A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Supramaximal Intensity Hypoxic Exercise and Vascular Function Assessment in Mice
In This Article
Summary
High-intensity training in hypoxia is a protocol that has been proven to induce vascular adaptations potentially beneficial in some patients and to improve athletes' repeated sprint ability. Here, we test the feasibility of training mice using that protocol and identify those vascular adaptations using ex vivo vascular function assessment.
Abstract
Exercise training is an important strategy for maintaining health and preventing many chronic diseases. It is the first line of treatment recommended by international guidelines for patients suffering from cardiovascular diseases, more specifically, lower extremity artery diseases, where the patients' walking capacity is considerably altered, affecting their quality of life.
Traditionally, both low continuous exercise and interval training have been used. Recently, supramaximal training has also been shown to improve athletes' performances via vascular adaptations, amongst other mechanisms. The combination of this type of training with hypoxia could bring an additional and/or synergic effect, which could be of interest for certain pathologies. Here, we describe how to perform supramaximal intensity training sessions in hypoxia on healthy mice at 150% of their maximal speed, using a motorized treadmill and a hypoxic box. We also show how to dissect the mouse in order to retrieve organs of interest, particularly the pulmonary artery, the abdominal aorta, and the iliac artery. Finally, we show how to perform ex vivo vascular function assessment on the retrieved vessels, using isometric tension studies.
Introduction
In hypoxia, the decreased inspired fraction of oxygen (O2) leads to hypoxemia (lowered arterial pressure in hypoxia) and an altered O2 transport capacity1. Acute hypoxia induces an increased sympathetic vasoconstrictor activity directed toward skeletal muscle2 and an opposed 'compensatory' vasodilatation.
At submaximal intensity in hypoxia, this 'compensatory' vasodilatation, relative to the same level of exercise under normoxic conditions, is well established3. This vasodilation is essential to ensure an augmented blood flow and maintenance (or limit the alteration) of oxygen delivery to the active muscles. Adenosine was shown to not have an independent role in this response, while nitric oxide (NO) seems the primary endothelial source since significant blunting of the augmented vasodilatation was reported with nitric oxide synthase (NOS) inhibition during hypoxic exercise4. Several other vasoactive substances are likely playing a role in the compensatory vasodilatation during a hypoxic exercise.
This enhanced hypoxic exercise hyperemia is proportional to the hypoxia-induced fall in arterial O2 content and is larger as the exercise intensity increases, for example during intense incremental exercise in hypoxia.
The NO-mediated component of the compensatory vasodilatation is regulated through different pathways with increasing exercise intensity3: if β-adrenergic receptor-stimulated NO component appears paramount during low-intensity hypoxic exercise, the source of NO contributing to compensatory dilatation seems less dependent on β-adrenergic mechanisms as the exercise intensity increases. There are other candidates for stimulating NO release during higher-intensity hypoxic exercise, such as ATP released from erythrocytes and/or endothelial-derived prostaglandins.
Supramaximal exercise in hypoxia (named repeated sprint training in hypoxia [RSH] in the exercise physiology literature) is a recent training method5 providing performance enhancement in team- or racket-sport players. This method differs from interval training in hypoxia performed at or near maximal speed6 (Vmax) since RSH performed at maximal intensity leads to a greater muscle perfusion and oxygenation7 and specific muscle transcriptional responses8. Several mechanisms have been proposed to explain the effectiveness of RSH: during sprints in hypoxia, the compensatory vasodilation and associated higher blood flow would benefit the fast-twitch fibers more than the slow-twitch fibers. Consequently, RSH efficiency is likely to be fiber-type selective and intensity dependent. We speculate that the improved responsiveness of the vascular system is paramount in RSH.
Exercise training has been extensively studied in mice, both in healthy individuals and in pathological mouse models9,10. The most common way to train mice is using a rodent treadmill, and the traditionally used regimen is low-intensity training, at 40%–60% of Vmax (determined using an incremental treadmill test11), for 30–60 min12,13,14,15. Maximal intensity interval training and its impact on pathologies have been widely studied in mice16,17; thus, interval training running protocols for mice have been developed. Those protocols usually consist of about 10 bouts of running at 80%–100% of Vmax on a rodent motorized treadmill, for 1–4 min, interspersed with active or passive rest16,18.
The interest in mice exercising at supramaximal intensity (i.e., above the Vmax) in hypoxia comes from previous results that the microvascular vasodilatory compensation and the intermittent exercise performance are both more increased at supramaximal than at maximal or moderate intensities. However, to our knowledge, there is no previous report of a supramaximal training protocol in mice, either in normoxia or in hypoxia.
The first aim of the present study was to test the feasibility of supramaximal intensity training in mice and the determination of a tolerable and adequate protocol (intensity, sprint duration, recovery, etc.). The second aim was to assess the effects of different training regimen in normoxia and hypoxia on the vascular function. Therefore, we test the hypotheses that (1) mice tolerate well supramaximal exercise in hypoxia, and (2) that this protocol induces a larger improvement in vascular function than exercise in normoxia but also than exercise in hypoxia at lower intensities.
Protocol
The local state's animal care committee (Service de la Consommation et des Affaires Vétérinaires [SCAV], Lausanne, Switzerland) approved all experiments (authorization VD3224; 01.06.2017) and all experiments were carried out in accordance with the relevant guidelines and regulations.
1. Animal housing and Preparation
- House 6 to 8 week-old C57BL/6J male mice in the animal facility for at least 1 week prior to the beginning of the experiments in order for the mice to get used to their new housing conditions. For practical reasons, mice of the same experimental group are usually housed together.
- Keep the mice in a temperature-controlled room (22 ± 1 °C) with a 12 h light/dark cycle with ad libitum access to food and water.
2. Determination of the Maximal Speed and Standard Assessment of Performance Improvement by Treadmill Incremental Test
NOTE: The following steps are critical to completing the training protocols.
- Use a motorized treadmill for mice where mice can be on multiple lanes alongside each other, with a 0° inclination and mounted with an electric grid set to 0.2 mA at the back of the lane, in order to encourage the mice to run.
- Prior to the first test, submit the mice to 4 days of acclimatization to the treadmill, according to the following protocol.
- On day 1, have mice run for 10 min at 4.8 m/min.
- On day 2, have the mice run for 10 min at 6 m/min.
- On day 3, have the mice run for 10 min at 7.2 m/min.
- On day 4, have the mice run for 10 min at 8.4 m/min.
- On day 5, submit the mice to an incremental test to exhaustion, according to the following protocol.
- Let the mice warm up for 5 min at 4.8 m/min (at a 0° inclination).
- Increase the speed by 1.2 m/min every 3 min (e.g., 5 min at 4.8 m/min, then 3 min at 6 m/min, 3 min at 7.2 m/min, 3 min at 8.4 m/min, etc.) until exhaustion, which is reached when the mouse either spends 3 consecutive seconds on the electric grid or receives 100 shocks (displayed by the apparatus).
- Write down the achieved speed (considered as the Vmax), duration, distance, number of shocks, and total time spent on the grid.
NOTE: Typically, Vmax was 28.8 ± 3.7 m/min. - Mid-training, resubmit the mice to this test in order to readjust the speeds of training to the updated Vmax of the mice (e.g., if the training protocol lasts 8 weeks, then perform a mid-training incremental test at 4 weeks. In that case, replace one of the scheduled trainings by the test), and do so again at the end of the study in order to assess performance improvements.
- Implement a 48 h rest period before and after this test.
NOTE: All the incremental tests were performed in the morning.
3. Hypoxic Environment
- For the training sessions in hypoxia, place the treadmill in the hypoxic box (Figure 1) linked to a gas mixer. Use a calibrated oximeter to regularly control the ambient fraction of oxygen (FiO2 [i.e., the level of hypoxia]) in the box.
- Set the gas mixer on 100% of nitrogen (N2) and use the oximeter to verify the level of hypoxia. Once FiO2 = 0.13, change the parameter of the gas mixer from 100% N2 to 13% O2.
- In order to avoid prolonged passive exposure to hypoxia, place the mice in a temporary smaller cage with litter and enrichment, and quickly place it in the box once FiO2 = 0.13 has been reached. Verify that the environment is still at 13% O2 after putting the cage in; if not, readjust it.
- Regularly verify the level of O2 over the course of a training session to make sure that it remains at FiO2 = 0.13 ± 0.002.
4. Normoxic Environment
- For the training sessions in normoxia, keep the treadmill in the hypoxic box, but remove the gloves so that there is ambient air (FiO2 = 0.21). The aim is to recreate the same training environment as the mice in hypoxia.
5. Supramaximal Intensity Training
- Place the mice on individual lanes in the treadmill (at a 0° inclination) and submit them to the following protocol.
- Have the mice warm up for 5 min at 4.8 m/min, followed by 5 min at 9 m/min.
- Set the speed of the sprints to 150% of the previously determined Vmax.
NOTE: Typically, the sprint velocity was 42.1 ± 5.5 m/min. - Train the mice for four sets of 5x 10 s sprints with 20 s of rest between each sprint. The interset rest is 5 min (Figure 2).
NOTE: Add a cooldown period if the total workload of the training session needs to match that of another training group.
- Perform this training 3x per week, with preferably 48 h between training sessions.
- Use cotton swabs as a complementary method to electric shocks to encourage the mice to run. Place a cotton swab in a slit at the top of the lane, between the mouse and the electric grid, and gently nudge the mouse when it reaches the back of the treadmill. This will avoid the delivery of electric shocks and stimulate the mice to run in a softer way.
6. Low-intensity Training
- Place the mice on individual lanes in the treadmill (at a 0° inclination) and submit them to the following protocol.
- Have the mice warm up for 5 min at 4.8 m/min, followed by 5 min at 7.2 m/min.
- Set the speed of the continuous running session to 40% of the previously determined Vmax.
NOTE: Typically, the continuous running velocity was 9.9 m/min. - Train the mice for 40 min.
- Perform this training 3x per week with preferably 48 h between training sessions.
- Use cotton swabs as a complementary method to electric shocks to encourage the mice to run.
7. Mice Euthanasia and Organ Extraction
- At the end of the training protocol and at least 24 h after the last incremental test, anesthetize the mouse in an induction chamber using isoflurane (4%–5% in O2 to induce anesthesia, and 1%–2% in 100% O2 to maintain anesthesia). Confirm proper anesthetization using the paw retraction reflex (firmly pinch the animal's paw; anesthesia is considered proper when the animal does not react to the stimulus).
- Using a 25 G needle, perform a percutaneous cardiac puncture, to collect maximum blood volume as previously described19.
- Perform a cervical dislocation and remove the skin of the mouse by cutting through the first layer of skin on the abdomen with round-tip scissors and pulling on the two sides of the incision (toward the head and the tail).
- Cut through the peritoneum under the ribcage on the left side of the mouse with thin-point-tip scissors to reach the spleen and extract it if needed.
NOTE: Dissect out muscles if needed. - Dissect out the pulmonary artery.
- Using both small scissors and forceps, remove the thoracic cage and clear the heart-lung area.
- With "self-closing" tweezers, pinch the heart as close as possible to the apex and pull gently to stretch the base of the aortic arch and the pulmonary artery.
- Using the right hand, insert curved tweezers under the pulmonary artery and the aorta, and then move the tweezers back a little to hold only the pulmonary artery (Figure 3).
- Use the left hand to insert another pair of tweezers to replace the one held with the right hand.
- Using sharp straight microscissors in the right hand, dissect the pulmonary artery as close to the heart as possible on one side, and as far away as possible on the other side.
NOTE: It does not matter which hand holds which instrument, although we have found it easier to cut with the right hand than with the left. - Put it in a 2 mL tube with cold phosphate-buffered saline (PBS) buffer and keep on ice.
- Perform a whole-body perfusion.
- At the top of the right lower limb of the mouse, use tweezers to clear out the external-internal right iliac artery down to the right femoral artery (under the inguinal ligament). Using sharp straight microscissors, make a full cut in the femoral artery.
- Insert a 5 mL 25 G syringe filled with cold PBS in the left ventricle of the heart and gently inject the cold PBS to remove the remaining blood from the vessels.
NOTE: Due to the extraction of the pulmonary artery, it is possible that PBS does not circulate all the way to the incision.
- Using tweezers, remove the soft tissue surrounding the aorta from the left and right inguinal ligaments to the heart as thoroughly as possible.
NOTE: The heart can be extracted for further analysis if necessary. - Using both tweezers and microscissors, dissect out the heart up to the lowest point of the external iliac artery (in both left and right limbs) and place the entirely dissected-out section in a 10 cm-diameter dish with cold PBS.
- Using tweezers and/or microscissors, finish cleaning the remaining fat around the aorta and arteries by gently pulling or cutting it away from the vessels.
- Using microscissors, cut the left iliac artery at the left-right iliac artery bifurcation and store it for further analysis.
- Using microscissors, cut the abdominal aorta under the left renal artery, and place the extracted vessel in cold PBS buffer on ice (Figure 4).
- Keep the remaining cleaned vessel, from the aortic arch to right above the left renal artery, in storage for further analysis.
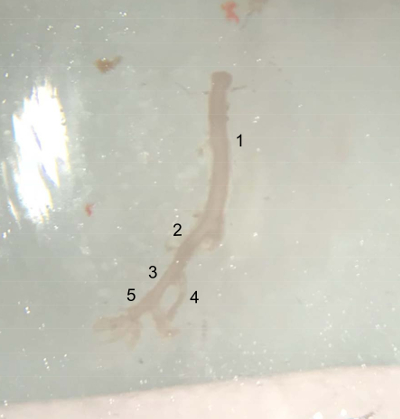
Figure 4: Picture of the dissected vessels. Extracted vessel from the top of the abdominal aorta (underneath the left renal artery) to the end of the right iliac artery, ready to be placed in cold PBS buffer on ice. (1) Abdominal aorta. (2) Right common iliac artery. (3) External iliac artery. (4) Internal iliac artery. (5) Femoral artery. Please click here to view a larger version of this figure.
8. Ex Vivo Vascular Function Assessment
NOTE: A wash corresponds to the emptying and refilling of the chambers with Krebs.
- According to a previously described protocol20, cut the isolated pulmonary artery, abdominal aorta, and right iliac artery segments into vascular rings of 1.0–2.0 mm long and mount each ring on two 0.1 mm-diameter stirrups passed through the lumen.
- Suspend the vessel rings in vertical organ chambers filled with 10 mL of modified Krebs-Ringer bicarbonate solution (118.3 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25.0 mM NaHCO3, and 11.1 mM glucose) maintained at 37 °C and aerated with 95% O2-5% CO2 (pH 7.4). One stirrup is anchored to the bottom of the organ chamber and the other one is connected to a strain gauge for the measurement of isometric force in grams.
- Bring the vessels to their optimal resting tension: stretch the rings to 0.5 g for the pulmonary artery, 1.5 g for the iliac artery, and 2 g for the abdominal aorta, and wash them after a 20 min period of equilibration. Repeat the stretch-equilibration-wash steps 1x.
- To test the viability of the vessels, contract the rings with 235 µL of KCl (10-1 M) for 10 min, wash them for another 10 min, and contract again with 235 µL of KCl (10-1 M) for about 20 min until reaching a plateau.
- Wash the vessels again for 10 min and add 58.4 µL of indomethacin (10-5 M) (an inhibitor of cyclooxygenase activity) for at least 20 min in order to avoid possible interference of endogenous prostanoids.
- Add cumulative doses of phenylephrine (Phe) from 10-9 (10 µL) to 10-4 M (or 10-9 to 10-5 M for the pulmonary artery; 9 µL for all concentrations above 10-9 M) to contract the vessels.
- After the last dose of Phe, wait for about 1 h until the vessels reach a relatively stable contraction state (plateau).
- Add cumulative doses of the endothelium-dependent vasodilator acetylcholine (ACh), from 10-9 to 10-4 M (58.4 µL for 10-9 M, and alternately 12.6 µL and 40 µL for all concentrations above 10-9 M), to induce nitric oxide (NO)-mediated relaxation.
- At the end of the relaxation curve, wash the vessels for 10 min, and add 58.4 µL of indomethacin (10-5 M), as well as 184 µL of NG-nitro-L-arginine (NLA, 10-4 M), which is an inhibitor of the NOS, for at least 20 min.
- Contract the vessels again with a unique dose of 10 µL of Phe (10-5 and 10-4 M for the pulmonary artery and 10-4 M for the abdominal aorta and the iliac artery) for 1 h, to induce a relatively stable contraction.
- Add a unique dose of 40 µL of ACh (10-4 M) until reaching a plateau.
- Wash the vessels again for 10 min, before adding 58.4 µL of indomethacin (10-5 M) and 184 µL of NLA (10-4 M) for 20 min.
- Contract the vessels with 10 µL of Phe (10-5 and 10-4 M) for 1 h.
- Add cumulative doses (10-9 [58.4 µL] to 10-4 M [40 µL for all concentrations above 10-9 M]) of the NO donor diethylamine (DEA)/NO, in order to assess the endothelium-independent NO-induced relaxation.
- At the end of the experiment, store the vessels in liquid nitrogen for future analyses if needed.
Results
To our knowledge, the present study is the first to describe a program of supramaximal intensity training in normoxia and in hypoxia for mice. In this protocol, mice ran four sets of five 10 s sprints with a 20 s recovery in between each sprint. The sets were interspersed with 5 min of recovery periods. It was unknown whether the mice would be capable of sustaining such a protocol and complete it properly. However, according to Figure 5, the body weight gain ...
Discussion
The first objective of this study was to assess the feasibility of hypoxic high-intensity training in mice and to determine the adequate characteristics of the protocol that would be well tolerated by mice. Purposely, since there is no data using supramaximal (i.e., more than Vmax) intensity training in mice, we had to perform trials based on previous protocols developed with athletes, which consisted of four to five sets of five all-out sprints (about 200% of Vmax), interspersed with 20 s active re...
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Danilo Gubian and Stephane Altaus from the Lausanne University Hospital (CHUV) mechanical workshop for helping create the hypoxic setup. The authors would also like to thank Diane Macabrey and Melanie Sipion for their help with training the animals.
Materials
| Name | Company | Catalog Number | Comments |
| Cotton swab | Q-tip | ||
| Gas mixer Sonimix 7100 | LSI Swissgas, Geneva, Switzerland | Gas-flow: 10 L/min and 1 L/min for O2 and CO2, respectively | |
| Hypoxic Box | Homemade | Made in Plexiglas | |
| Motorized rodents treadmill Panlab LE-8710 | Bioseb, France | ||
| Oximeter Greisinger GOX 100 | GREISINGER electronic Gmbh, Regenstauf, Germany | ||
| Sedacom software | Bioseb, France | ||
| Strain gauge | PowerLab/8SP; ADInstruments |
References
- Calbet, J. A., et al. Determinants of maximal oxygen uptake in severe acute hypoxia. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 284 (2), 291-303 (2003).
- Hanada, A., Sander, M., González-Alonso, J. Human skeletal muscle sympathetic nerve activity, heart rate and limb haemodynamics with reduced blood oxygenation and exercise. The Journal of Physiology. 551, 635-647 (2003).
- Casey, D. P., Joyner, M. J. Compensatory vasodilatation during hypoxic exercise: mechanisms responsible for matching oxygen supply to demand. The Journal of Physiology. 590 (24), 6321-6326 (2012).
- Casey, D. P., et al. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. The Journal of Physiology. 588, 373-385 (2010).
- Girard, O., Brocherie, F., Millet, G. P. Effects of Altitude/Hypoxia on Single- and Multiple-Sprint Performance: A Comprehensive Review. Sports Medicine. 47 (10), 1931-1949 (2017).
- Faiss, R., Girard, O., Millet, G. P. Advancing hypoxic training in team sports: from intermittent hypoxic training to repeated sprint training in hypoxia. British Journal of Sports Medicine. 47, 45-50 (2013).
- Brocherie, F., Girard, O., Faiss, R., Millet, G. P. Effects of Repeated-Sprint Training in Hypoxia on Sea-Level Performance: A Meta-Analysis. Sports Medicine.(Auckland, N.Z). 47 (8), 1651-1660 (2017).
- Brocherie, F., et al. Repeated maximal-intensity hypoxic exercise superimposed to hypoxic residence boosts skeletal muscle transcriptional responses in elite team-sport athletes. Acta Physiologica. 222 (1), 12851 (2018).
- Pellegrin, M., et al. New insights into the vascular mechanisms underlying the beneficial effect of swimming training on the endothelial vasodilator function in apolipoprotein E-deficient mice. Atherosclerosis. 190 (1), 35-42 (2007).
- Picard, M., et al. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. Journal of Applied Physiology. 115 (10), 1562-1571 (2013).
- Ayachi, M., Niel, R., Momken, I., Billat, V. L., Mille-Hamard, L. Validation of a Ramp Running Protocol for Determination of the True VO2max in Mice. Frontiers in Physiology. 7, (2016).
- Pellegrin, M., et al. Running Exercise and Angiotensin II Type I Receptor Blocker Telmisartan Are Equally Effective in Preventing Angiotensin II-Mediated Vulnerable Atherosclerotic Lesions. Journal of Cardiovascular Pharmacology and Therapeutics. 22 (2), (2016).
- Semin, I., Acikgöz, O., Gönenc, S. Antioxidant enzyme levels in intestinal and renal tissues after a 60-minute exercise in untrained mice. Acta Physiologica Hungarica. 88 (1), 55-62 (2001).
- Cho, J., et al. Treadmill Running Reverses Cognitive Declines due to Alzheimer Disease. Medicine & Science in Sports & Exercise. 47 (9), 1814-1824 (2015).
- Schill, K. E., et al. Muscle damage, metabolism, and oxidative stress in mdx mice: Impact of aerobic running. Muscle & Nerve. 54 (1), 110-117 (2016).
- Cho, J., Kim, S., Lee, S., Kang, H. Effect of Training Intensity on Nonalcoholic Fatty Liver Disease. Medicine & Science in Sports & Exercise. 47 (8), 1624-1634 (2015).
- Sabatier, M. J., Redmon, N., Schwartz, G., English, A. W. Treadmill training promotes axon regeneration in injured peripheral nerves. Experimental Neurology. 211 (2), 489-493 (2008).
- Rolim, N., et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic Research in Cardiology. 110 (4), 44 (2015).
- Lab Animal Research. Blood Withdrawal I. JoVE Science Education Database Available from: https://www.jove.com/science-education/10246/blood-withdrawal-i (2018)
- Peyter, A. -. C., et al. Muscarinic receptor M1 and phosphodiesterase 1 are key determinants in pulmonary vascular dysfunction following perinatal hypoxia in mice. American Journal of Physiology-Lung Cellular and Molecular Physiology. 295 (1), 201-213 (2008).
- Faiss, R., et al. Significant Molecular and Systemic Adaptations after Repeated Sprint Training in Hypoxia. PLOS ONE. 8 (2), (2013).
- Faiss, R., et al. Repeated Double-Poling Sprint Training in Hypoxia by Competitive Cross-country Skiers. Medicine & Science in Sports & Exercise. 47 (4), 809-817 (2015).
- Billat, V. L., Mouisel, E., Roblot, N., Melki, J. Inter- and intrastrain variation in mouse critical running speed. Journal of Applied Physiology. 98 (4), 1258-1263 (2005).
- Ferguson, S. K., et al. Effects of living at moderate altitude on pulmonary vascular function and exercise capacity in mice with sickle cell anemia. The Journal of Physiology. , (2018).
- Lightfoot, J. T., Turner, M. J., Debate, K. A., Kleeberger, S. R. Interstrain variation in murine aerobic capacity. Medicine & Science in Sports & Exercise. 33 (12), (2001).
- Wojewoda, M., et al. Running Performance at High Running Velocities Is Impaired but V'O2max and Peripheral Endothelial Function Are Preserved in IL-6-/- Mice. PLOS ONE. 9 (2), (2014).
- Muller, C. R., Américo, A. L. V., Fiorino, P., Evangelista, F. S. Aerobic exercise training prevents kidney lipid deposition in mice fed a cafeteria diet. Life Sciences. 211, 140-146 (2018).
- Petrosino, J. M., et al. Graded Maximal Exercise Testing to Assess Mouse Cardio-Metabolic Phenotypes. PLOS ONE. 11 (2), 0148010 (2016).
- Poole, D. C., Jones, A. M. Oxygen Uptake Kinetics. Comprehensive Physiology. , (2012).
- Copp, S. W., Hirai, D. M., Musch, T. I., Poole, D. C. Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. The Journal of Physiology. 588, 5077-5087 (2010).
- Kregel, K., et al. . Resource Book for the Design of Animal Exercise Protocols. , (2006).
- Lamy, S., et al. Air puffs as refinement of electric shocks for stimulation during treadmill exercise test. The FASEB Journal. 30, 1014 (2016).
- Koenen, K., et al. Sprint Interval Training Induces A Sexual Dimorphism but does not Improve Peak Bone Mass in Young and Healthy Mice. Scientific Reports. 7, (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved