS to the flask, dropwise, with constant stirring. Stir the solution mixture for 24 h at the same temperature (40 °C).
- Transfer the contents of the flask to a polytetrafluoroethylene cartridge. Leave the solution for 24 h in a 120 °C oven.
- Filter the resulting precipitate, using a Büchner funnel, and wash it with distilled water (at least 1 L).
- Dry the obtained solid at room temperature and calcine for 6 h at 600 ° C, using a muffle furnace in an air atmosphere.
3. Methods of Characterization
- Low-temperature nitrogen adsorption/desorption measurements
- Use an automatic sorption analyzer to obtain N2 adsorption/desorption isotherms at 77 K.
- Use an appropriate glass tube for nitrogen sorption measurements. Before adding the porous sample to the glass tube, clean the tube in an ultrasonic washer and rinse it first with distilled water and, next, with anhydrous ethanol.
- Heat the glass tube at 150 °C for 3 h and fill the tube with compressed nitrogen. Weigh the empty glass tube under the nitrogen conditions before the measurement to minimize the weight error.
- Place the sample in the glass tube and weigh the total mass (the mass of the sample with the glass tube).
- Prior to the measurements, degas the sample. Place the glass tube with the sample in the degassing port of the sorption analyzer. Apply the following process conditions: a pressure of at least 0.01 mmHg, a temperature of 423 K, and a duration of 24 h. In the degassing port, connect the sample to the vacuum and heat it to the set temperature (423 K). After degassing, fill the sample with nitrogen and transfer it to the analysis port.
- Transmission electron microscopy
- Use the TEM microscope with the 120 kV (for SBA-15) and 200 kV (for the OMC material) accelerating voltages to collect the good quality TEM images.
- For preparing a monodisperse film of the sample, disperse the sample (1 mg) in ethanol (1 mL). Perform the dispersion procedure in a microcentrifuge tube by placing it in an ultrasonic bath for 3 min.
- Place two drops of the dispersion on a TEM copper grid using a micropipette. Transfer the TEM grid to the TEM microscope and start the TEM imaging.
- Energy dispersive X-ray spectroscopy
- Use a scanning electron microscope equipped with an X-ray detector to acquire an energy dispersive X-ray spectrum of the samples.
- Apply an acceleration voltage of 15 kV to harvest the spectrum. Select the silicon as the optimization element for SBA-15 and the carbon for the OMC sample.
- Potentiometric titration measurement
- Use an automatic burette to perform the potentiometric titration experiment. Add the titrant in small and controlled portions (according to the titration software and procedure). Provide the smallest increment, at least 1 μL, by an automatic dosing intrument.
- Disperse 0.1 g of the sample in 30 mL of an electrolyte solution (water solution of 0.1 M NaCl). Use the magnetic stirrer and isothermal conditions (293 ± 0.1 K) during the dispersion procedure.
- Add 1 - 2 mL of titrant (0.1 M NaOH solution) to the suspension.
NOTE: Perform the addition in small aliquots (each to be about 0.05 mL). The automatic burette The procedure should provide at least a dozen experimental points in the pH range from 1 to 14. - Calculate the surface density of the charge Qs, using the following formula.
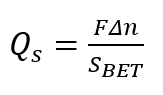 (1)
(1)
Here,
Δn = the change in H+/OH- balance reduced per mass of sample;
SBET = the Brunauer-Emmett-Teller (BET) surface area of the porous solid state;
F = the Faraday number.
- Capillary rise method for wettability measurements
- To determine the contact angle inside the pores of the studied samples, use the capillary rise method.
NOTE: This method is based on the measurement of the mass rise of the liquid, which is penetrating the porous bed, as the function of the time. The main assumption of this method is based on the fact that penetrating liquid is advancing into the porous column and that this column consists of intergranular capillaries with a certain average radius. Thus, every relation derived for single capillary is valid for the layer of the porous powder. In a single vertical capillary, the wetting liquid floats against the gravitational forces as a result of the difference of pressure between the liquid and the vapor in the pores (capillary pressure). In this meaning, the penetration of the liquid into the porous bed allows the determination of the dynamic advancing contact angle inside the pores. - Apply the modified Washburn’s equation17,18, expressed as follows.
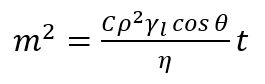 (2)
(2)
Here,
m = the mass of the measured liquid;
C = the geometric parameter dependent on the distribution, shape, and size of the pores;
ρ = the density;
γl = the surface tension;
η = the viscosity of the penetrating liquid;
θ = the contact angle;
t = time. - Using equation (2), estimate the values of the advancing contact angles inside the studied pores.
- Prepare the force tensiometer. For powders, use a glass tube with a diameter of 3 mm and a ceramic sinter; for liquid, use a vessel with a diameter of 22 mm and a maximum volume of 10 mL.
- Measure 0.017 g of the sample.
- Start the computer program connected to the tensiometer. Put a vessel with the liquid on a motor-driven stage and suspend the glass tube with the sample on an electronic balance.
- Start the motor and start approaching the liquid in the vessel with the sample at a low constant rate of 10 mm/min; set the immersion depth of the sample tube into the liquid equal to 1 mm.
- From this moment, the dependence m2 = f(t) registers in the computer program.
- Stop the experiment when the dependence m2 = f(t) starts to show the characteristic plateau.
- Check for accuracy by repeating this procedure 3x - 5x.
- To determine the contact angle inside the pores of the studied samples, use the capillary rise method.
- Dielectric relaxation spectroscopy
- To describe the melting behavior of confined water inside the studied porous matrices, perform the temperature measurements of electric capacitance C of the sample present in a parallel plate capacitor made of stainless steel19,20,21. To measure the capacitance C as the function of the temperature and the frequency of the applied cyclic electric field, use an impedance analyzer.
NOTE: The complex electric permittivity is defined as ε* = ε` + iε``, where ε` = C/C0 is the real, and ε`` = tgδ·ε` is an imaginary part of the permittivity, where C0 is the capacitance of the empty capacitor and tgδ are the dielectric losses. - Put the measured sample into the plate capacitor.
- Select a frequency range from 100 Hz to 1 MHz and a temperature from 140 K to 305 K. Control the rate of temperature change with the temperature controller; set the temperature rate as equal to 0.8 K/min during the cooling and 0.6 K/min during the heating process.
- To describe the melting behavior of confined water inside the studied porous matrices, perform the temperature measurements of electric capacitance C of the sample present in a parallel plate capacitor made of stainless steel19,20,21. To measure the capacitance C as the function of the temperature and the frequency of the applied cyclic electric field, use an impedance analyzer.
Results
To characterize the porous structure of the investigated samples of OMC and SBA-15, the N2 adsorption-desorption isotherms were recorded at 77 K. The experimental N2 gas adsorption-desorption isotherms characterizing the investigated systems, as well as the pore size distributions (PSD) obtained from the adsorption and desorption data, are presented in Figure 1A-D. The position of the inflection points on the sorption is...
Discussion
The critical steps during the preparation of the ordered mesoporous carbon material include the preparation of the ordered mesoporous silica materials as the template with well-defined structural properties that affect the properties of the final materials and a tempering/carbonization step under a nitrogen atmosphere. The modification of the typical method of preparation of the mesoporous ordered silicates with cylindrical pores28 concerns the application of an untypical structure-directing agent...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank the National Center of Science for providing financial support with grant no. DEC-2013/09/B/ST4/03711 and UMO-2016/22/ST4/00092. The authors are also grateful for the partial support from the Poland Operational Program Human Capital PO KL 4.1.1, as well as from the National Centre for Research and Development, under research grant no. PBS1/A9/13/2012. The authors are especially grateful for Prof. L. Hołysz from Interfacial Phenomena Division, Faculty of Chemistry, Maria Curie-Skłodowska University, Lublin, Poland, for her kindness and enabling the measurements of the wettability in the SBA-15 nanopores.
Materials
| Name | Company | Catalog Number | Comments |
| 1,3,5-trimethylbenzene | Sigma-Aldrich, Poland | M7200 Sigma-Aldrich | Mesitylene, also known as 1,3,5-trimethylbenzene, reagent grade, assay: 98%. |
| anhydrous ethanol | POCH, Avantor Performance Materials Poland S.A. | 396480111 | Assay, min. 99.8 %, analysis-pur (a.p.) |
| ASAP 2020. Accelerated Surface Area and Porosimetry System | Micromeritics Instrument Corporation, Norcross, GA, USA | Samples were outgassed before analysis at 120 oC for 24 hours in degas port of analyzer. The dead space volume was measured for calibration on experimental measurement using helium as a adsorbate. | |
| Automatic burette Dosimat 665 | Metrohm, Switzerland | The surface charge properties were experimentally determined by potentiometric titration of the suspension at constant temperature 20°C maintained by the thermostatic device. Prior to potentiometric titration measurements, the solid samples were dried by 24 hours at 120 oC. The initial pH was established by addition of 0.3 cm3 of 0.2 mol/L HCl. T The 0.1 mol/L NaOH solution was used as a titrant, added gradually by using automatic burette. | |
| Digital pH-meter pHm-240 | Radiometer, Copenhagen | Device coupled with automatic burette | |
| ethyl alcohol | POCH, Avantor Performance Materials Poland S.A. | 396420420 | Assay, min. 96 %.analysis-pur (a.p.) |
| glucose | POCH, Avantor Performance Materials Poland S.A. | 459560448 | assay 99.5% |
| Hydrochloric acid | POCH, Avantor Performance Materials Poland S.A. | 575283115 | Hydrochloric acid, 35 - 38% analysis-pur (a.p.) |
| HOPG graphite substrate | Spi Supplies | LOT#1170906 | HOPG SPI-2 Grade, 20x20x1 mm |
| Impedance analyzer Solartron 1260 | Solartron | ||
| Pluronic PE 6400 polymer | BASF (Polska) | (EO13PO70EO13) | |
| Pluronic PE10500 | BASF Canada Inc. | Molar mass 6500 g/mol | |
| potassium hydroxide | Sigma-Aldrich, Poland | P5958 Sigma-Aldrich | BioXtra, ≥85% KOH basis |
| SEM microscope | JEOL JSM-7001F | Scanning Electron Microscope with EDS detector | |
| Sigma Force Tensiometer 701 | KSV, Sigma701, Biolin Scientific | force tensiometer | |
| Sulfuric acid (VI) | POCH, Avantor Performance Materials Poland S.A. | 575000115 | |
| surface glass type KS 324 Kavalier | Megan Poland | 80 % of SiO2 , 11% of Na2O and 9% of CaO | |
| Tecnai G2 T20 X-TWIN | FEI, USA | Transmission Electron Microscope with EDX detector. | |
| TEM microscope | JEOL JEM-1400 | ||
| temperature controller ITC503 | Oxford Instruments | ||
| Tetraethylorthosilicate | Sigma-Aldrich, Poland | 131903 | Tetraethyl silicate, TEOS, reagent grade, assay 98% |
| Ultrapure water | Millipore, Merck KGaA, Darmstadt, Germany | SIMSV0001 | Simplicity Water Purification SystemUltrapure Water: 18.2 MegOhm·cm, TOC: <5 ppb |
References
- Tao, Y., Kanoh, H., Abrams, L., Kaneko, K. Mesopore-Modified Zeolites: Preparation, Characterization, and Applications. Chemical Reviews. , 896-910 (2006).
- Wan, Y., Zhao, D. On the Controllable Soft-Templating Approach to Mesoporous Silicates. Chemical Reviews. 107, 2821-2860 (2007).
- Khder, A. E. S., Hassan, H. M. A., El-Shall, M. S. Acid catalyzed organic transformations by heteropolytungstophosphoric acid supported on MCM-41. Applied Catalysis A. 411, 77-86 (2012).
- Zhao, D. D., et al. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science. 279, 548-552 (1998).
- Linssen, T., Cassiers, K., Cool, P., Vansant, E. Mesoporous templated silicates: an overview of their synthesis, catalytic activation and evaluation of the stability. Advances in Colloid and Interface Science. 103, 121-147 (2003).
- Eftekhari, A., Fan, Z. Ordered mesoporous carbon and its applications for electrochemical energy storage and conversion. Materials Chemistry Frontiers. 1, 1001-1027 (2017).
- Sing, K. Characterization of porous materials: past, present and future. Colloids and Surfaces A. 241, 3-7 (2004).
- Huo, Q., Margolese, D. I. Generalized synthesis of periodic surfactant/inorganic composite materials. Nature. 368, 317-321 (1994).
- Selvaraj, M., Kawi, S., Park, D. W., Ha, C. S. Synthesis and characterization of GaSBA-15: Effect of synthesis parameters and hydrothermal stability. Microporous and Mesoporous Materials. , 586-595 (2009).
- Leonard, A., et al. Toward a better control of internal structure and external morphology of mesoporous silicas synthesized using a nonionic surfactant. Langmuir. 19, 5484-5490 (2003).
- Liang, C., Li, Z., Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angewandte Chemie International Edition. 47, 3696-3717 (2008).
- Babić, B., et al. New mesoporous carbon materials synthesized by a templating procedure. Ceramics International. 39 (4), 4035-4043 (2013).
- Allen, S. J., Whitten, L., Mckay, G. The Production and Characterization of Activated Carbons: A Review. Developments in Chemical Engineering and Mineral Processing. 6, 231-261 (1998).
- Kwak, G., et al. Preparation Method of Co3O4 Nanoparticles Using Ordered Mesoporous Carbons as a Template and Their Application for Fischer-Tropsch Synthesis. The Journal of Physical Chemistry C. 117 (4), 1773-1779 (2013).
- Koo, H. M., et al. Effect of the ordered meso-macroporous structure of Co/SiO2 on the enhanced activity of hydrogenation of CO to hydrocarbons. Catalysis Science and Technology. 6, 4221-4231 (2016).
- Jun, S., Joo, S. H., Ryoo, R., Kruk, M., Jaroniec, M. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. Journal of the American Chemical Society. 122 (43), 10712-10713 (2000).
- Washburn, E. W. The dynamics of capillary flow. Physical Review Series2. 17, 273 (1921).
- Śliwińska-Bartkowiak, M., Sterczyńska, A., Long, Y., Gubbins, K. E. Influence of Microroughness on the Wetting Properties of Nano-Porous Silica Matrices. Molecular Physics. 112, 2365-2371 (2014).
- Śliwińska-Bartkowiak, M., et al. Melting/freezing behavior of a fluid confined in porous glasses and MCM-41: dielectric spectroscopy and molecular simulation. Journal of Chemical Physics. 114, 950-962 (2001).
- Coasne, B., Czwartos, J., Śliwińska-Bartkowiak, M., Gubbins, K. E. Freezing of mixtures confined in silica nanopores: experiment and molecular simulation. Journal of Chemical Physics. 133, 084701-084709 (2010).
- Chełkowski, A. . Dielectric Physics. , (1990).
- Radhakrishnan, R., Gubbins, K. E., Śliwińska-Bartkowiak, M. Global phase diagrams for freezing in porous media. Journal of Chemical Physics. 116, 1147-1155 (2002).
- Gubbins, K. E., Long, Y., Śliwińska-Bartkowiak, M. Thermodynamics of confined nano-phases. Journal of Chemical Thermodynamics. 74, 169-183 (2014).
- Radhakrishnan, R., Gubbins, K. E., Śliwińska-Bartkowiak, M. Effect of the fluid-wall interaction on freezing of confined fluids: Toward the development of a global phase diagram. Journal of Chemical Physics. 112, 11048 (2000).
- Cassie, A. B. D., Baxter, S. Wettability of porous surfaces. Transactions of the Faraday Society. 40, 546 (1944).
- Sing, K. Adsorption methods for the characterization of porous materials. Advances in Colloid and Interface Science. 76, 3-11 (1998).
- Sing, K. The use of nitrogen adsorption for the characterisation of porous materials. Colloids and Surfaces A. 187, 3-9 (2001).
- Yu, C., Fan, J., Tian, B., Zhao, D. Morphology Development of Mesoporous Materials: a Colloidal Phase Separation Mechanism. Chemistry of Materials. 16 (5), 889-898 (2004).
- Liu, D., et al. Enhancement of Electrochemical Hydrogen Insertion in N-Doped Highly Ordered Mesoporous Carbon. The Journal of Physical Chemistry C. 118 (5), 2370-2374 (2014).
- Choi, W. C., et al. Platinum Nanoclusters Studded in the Microporous Nanowalls of Ordered Mesoporous Carbon. Advanced Materials. 17, 446-451 (2005).
- Rouquerol, F., Rouquerol, J., Sing, K. . Adsorption by Powders and Porous Solids: Principles, Methodology and Application. , (1999).
- Gregg, S. J., Sing, K. S. W. . Adsorption, Surface Area and Porosity. , (1982).
- Llewellyn, P. L., Rouquerol, F., Rouquerol, J., Sing, K. S. W., Unger, K. K., Kreysa, G., Baselt, J. P. Critical appraisal of the use of nitrogen adsorption for the characterization of porous carbons. Characterization of Porous Solids V. , 421-427 (2000).
- Sing, K. S. W. The use of gas adsorption for the characterization of porous solids. Colloids and Surfaces. 38, 113-124 (1989).
- Rouquerol, J. Recommendations for the characterization of porous solids. Pure & Applied Chemistry. 66, 1739-1758 (1994).
- Marega, C. A direct SAXS approach for the determination of specific surface area of clay in polymer-layered silicate nanocomposites. The Journal of Physical Chemistry B. 116, 7596-7602 (2012).
- Tsao, C. S., et al. Neutron Scattering Methodology for Absolute Measurement of Room-Temperature Hydrogen Storage Capacity and Evidence for Spillover Effect in a Pt-Doped Activated Carbon. The Journal of Physical Chemistry Letters. 1, 1569-1573 (2010).
- Mattson, J. S., Mark, H. B. . Activated Carbon: Surface Chemistry and Adsorption from Solution. , (1971).
- László, K., Szucs, A. Surface characterization of polyethyleneterephthalate (PET) based activated carbon and the effect of pH on its adsorption capacity from aqueous phenol and 2,3,4-trichlorophenol solutions. Carbon. 39, 1945-1953 (2001).
- Garten, V. A., Weiss, D. E., Willis, J. B. A new interpretation of the acidic and basic structures in carbons. Australian Journal of Chemistry. 10, 309-328 (1957).
- Boehm, H. P. Surface oxides on carbon and their analysis: A critical assessment. Carbon. 40, 145-149 (2002).
- Menendez, J. A., Phillips, J., Xia, B., Radovic, L. R. On the modification and characterization of chemical surface properties of activated carbon: In the search of carbons with stable basic properties. Langmuir. 12, 4404-4410 (1996).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved